Significance
A key challenge in biomedical research is the ability to specifically target cells and tissues. Targeting typically relies on identifying a suitable marker, e.g., a highly expressed receptor, and choosing a ligand that strongly and specifically binds to the marker. However, this procedure fails when a suitable marker unique to the targeted cells cannot be identified, notably in many forms of cancer. We show that properly designed multivalent targeting of multiple cognate receptor types results in a specificity toward a chosen receptor density profile, thus demonstrating a general route toward targeting cells without particularly dominant markers.
Keywords: multivalency, Monte Carlo simulations, drug delivery, endocytosis, statistical mechanics
Abstract
Cells can often be recognized by the concentrations of receptors expressed on their surface. For better (targeted drug treatment) or worse (targeted infection by pathogens), it is clearly important to be able to target cells selectively. A good targeting strategy would result in strong binding to cells with the desired receptor profile and barely binding to other cells. Using a simple model, we formulate optimal design rules for multivalent particles that allow them to distinguish target cells based on their receptor profile. We find the following: (i) It is not a good idea to aim for very strong binding between the individual ligands on the guest (delivery vehicle) and the receptors on the host (cell). Rather, one should exploit multivalency: High sensitivity to the receptor density on the host can be achieved by coating the guest with many ligands that bind only weakly to the receptors on the cell surface. (ii) The concentration profile of the ligands on the guest should closely match the composition of the cognate membrane receptors on the target surface. And (iii) irrespective of all details, the effective strength of the ligand–receptor interaction should be of the order of the thermal energy , where is the absolute temperature and is Boltzmann’s constant. We present simulations that support the theoretical predictions. We speculate that, using the above design rules, it should be possible to achieve targeted drug delivery with a greatly reduced incidence of side effects.
The fact that most cells can be recognized from the outside is advantageous for the normal functioning of an organism, but it can be a disadvantage when specific cells are being targeted by pathogens. Cells betray their identity (and state of health) by the composition profile of molecules that are exposed on their outer surface. In what follows, we call these molecules “receptors,” irrespective of whether they are receptors in the biological sense (they are receptors for the ligands that will be used to recognize them). It would clearly be advantageous if diseased cells could be selectively targeted by a drug-delivery vehicle on the basis of their receptor profile. Here, the crucial word is “selective”: We wish to target only those cells that have the correct receptor profile, as binding of drug-delivery vehicles to other cells may lead to undesired side effects.
Targeted drug delivery is based on identifying a specific marker (peptide, sugar) that is unique to the targeted group of cells. Binding to a single marker type can be effective if this molecule is presented in sufficient quantities on the outer surface of the targeted cell. However, in many cases of practical importance (e.g., many types of cancer), the markers that are known are not unique to cancer cells, but just overexpressed. Over the past 20 y many nanoparticle-based targeting methods have been developed. However, thus far, effective tumor drug delivery is hampered by the lack of reliable, unique markers (1, 2).
To recognize the simultaneous presence of a mixture of different receptors on the host surface, we need to use a “guest” particle (e.g., drug-delivery vehicle) that is coated with a mixture of cognate ligands schematically shown in Fig. 1. In its simplest form (the binding of dimeric bispecific antibodies compared with monomeric antibodies), this problem has been studied theoretically (3) and experimentally (4). The in vitro experiments showed that the use of bispecific antibodies led to a higher specificity than can be achieved with their standard, monomeric counterparts. However, antibodies are not very good at distinguishing between surfaces that have different receptor concentrations. Such selectivity can be achieved by exploiting multivalency. A series of recent experimental and theoretical papers have shown that multivalent carriers (nanoparticles or polymers) can distinguish target surfaces (cells) on the basis of their receptor concentration, rather than just on the basis of the presence of a suitable receptor (5–9).
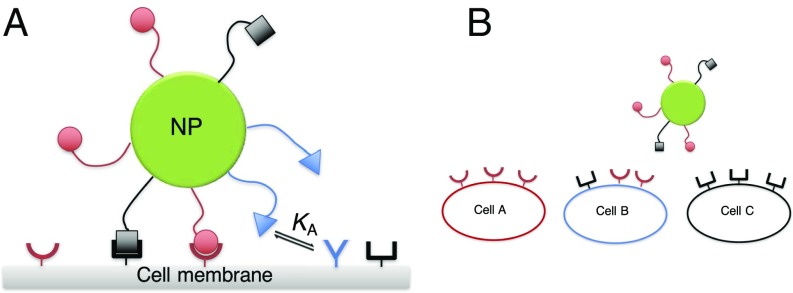
Fig. 1.
This schematic figure aims to explain the challenge addressed in this paper. A shows a schematic drawing of a multivalent nanoparticle binding to a surface containing several distinct receptors (represented as different concave shapes). B shows the challenge: How to target cell B selectively, in the presence of cell types A and C.
The use of multivalent particles coated with a single type of ligand is very effective, provided that a cognate receptor has been identified that is sufficiently overexpressed in targeted cells. But often the situation is not that clear cut (Fig. 1). In general, it is essential to exploit all of the information that we have about the concentration of various receptors on the cell surface and then design guest particles that target this specific receptor profile. In this paper, we show that the design rules for such multicomponent targeting are surprisingly simple and therefore hopefully useful. Specifically, we show that the individual ligand–receptor binding strength needs to be weak, such that when the guest particle is within interaction range of the surface, each ligand is unbound 30% of the time. To target a specific receptor profile selectively, many weak ligands work better than a few strong ones. We derive our results using a simple analytical theory and validate our approach using coarse-grained simulations. As an example, we present simulation results (Fig. 2) showing that correctly functionalized guest particles are more successful at entering cells with matching receptor compositions than those with suboptimal compositions.
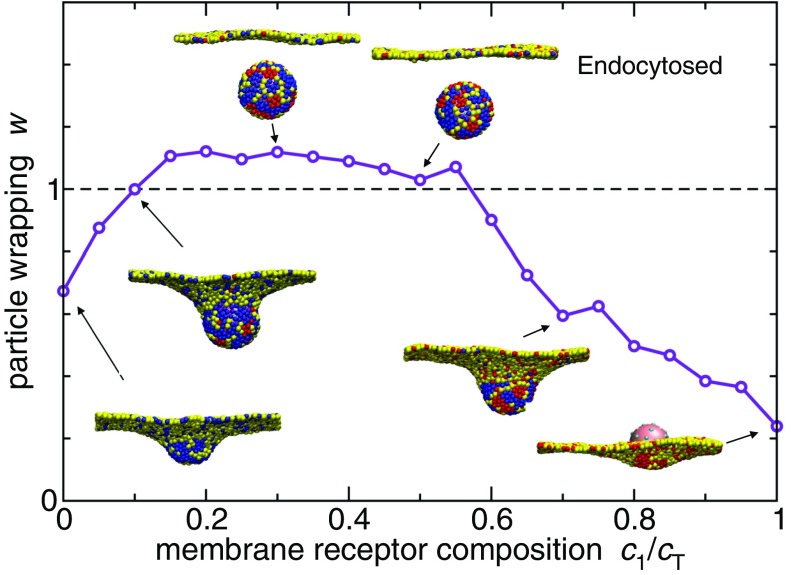
Fig. 2.
Simulation results of nanoparticle endocytosis. We consider a system with two ligand types on the particle and two cognate receptor types in the membrane with concentrations and , respectively. The red and blue beads in the membrane denote receptors of type 1 and type 2, respectively. The yellow beads are inert (no binding to the ligands). The total concentration of receptors is kept fixed at , but the composition is varied. The curve shows the coverage of the particle by the membrane beads (particle wrapping). When the wrapping exceeds 1, the particle is fully covered and has therefore undergone endocytosis. Snapshots show corresponding system configurations. The nanoparticle is covered with 40 randomly distributed immobile ligands with a “ligand” profile . The interaction strength of a ligand patch is determined as , with for the above results.
Of course, there is a wide variety of possible host–guest interactions that we could have considered: The receptors could be mobile or immobile, clustered or mixed, etc. In what follows we assume that the host cell is large compared with the guest particle and that the receptors are mobile on its surface. In this case, the chemical potential of the receptors is effectively fixed and, as shown in SI Appendix, the theoretical expressions for the binding free energy become surprisingly simple. The other situations that we mention can also be modeled, and the overall conclusions remain the same, except in the limit where so many guest particles bind to the host surface that they deplete the receptor “reservoir.” Hence, for the sake of simplicity, we consider just the easiest case. We do not specify the precise nature of the guest particle: It could be a functionalized nanoparticle, polymer, or self-assembled DNA-origami structure. Again, for the present analysis the distinction is immaterial. What is important is that the ligands are flexible and can reach multiple receptors on the surface equally well.
Model
The membrane surface is covered by a population of receptors of different types with concentrations . Similarly, a multivalent particle is characterized by the ligand composition , where is the total number of ligands on the guest particle and specifies their relative profile; i.e., the relative coverage of the particle with ligands of type is . We are interested in the case where guest nanoparticles interact with a fluctuating number of receptors on the surface. Hence, following the Bell adhesion model (10), the number of receptors in contact with the nanoparticle is not fixed, but their chemical potential is. In contrast, the number of ligands on the nanoparticle that can interact with the surface is fixed. The nanoparticle is attracted to the surface only through ligand–receptor interactions. Apart from that, the particle behaves like a hard sphere (Fig. 1). The ligand–receptor binding is valence limited; i.e., only a single ligand can bind to a receptor and vice versa. The model is an extension of the model of refs. 5 and 7, generalized to include different ligand/receptor types.
To calculate the binding free energy we need to consider all possible bonding combinations between receptors and ligands. To simplify the description, we neglect the interactions between different receptors and we assume that different ligands bind independently (except that no two ligands can bind to the same receptor). The probability that a single ligand and a single receptor form a bond depends on the equilibrium constant for their association in solution and on the free-energy cost , which is due to the loss of configurational entropy of the ligand upon binding. The configurational entropy term obviously depends on the distance between the receptor and the grafting point of the ligand, which we capture in the simulations. However, it turns out that the distance dependence of is not important in a simple theoretical description: In SI Appendix we show that we can treat the configurational term as if it were a constant () for all receptors within a distance of the ligands and infinite elsewhere. The parameter represents the ligand–receptor interaction range and is determined by the length of the polymeric linker.
For a ligand within the interaction range of the receptor-decorated surface, the ratio between the probabilities of being in the bound and free (unbound) states is
| [1] |
We have defined the effective association constant matrix , which includes the configurational contribution. Note that the first index in always refers to a ligand, and the second index always refers to a receptor. Thus, describes the equilibrium constant for binding between ligand and its cognate receptor . Emphatically, it does not mean that ligand and receptor are the same species. Similarly, describes the “cross”-binding of ligand with the receptor cognate to ligand . is, in general, not the same as , which describes the cross-binding of ligand with receptor cognate to ligand . Using the fact that probabilities must add to unity, , we can directly determine the probability that a given ligand is unbound, .
As shown in SI Appendix, the fact that the receptors do not interact with each other and are in contact with a reservoir (the remainder of the cell surface) simplifies the expression of the binding free energy between a guest particle and a host membrane. Moreover, we assume that different ligands are uncorrelated; hence the binding free energy of the guest particle is simply a sum of individual ligand contributions, , with the binding free energy per ligand:
| [2] |
We note that this expression could be interpreted as (minus) the cross-entropy between the two distributions and . The total binding free energy for a guest particle near the cell surface is , where is the zero-bond free-energy cost of bringing a guest particle into a position to start forming bonds with the host membrane. For what follows, is unimportant, because we assume that it is the same irrespective of the receptor composition or the ligand profile, and thus it drops out of the expressions for free-energy difference that determine the selectivity. We note that the host–guest binding free energy is related to the widely used “avidity” constant , measuring the association between multivalent entities in units of standard molar concentration M.
Furthermore, the same free-energy expression (Eq. 2) also governs the free-energy change upon a passive particle endocytosis (Fig. 2) where ligands are not flexible, but the membrane itself is. In this case simply refers to the particle endocytosis free-energy change when there are no receptors present, and again captures bonding with mobile receptors.
Selectivity Optimization
Expression 2 describes how the binding free energy depends on the receptor composition , particle profile , and the interaction matrix .
Our aim is to design a guest particle that binds more strongly to cells with the specified receptor profile than to any other. Among all possible receptor compositions c, the targeted composition should thus be the one with the minimum binding free energy. This yields the condition
| [3] |
Note that this equation does not imply that we optimize the receptor composition of the target cell (after all, this composition is given). Rather, we vary the parameters that characterize the guest particle (namely p and K) to make the guest particle bind more strongly to the target receptor composition than to any other. Because there are several combinations of p and K that can satisfy this condition, we need to further select the one that is the most selective, i.e., the one that results in the free-energy minimum with the largest curvature.
Our optimization condition is therefore to maximize the selectivity , defined as
| [4] |
subject to the constraint of Eq. 3. in Eq. 4 is the (Hessian) matrix of second derivatives of the free energy with respect to composition . As can be seen from Eq. 4, the selectivity is defined as the relative curvature of the free-energy functional at its minimum.
It is important to define the selectivity as the relative, rather than the absolute, curvature. The absolute value of the free energy can be trivially controlled by changing the number of bonds , Eq. 2. Therefore, by optimizing for the relative curvature, we obtain the largest possible curvature at a given absolute value of the free energy . The binding can always be made stronger by increasing the total receptor concentration (5–7, 11); therefore, to make a meaningful comparison, we compare systems with the same total receptor concentration on the membranes.
In general, finding the maximal selectivity by solving for the above conditions is nontrivial and must be performed numerically. However, we can greatly simplify the problem and find a closed-form solution by also requiring that the binding free energy is optimized with respect to the ligand profile :
| [5] |
This is in principle not a necessary condition for selective targeting; however, we expect it to be a practically useful condition; we wish to design guests that are robust to small variations in the ligand profile. Robustness is important for practical applications when the guest particle manufacturing-process tolerances must also be considered. The additional constraint results in a simple closed-form solution whereas the optimal selectivity decreases only marginally (SI Appendix).
We can determine the optimal ligand profile and interaction matrix analytically. The procedure that we use is discussed in SI Appendix; here we outline only the main results. Our first result is that all ligands should have the same probability to be unbound,
| [6] |
and that each ligand contributes an equal amount, , to the total binding free energy. Hence, any ligand profile will yield the same free energy, . In a sense, this result is trivial: It simply states that if all ligands are equally likely to bind, a small change in the ligand profile will not change the overall host–guest binding free energy. This result should not be viewed as a design rule to “target” guest particles by cells (in fact, the rule states that, in the optimal case, the cells cannot distinguish between different particles). Rather, we are interested in the opposite problem, namely the targeting of cells by guest particles. That problem does have a unique, nontrivial solution.
Minimizing the free-energy functional Eq. 2 with respect to the particle profile and targeted composition determines the optimal ligand profile . For a symmetric interaction matrix (or when off-diagonal terms are small) the ligand profile should match the cognate receptor composition: . Finally, the selectivity can be decomposed into a part that depends only on and a reduced Hessian that does not,
| [7] |
where is the “dimensionality” (the number of distinct receptor types). Optimizing the selectivity, , we find that the nontrivial solution satisfies , and hence
| [8] |
Eq. 8 is our most important result. It states that the binding free energy of each ligand to the targeted surface should be irrespective of the details of the system. Equivalently, Eq. 8 states that when the guest particle is adsorbed on the targeted surface, each ligand should be unbound of the time: .
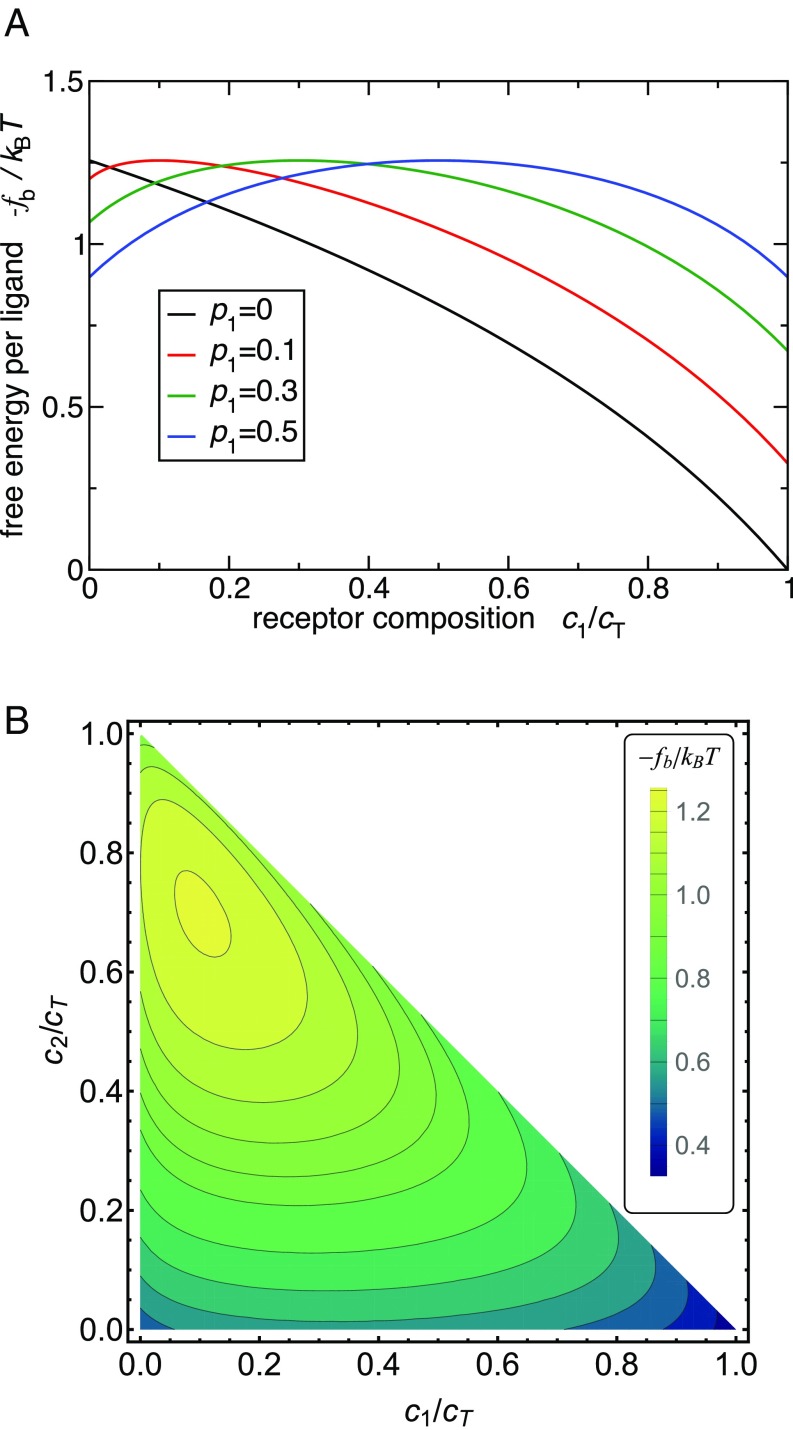
Fig. 3 shows the variation of the binding free energy with changing receptor composition obtained from the analytical model. In practice we might wish to distinguish a host surface with 20–80% receptor composition from a surface with inverted 80–20% composition. In Fig. 3 we see that the difference in the corresponding bond strength is about per ligand. This may not seem to be much, but multivalent guests will hold 10–20 ligands (or more) if we require a total guest binding free energy of the order of . The difference thus becomes substantial , corresponding to orders of magnitude difference in adsorption and, therefore, very strong targeting efficacy as shown in Fig. 4.
Fig. 3.
Optimal targeting using the analytical theory. (A) The binding free energy per ligand as a function of the cell receptor composition for two ligand/receptor types. Different curves correspond to different guest profiles . (B) Targeting with three ligand types. Contour plot shows the binding free energy as a function of the receptor composition and . The ligand profile is chosen as . We have used Eq. 2 to calculate the free energy, assuming a diagonal interaction matrix and optimal .
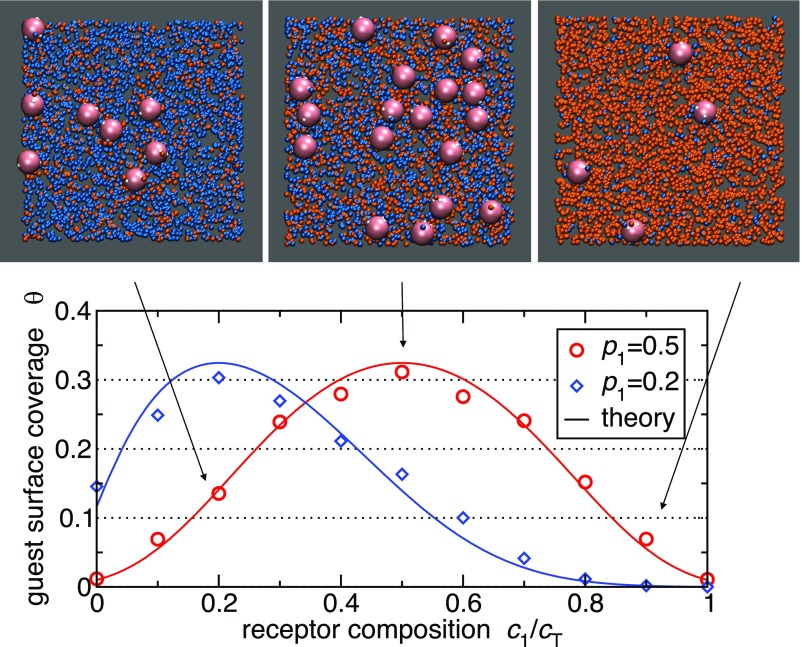
Fig. 4.
Grand canonical Monte Carlo simulation results of guest adsorption (symbols) and comparison with analytical theory (solid lines). The simulated surface has two different types of receptors embedded (colored orange and cyan, respectively). The total concentration of receptors is kept fixed at , with the guest particle radius. Each guest particle has 10 ligands (each represented as a soft blob with radius ) with a profile of (red circles) and (blue diamonds) and cognate bond energy . The guest chemical potential corresponds to a bulk solution concentration of . Theoretical adsorption curves (solid lines) are obtained by inserting the free-energy expression Eq. 2 at into a standard Langmuir adsorption model, assuming each guest occupies an area of . The surface coverage is the number of adsorbed guests per guest area . The three snapshots correspond to the plotted data (red circles) at composition and guest profile .
Furthermore, Fig. 3B demonstrates that increasing the number of ligand types increases the selectivity because the optimal binding region becomes a smaller fraction of the total parameter space.
Design Rules
Our analytical calculations suggest the following simple design rules to make multivalent guest particles that target a particular receptor profile:
-
∙
: Ideally, the profile of the nanoparticle should match the density composition of the targeted cell. As shown in SI Appendix, this is not a condition on the average ligand profile. It really means that, ideally, every nanoparticle should have precisely the optimal number of ligands. In fact, if only the averages are fixed and the number of ligands is Poisson distributed, most of the selectivity is lost.
-
∙
: The value of should be inversely proportional to the density . It is useful to avoid cross-binding (i.e., interaction matrix should be diagonal). The optimal binding free energy per ligand , which states that ligand binding should be weak, with each ligand independently having the probability of being bound at most 70%.
-
∙
The greater the number is of distinct ligand–receptor types, the higher the potential selectivity.
-
∙
The overall binding free energy of the particle is proportional to the number of ligands per guest particle (valency ). Valency should be chosen to give a desired absolute value of guest adsorption strength; for Langmuir adsorption the optimal will be close to the chemical potential of the guests in solution .
The major assumption underlying the derivation of the design rules is that all pairs of ligands and receptors can in principle form a bond. This assumption is fulfilled when both the ligands and the receptors are mobile, which is often the case in biological systems. However, our results also apply in other situations with some additional restrictions: (i) For mobile ligands on the guest but immobile receptors on the membrane, the theory is relevant when the receptor density is large enough. If is the surface area of the membrane in contact with the guest particle, the receptor concentration should be large enough, , such that all ligands can find their binding partners. (ii) In the case of mobile receptors and immobile ligands, the ligand profile presented to the surface receptors must be independent of the guest particle orientation. This can be achieved when using a long, flexible ligand , or when targeting a deformable membrane that can wrap around a particle (Fig. 2), or by carefully uniformly coating the guest particle with ligands such that every “face” presents the same ligand profile. (iii) When both ligands and receptors are immobile, both constraints i and ii apply and in addition each ligand must be able to find a receptor within its interaction range : .
Monte Carlo Simulation Results
The above analytical model is highly idealized. However, the coarse-grained simulation results of both particle endocytosis (Fig. 2) and adsorption (Fig. 4) support the predictions of our analytical model. Our simulations clearly show that our design rules, even though derived from a simple model, are nevertheless directly applicable to more complex and realistic systems where ligand interactions, correlations, and membrane elasticity cannot be neglected.
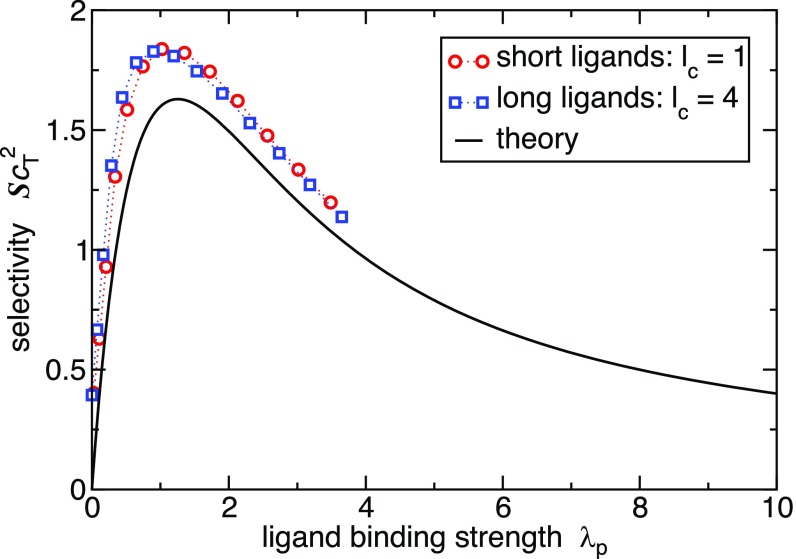
The simulation snapshots of multivalent nanoparticle targeting in Fig. 4 give a pictorial illustration of the effect of optimizing the ligand concentration profile to target a mixed receptor surface. The adsorption isotherm is well captured by the simple analytical model. Furthermore, Fig. 5 shows the selectivity, obtained from the analytical theory (Eq. 4) and simulations, plotted as a function of the ligand binding strength . Clearly, the simulation results support the analytical value for the optimal ligand strength .
Fig. 5.
Selectivity dependence on the mean bond strength . The theoretical curve is given by Eq. 7. The simulation results were obtained by calculating the free-energy profile of a simulated guest particle and obtaining the relative curvature, and hence the selectivity , from a parabolic fit to the free-energy profile. The mean bond strength from simulations is defined as the free energy per bond at the targeted composition . The simulated guest parameters are chosen according to our analytical design rules,
Conclusions
In this paper, we outline simple rules to design ligand-coated particles that can target cell surfaces on the basis of a receptor profile, rather than on the recognition of a single receptor. The receptor profile of a cell surface can be viewed as a “bar code” that is selectively recognized by the ligand profile of the guest particle. Here we have shown that properly designed multivalent targeting of multiple cognate receptor types results in a specificity toward a chosen receptor composition, thus demonstrating a general route toward targeting cells without particularly dominant markers.
We have assumed a generic case where background (untargeted) cell populations contain all possible receptor compositions. However, the selectivity can be increased further if only a few distinct cell populations are present and their receptor compositions are known in advance. In this case the optimal targeting strategy is obtained by maximizing the free-energy difference between discreet populations rather than the free-energy curvature. We also note that, although in this paper we focus on the targeting of cells, our model can also be used to understand how imprinted polymers can be used to sort cells (12, 13).
Materials and Methods
Endocytosis Simulations.
We perform Monte Carlo simulations with a coarse-grained membrane model (14) and a patchy hard-sphere model (15) for the nanoparticle. The nanoparticle has two different types of circular patches modeling coverage with two different ligand types. The membrane is composed of individual beads that can be either inert (representing normal lipids) or “receptor” beads that bind to the cognate patches on the particle, but are otherwise identical to the inert beads. The receptor beads can interact with the patches via a square-well attraction with width equal to the diameter of individual beads , where denotes the well depth. The particle wrapping is calculated as the number of membrane beads within a distance of the particle surface , normalized by the fully wrapped triangular lattice, , with the particle radius.
The simulations are performed using standard Monte Carlo translational moves in the ensemble and no applied external pressure (to be precise, our membrane system is metastable at zero applied external pressure, and the thermodynamically stable configuration is an infinitely large box; however, on a simulation timescale, a flat membrane is stable). The box size is with periodic boundary conditions in lateral directions. The box size in the vertical direction is sufficiently large to ensure that none of the particles ever interacts with the hard ceiling or the floor. The simulations started with the particle center of mass at height above the membrane and were run for cycles, where in each cycle on average one translational/rotational move per every bead and particle is attempted.
Multivalent Particle Adsorption.
We performed grand canonical Monte Carlo simulations where the chemical potential of the guest particles in solution is fixed to a value that results in a desired guest density in solution. Guest particles are represented as hard spheres with radius and attached polymeric ligand arms that are modeled as a series of soft blobs of size (16). Receptors are represented as points on the hard surface and can bind to ligands with a valence-limited harmonic bond and the interaction matrix determines the individual binding/unbinding probabilities. Standard Monte Carlo moves are used to displace and add/delete guests into the system, and Rosenbluth sampling is used to change polymeric ligand conformations. The model and technique are an extension of ref. 5 to multiple ligand/receptor types.
Free energies are calculated using the Wang–Landau sampling technique (17). First, we bias the sampling in the number of formed ligand–receptor bonds to obtain the absolute value of the bound guest free energy relative to a common reference point: a single unbound guest particle within interaction range of the surface ( for single-blob ligands and can be well approximated as the average height of a guest with a single formed bond). We then bias the simulation in the receptor composition to obtain the curvature of the free energy. The selectivity is calculated by fitting a quadratic function to the free-energy profile and normalizing by the absolute value as in Eq. 4. Langmuir isotherm (Fig. 4) zero-bond free energy is approximated as the translational entropy of a guest within a lattice site of size and height : .
Supplementary Material
Acknowledgments
We thank Bortolo Mognetti and Stefano Angioletti-Uberti for in-depth discussions on multivalency and Andđela Šarič for help with endocytosis simulations. This work was supported by the European Union through European Training Network NANOTRANS Grant 674979 and by the Herchel Smith fund.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704226114/-/DCSupplemental.
References
- 1.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm S, et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:14–26. [Google Scholar]
- 3.Caplan MR, Rosca EV. Targeting drugs to combinations of receptors: A modeling analysis of potential specificity. Ann Biomed Eng. 2005;33:1113–1124. doi: 10.1007/s10439-005-5779-1. [DOI] [PubMed] [Google Scholar]
- 4.Robinson MK, et al. Targeting ErbB2 and ErbB3 with a bispecific single-chain Fv enhances targeting selectivity and induces a therapeutic effect in vitro. Br J Cancer. 2008;99:1415–1425. doi: 10.1038/sj.bjc.6604700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Veracoechea FJ, Frenkel D. Designing super selectivity in multivalent nano-particle binding. Proc Natl Acad Sci USA. 2011;108:10963–10968. doi: 10.1073/pnas.1105351108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubacheva GV, et al. Superselective targeting using multivalent polymers. J Am Chem Soc. 2014;136:1722–1725. doi: 10.1021/ja411138s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dubacheva GV, Curk T, Auzély-Velty R, Frenkel D, Richter RP. Designing multivalent probes for tunable superselective targeting. Proc Natl Acad Sci USA. 2015;112:5579–5584. doi: 10.1073/pnas.1500622112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson CB, Mowery P, Owen RM, Dykhuizen EC, Kiessling LL. Selective tumor cell targeting using low-affinity, multivalent interactions. ACS Chem Biol. 2007;2:119–127. doi: 10.1021/cb6003788. [DOI] [PubMed] [Google Scholar]
- 9.Kiessling LL, Grim JC. Glycopolymer probes of signal transduction. Chem Soc Rev. 2013;42:4476–4491. doi: 10.1039/c3cs60097a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bell GI, Dembo M, Bongrand P. Cell adhesion. Competition between nonspecific repulsion and specific bonding. Biophys J. 1984;45:1051–1064. doi: 10.1016/S0006-3495(84)84252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Shaw DE. A simple model of multivalent adhesion and its application to influenza infection. Biophys J. 2016;110:218–233. doi: 10.1016/j.bpj.2015.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ren K, Zare RN. Chemical recognition in cell-imprinted polymers. ACS Nano. 2012;6:4314–4318. doi: 10.1021/nn300901z. [DOI] [PubMed] [Google Scholar]
- 13.Ren K, Banaei N, Zare RN. Sorting inactivated cells using cell-imprinted polymer thin films. ACS Nano. 2013;7:6031–6036. doi: 10.1021/nn401768s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan H, Huang C, Li J, Lykotrafitis G, Zhang S. One-particle-thick, solvent-free, coarse-grained model for biological and biomimetic fluid membranes. Phys Rev E. 2010;82:011905. doi: 10.1103/PhysRevE.82.011905. [DOI] [PubMed] [Google Scholar]
- 15.Kern N, Frenkel F. Fluid-fluid coexistence in colloidal systems with short-ranged strongly directional attraction. J Chem Phys. 2003;118:9882–9889. [Google Scholar]
- 16.Pierleoni C, Capone B, Hansen J-P. A soft effective segment representation of semidilute polymer solutions. J Chem Phys. 2007;127:171102. doi: 10.1063/1.2803421. [DOI] [PubMed] [Google Scholar]
- 17.Wang F, Landau DP. Efficient, multiple-range random walk algorithm to calculate the density of states. Phys Rev Lett. 2001;86:2050–2053. doi: 10.1103/PhysRevLett.86.2050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.