Abstract
In animal and fungal cells, the monomeric GTPase Cdc42p is a key regulator of cell polarity that itself exhibits a polarized distribution in asymmetric cells. Previous work showed that in budding yeast, Cdc42p polarization is unaffected by depolymerization of the actin cytoskeleton (Ayscough et al., J. Cell Biol. 137, 399–416, 1997). Surprisingly, we now report that unlike complete actin depolymerization, partial actin depolymerization leads to the dispersal of Cdc42p from the polarization site in unbudded cells. We provide evidence that dispersal is due to endocytosis associated with cortical actin patches and that actin cables are required to counteract the dispersal and maintain Cdc42p polarity. Thus, although Cdc42p is initially polarized in an actin-independent manner, maintaining that polarity may involve a reinforcing feedback between Cdc42p and polarized actin cables to counteract the dispersing effects of actin-dependent endocytosis. In addition, we report that once a bud has formed, polarized Cdc42p becomes more resistant to dispersal, revealing an unexpected difference between unbudded and budded cells in the organization of the polarization site.
INTRODUCTION
The Rho-family GTPase Cdc42p is critical for cell polarity in both yeast and mammalian cells (Pringle et al., 1995; Etienne-Manneville, 2004). In Saccharomyces cerevisiae, cell cycle commitment in late G1 triggers cell polarization in preparation for bud formation (Lew and Reed, 1993). This process involves the concentration of Cdc42p together with many other proteins in a “cap” at the presumptive bud site, the polarization of actin cables toward that site, the clustering of cortical actin patches (thought to be sites of endocytosis; Kaksonen et al., 2003) at that site, and the assembly of a ring of septin filaments surrounding the Cdc42p cap (Pruyne and Bretscher, 2000). Cdc42p is essential for polarization of cytoskeletal filaments (Adams et al., 1990), and it is believed that the concentration of Cdc42p at the presumptive bud site is critical for effective cell polarization. Consistent with this view, experiments in mammalian cells demonstrated that whereas activated, membrane-targeted Cdc42p distributed throughout the cell cortex did not induce cytoskeletal changes, spatial clustering of that Cdc42p sufficed to induce dramatic actin polarization (Castellano et al., 1999). Thus, the molecular basis for Cdc42p polarization is of fundamental importance to polarity establishment.
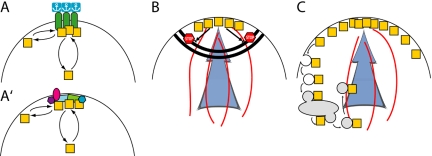
Once a cell is polarized, many cortical proteins are asymmetrically distributed. Three general classes of models have been invoked to explain such polarization (Figure 1). In the first model (Figure 1A), a preexisting stably polarized “anchor” interacts (directly or indirectly) with the protein of interest, thereby increasing its local concentration. In yeast, a subset of “bud site selection” proteins are integral membrane proteins with large extracellular domains that are thought to interact with the rigid cell wall in a manner that renders them immobile in the plane of the membrane (Harkins et al., 2001). These proteins are deposited at the poles of the cell during bud formation, allowing them to serve as “landmarks” that can anchor proteins to those sites in the following cell cycle (Schenkman et al., 2002).
Figure 1.
Mechanisms underlying polarized protein distribution. A protein of interest (squares) displaying an asymmetric distribution at the cell cortex can attain that polarization via one or more of the indicated mechanisms. (A) A preexisting asymmetric distribution of an anchored interacting factor can concentrate the protein of interest. Here, the interacting factor is shown connected to an extracellular anchor that might be a site of cell-cell contact, or an immobilizing extracellular matrix, or the yeast cell wall. A variant of the anchor model (A′) is that a dynamic patch of interacting proteins is assembled by a symmetry-breaking process and serves to localize the protein of interest. Like the anchor model, this does not require input from the cytoskeleton. However, the anchor-like patch is not fixed but dynamic, and can assemble de novo at random locations. (B) A diffusion barrier (stop signs indicate blocked movement of the squares), such as the claudin-dependent tight junction, or the yeast septin-dependent neck cortex, can keep a cortical protein from crossing the fence (thick double black line). For this to produce a polarized distribution, the protein must be delivered to only one side of the fence (indicated by the blue arrow), along polarized cytoskeletal elements (red lines). (C) Polarized delivery (blue arrow and red lines as in B) can yield a polarized distribution in the absence of a diffusion barrier if active recycling of the protein (here indicated by endocytic internalization, passage through a recycling endosome, and reexport along the polarized cytoskeleton) is fast enough to counter diffusion.
In the second model (Figure 1B), a “fence” of membrane-associated filaments forms a diffusion barrier such that proteins delivered to one compartment cannot cross the fence to another compartment. In yeast, the septin filament system is thought to act as a fence between the cortex of the bud and that of the mother, maintaining the asymmetric distributions of cortical proteins delivered to only one side (Barral et al., 2000; Takizawa et al., 2000). In the third model (Figure 1C), asymmetric distribution arises through a dynamic process in which the polarized cytoskeleton delivers cortical proteins to the “front” of the cell, and endocytic retrieval of the proteins occurs before they can diffuse too far from that site. Recycling of the endocytosed proteins to the front of the cell maintains the polarized distribution. In yeast, endocytosis of integral membrane SNARE proteins was required for their asymmetric distribution, indicating that they are polarized by this mechanism (Valdez-Taubas and Pelham, 2003). In all cases, a polarized cytoskeleton is needed to set up the asymmetry: the anchors and fences must be deposited or assembled at the right location, and polarized delivery to right side of the fence or the front of the cell is key for the latter two models.
Which of these models accounts for Cdc42p polarization to the presumptive bud site? In a classic study, Ayscough et al. (1997) demonstrated that although F-actin was required for polarization of many proteins, depolymerization of all F-actin did not prevent Cdc42p polarization, suggesting that polarized delivery of Cdc42p or of other factors is not required for polarization of Cdc42p. The simplest explanation for that result is that Cdc42p is polarized via interactions with previously deposited anchors, and indeed the bud site selection landmarks are known to influence the location to which Cdc42p becomes polarized (Pringle et al., 1995). However, mutations that eliminate the landmarks themselves or other factors required for recognition of the landmarks do not prevent polarization, but only randomize the site at which polarization takes place (Chant, 1999). We recently showed that even in cells lacking effective landmarks to serve as anchors, Cdc42p could become polarized in the complete absence of F-actin or microtubules (Irazoqui et al., 2003). This result indicated that Cdc42p polarization could occur without preexisting anchors and without the (actin-mediated) polarized delivery required by the fence and recycling models, indicating that this key regulator of polarity was itself polarized by a novel mechanism. Other data suggested that, together with interacting scaffolds and effectors including Bem1p, Cdc42p triggered the cooperative assembly of an anchor-like patch de novo (Irazoqui et al., 2003; Figure 1A′).
A central finding supporting anchor models (Figure 1, A and A′) for Cdc42p polarization was the ability of Cdc42p to polarize in the complete absence of F-actin. However, we now report that unlike loss of all F-actin, selective loss of actin cables causes the dispersal of Cdc42p from the prebud site in unbudded cells. Such dispersal is reduced in an endocytosis mutant, suggesting that endocytic internalization of polarity factors by actin patches must be counteracted by polarized delivery along actin cables to maintain polarization of Cdc42p. Intriguingly, the polarized cap of Cdc42p in cells that have already formed a bud is much less sensitive to actin cable perturbation, suggesting that there are significant and previously unsuspected differences in the organization of the Cdc42p cap in budded and unbudded cells.
MATERIALS AND METHODS
Yeast Strains, Elutriation, and Lat Treatment
Standard media were used for yeast growth (Guthrie and Fink, 1991). The yeast strains used are listed in Table 1. Lat A and Lat B were purchased from Molecular Probes (Eugene, OR) and kept as 200× (Latrunculin A [Lat A]) and 100× (Lat B) stocks in dimethyl sulfoxide (DMSO) at -20°C. Centrifugal elutriation to isolate early G1 cells was performed as described previously (Lew and Reed, 1993).
Table 1.
Yeast strains used in this study
| Strain | Relevant genotype | Reference/Source |
|---|---|---|
| ABY551 | a/α MYO2::HIS3/MYO2::HIS3 | Schott et al. (1999) |
| ABY553 | a/α myo2-16::HIS3/myo2-16::HIS3 | Schott et al. (1999) |
| ABY971 | a/α tpm1-2::LEU2/tpm1-2::LEU2 tpm2Δ::HIS3/tpm2Δ::HIS3 | Pruyne et al. (1998) |
| ABY973 | a/α tpm2::HIS3/tpm2::HIS3 | Pruyne et al. (1998) |
| DDY342 | α act1-113::HIS3 | Ayscough et al. (1997) |
| DDY345 | α act1-117::HIS3 | Ayscough et al. (1997) |
| DDY354 | aACT1::HIS3 | Ayscough et al. (1997) |
| DDY595 | abar1 sla2-41 | Wesp et al. (1997) |
| DLY5a | a/α bar1/BAR1 | Lew and Reed (1993) |
| DLY3644a | ahis3 HIS2 GAL1p-SWE1::LEU2 | This work |
| DLY7543b | a/α MYO2::HIS3/MYO2::HIS3 GAL1p-SWE1myc::URA3 | This work |
| DLY7544c | a/α myo2-16::HIS3/myo2-16::HIS3 GAL1p-SWE1-myc::URA3 | This work |
| GPY10a | abar1 rdi1::URA3 | David Stone |
| RH1800 | abar1 | Friant et al. (2000) |
Strains in the BF264-15Du background (ura3Δns leu2-3112 trp1-1a ade1 his2)
Generated by transforming ABY551 with pRS306-GAL1-SWE1myc (McMillan et al., 1998)
Generated by transforming ABY553 with pRS306-GAL1-SWE1myc (McMillan et al., 1998)
Fluorescence Staining and Microscopy
To visualize Cdc42p and Sec4p, cells were fixed for a total of 3 h in 3.6% formaldehyde, permeabilized with 0.5% SDS, and processed for immunofluorescence as previously described (Redding et al., 1991; Lehman et al., 1999). Anti-Cdc42p antibody (used at 1:200 dilution) and anti-Sec4p antibody (used at 1:100 dilution) were generously provided by Patrick Brennwald (UNC, Chapel Hill, NC). To visualize F-actin, cells were fixed as above, treated with acetone, and processed for staining with Alexafluor488-phalloidin as previously described for rhodamine-phalloidin (Pruyne et al., 1998). Cells were examined using a Zeiss Axioskop (Carl Zeiss, Thornwood, NY) with a 100× oil immersion objective. Images were captured using an ORCA cooled charge-coupled device camera (Hamamatsu, Bridgewater, NJ), interfaced with MetaMorph software (Universal Imaging, Silver Spring, MD). Images were processed for presentation using Photoshop (Adobe Systems, San Jose, CA). Individual cells or groups of cells in the correct focal plane were grouped together from one or more fields to assemble the figures.
RESULTS
Partial Actin Depolymerization Results in Loss of Cdc42p Polarity
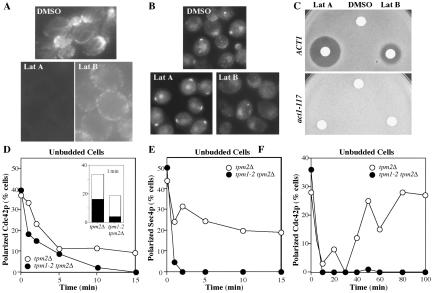
The role of F-actin in Cdc42p polarization has been addressed using Lat A, a G-actin–binding compound isolated from the Red Sea sponge Latrunculia magnifica (Spector et al., 1983). Treatment of yeast cells with 100 μM (or more) Lat A results in the rapid disassembly of all detectable F-actin structures (Figure 2A), blocking both polarized growth and endocytosis (Ayscough et al., 1997; Karpova et al., 2000). Because of the high cost of Lat A, we sought to extend our findings using its less expensive relative, Lat B. Lat B is not as potent as Lat A, and although detectable cables are lost after exposure to 100 μM Lat B, some F-actin patches remain (Figure 2A; McMillan et al., 1999). We were surprised to find that unlike Lat A, treatment of cells with Lat B led to a dispersal of Cdc42p from the prebud site (Figure 2B), with most unbudded cells losing polarized Cdc42p staining within an hour of Lat B addition.
Figure 2.
Loss of Cdc42p polarization upon partial actin depolymerization. (A) Wild-type cells (DLY5) were grown to exponential phase in YEPD at 30°C and treated with DMSO (control), Lat A (100 μM), or Lat B (100 μM) for 2 h. Cells were fixed and processed to visualize F-actin with Alexafluor488-phalloidin. (B) Cells treated as above except in this case with 200 μM Lat B were processed to visualize Cdc42p by indirect immunofluorescence. (C) Lawns of wild-type (ACT1: DDY354) and latrunculin-resistant actin mutant (act1–117: DDY345) cells were spread on YEPD plates, sterile paper discs spotted with 10 μl DMSO (control), Lat A (2 mM), or Lat B (4 mM) were placed on the agar, and the plates were incubated for 2 d at 30°C. Growth inhibition by Lat A and (less potently) Lat B produced a clear zone or halo around the discs, but act1-117 mutants were completely resistant to both Lat A and Lat B. Identical results were obtained with act1-113 mutants (DDY342). (D and E) Tropomyosin mutant (tpm1-2 tpm2Δ: ABY971) and control (tpm2Δ: ABY973) cells were grown to exponential phase in YEPD at 24°C and shifted to 34.5°C at t = 0. Rapid temperature shift was performed as described by Pruyne et al. (1998). Samples were fixed and double-stained to visualize Cdc42p (D) and Sec4p (E) by indirect immunofluorescence. More than 190 unbudded cells were scored for the presence of polarized signal at each time point. In the case of Cdc42p the polarized signal, though present, was diminished in the tropomyosin mutant after 1 min of shift: the inset in D indicates the proportion of cells with a bright polarized patch (black bar) versus a faint patch or extended crescent (white bar) at this time. (F) Cdc42p staining to a bright patch was quantitated in >100 unbudded cells from a separate experiment extending the temperature shift to longer times.
One interpretation of these findings is that Lat B has another target in addition to actin and that this second target causes dispersal of Cdc42p. However, we found that actin mutants previously shown to be resistant to Lat A (Ayscough et al., 1997) were also resistant to Lat B (Figure 2C), suggesting that Lat B exerts its effects through actin. Consistent with this view, lower doses of Lat A also caused some Cdc42p dispersal (see Supplementary Figure 1).
It is not clear from our data whether the dispersed Cdc42p remains at the cortex or redistributes to intracellular membranes, because immunofluorescence protocols do not reveal detectable unpolarized pools. Studies using GFP-Cdc42p, in contrast, detect abundant fluorescence throughout the cortex as well as on intracellular membranes (Richman et al., 2002; Wedlich-Soldner et al., 2004), but in that case the polarized signal is only a small fraction of the total, and redistribution would be hard to detect.
Selective Loss of Actin Cables Results in Loss of Cdc42p Polarity
Why would partial F-actin depolymerization disperse Cdc42p when full depolymerization does not? Because actin cables appear to be more sensitive to Lat B than cortical patches, one possibility was that selective disruption of cables by Lat B was responsible for dispersing Cdc42p. To test this hypothesis, we made use of temperature-sensitive tropomyosin mutants, which selectively lose actin cables but not patches upon shift to restrictive temperature, leading to the dispersal of polarized Sec4p (a secretory vesicle marker) within a minute after shift (Figure 2E; Pruyne et al., 1998). We found that polarized Cdc42p was dispersed from the prebud site within 10 min upon shift of tropomyosin mutant cells to restrictive temperature (Figure 2D). A complication with this experiment is that even wild-type cells transiently disperse polarized Cdc42p upon temperature shift (Figure 2D; Ho and Bretscher, 2001). However, dispersal of Cdc42p from the prebud site was reproducibly more rapid in tropomyosin mutants than in wild-type cells. Indeed, considerable Cdc42p dispersal had occurred after only 1 min at restrictive temperature in the tropomyosin mutant cells, and most of the cells still displaying polarized staining for Cdc42p had fainter or more diffuse staining compared with the bright Cdc42p spots in the wild-type cells (Figure 2D, inset). Thus, selective loss of actin cables led to rapid dispersal of Cdc42p from the polarization site in unbudded cells. Moreover, unbudded wild-type cells recovered polarized Cdc42p staining by 50 min after temperature shift, but the unbudded tropomyosin mutant cells never recovered polarized Cdc42p (Figure 2E), indicating that cables are required for regaining and/or maintaining polarity.
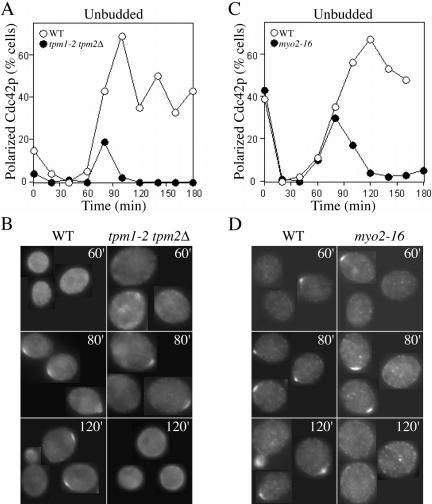
To ask whether loss of actin cables prevented the establishment as well as the maintenance of Cdc42p polarity, we isolated early G1 wild-type and tropomyosin mutant cells from asynchronous populations using centrifugal elutriation, incubated them at restrictive temperature, and examined Cdc42p localization as cells progressed through the cell cycle. The 50-min temperature shift recovery period in such cells occurs before cell cycle commitment and polarization. Wild-type and tropomyosin mutant cells both initially polarized Cdc42p at 80 min, but although wild-type cells retained polarized Cdc42p and went on to bud, the tropomyosin mutants lost polarized Cdc42p staining within 20 min and never formed a bud (Figure 3, A and B). Thus, Cdc42p polarization to the presumptive bud site can occur in the absence of actin cables, but it is not maintained.
Figure 3.
Selective loss of actin cables or Myo2p function impairs maintenance but not establishment of Cdc42p polarization. (A and B) Tropomyosin mutant (tpm1-2 tpm2Δ: ABY971) and wild-type (ABY551) cells were grown to exponential phase in synthetic complete medium without histidine at 24°C, and small daughter cells were isolated by centrifugal elutriation, harvested by centrifugation, and resuspended in prewarmed YEPD at 35°C (t = 0). Samples were fixed at 20-min intervals and processed to visualize Cdc42p by indirect immunofluorescence. (A) Quantitation: >100 cells were scored for the presence or absence of Cdc42p polarization. (B) Representative cells from the indicated time points. (C and D) Myosin mutant (myo2-16: ABY553) and wild-type (ABY551) cells were treated as above except that the restrictive temperature was 37°C. (C) Quantitation: >100 cells were scored for the presence or absence of Cdc42p polarization. (D) Representative cells from the indicated time points.
Only 20% of tropomyosin-mutant cells showed a clear Cdc42p polarization at 80 min, compared with 43% of control WT cells. Thus, the Cdc42p dispersal mechanism may be sufficiently effective to completely block Cdc42p polarization in many cells. Alternatively, all cells may be able to establish an initial Cdc42p polarity, which is then rapidly dispersed. As polarization of Cdc42p is triggered by a cell cycle cue and the population of cells was not perfectly synchronous with respect to cell cycle stage, we might only detect a robust Cdc42p cap in the 20% of cells that were in just the right cell cycle stage in this experiment.
One way that actin cables could contribute to Cdc42p polarity is by promoting polarized trafficking of cargo by myosin motors. The type V myosin Myo2p transports secretory vesicles and other cargo along actin cables (Schott et al., 1999, 2002). To test whether Myo2p-mediated trafficking was important for maintaining Cdc42p polarization at the prebud site, we repeated the synchrony experiment using temperature-sensitive myo2-16 mutants. As shown in Figure 3, C and D, myo2-16 cells initially polarized Cdc42p at the same time as wild-type controls, but then lost polarized Cdc42p staining and failed to form buds. A significantly higher fraction of cells were observed to polarize Cdc42p in the myosin mutants (Figure 3, C and D) compared with the tropomyosin mutants (Figure 3, A and B). This observation may reflect the presence of residual Myo2p activity in the mutant, or perhaps a contribution from additional actin cable-mediated pathways (e.g., the related myosin Myo4p). Regardless, these experiments indicate that Myo2p-mediated polarized traffic along actin cables is required for maintenance (though not for initial establishment) of Cdc42p polarity at the prebud site.
Dispersal of Cdc42p Involves Endocytosis
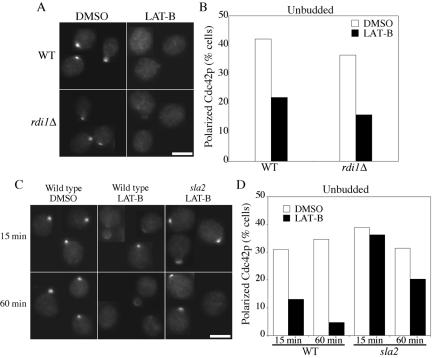
Cdc42p is prenylated and peripherally associated with cellular membranes, but significant cytoplasmic pools also exist (Ziman et al., 1993). It is thought that the guanine nucleotide dissociation inhibitor (GDI) Rdi1p removes Cdc42p from the membrane to the cytosolic pool (Masuda et al., 1994; Koch et al., 1997). Thus, the Cdc42p dispersal after selective elimination of actin cables could occur via direct displacement of Cdc42p from the membrane by Rdi1p, or by endocytic uptake of Cdc42p (or of anchoring factors for Cdc42p) from the presumptive bud site. We found that dispersal of Cdc42p in response to Lat B was unaffected by deletion of RDI1 (Figure 4, A and B). To test whether Cdc42p dispersal required endocytosis, we used temperature-sensitive sla2 mutants (also called end4) that have a defect in the internalization step of endocytosis (Raths et al., 1993). Sla2p is a homolog of the mammalian huntingtin-interacting protein (HIP1; Henry et al., 2002) and is localized to actin patches (Yang et al., 1999). Sla2p binds to clathrin and is thought to be a conserved endocytic adaptor protein (Henry et al., 2002; Kaksonen et al., 2003). Wild-type and sla2 mutant cells were shifted to restrictive temperature for 1 h to inactivate Sla2p (in the mutant) and allow recovery of Cdc42p polarity after the shift. Lat B was then added for 15 or 60 min and Cdc42p polarity was examined (Figure 4, C and D). Dispersal of Cdc42p from the prebud site was significantly reduced in sla2 mutants compared with the controls, at both time points (Figure 4, C and D). Thus, Cdc42p dispersal occurs primarily by endocytosis, and F-actin cables are required to counteract this effect. As endocytosis is also dependent on F-actin (Ayscough et al., 1997), complete actin depolymerization by Lat A eliminates the dispersal mechanism and hence obviates the need for actin cables in maintaining Cdc42p polarization.
Figure 4.
Cdc42p dispersal involves endocytosis but not Rdi1p. (A and B) Wild-type (DLY1) and rdi1Δ (GPY10) cells were grown to exponential phase in YEPD at 30°C, treated with DMSO (control) or Lat B (200 μM) for 1 h, and then fixed and processed to visualize Cdc42p by indirect immunofluorescence. (A) Representative cells. Bar, 5 μm. (B) Quantitation: >100 cells were scored in each sample. (C and D) Wild-type (RH1800) and temperature-sensitive sla2 (sla2-41 = end4-1: DDY595) cells were grown to exponential phase in YEPD at 24°C and shifted to 37°C (restrictive temperature) for 1 h, after which Cdc42p polarity had largely recovered from the temperature shift. Lat B (200 μM) was added for an additional 15 or 60 min at 37°C, as indicated, leading to dispersal of Cdc42p in the wild-type but not in the sla2 cells. (C) Representative cells. Bar, 5 μm. (D) Quantitation: >100 cells were scored in each sample.
Cdc42p Polarization Becomes More Resistant to Dispersal after Bud Emergence
The studies described above focused on Cdc42p polarization to the presumptive bud site in unbudded cells. After bud emergence, Cdc42p remains polarized to the bud tip until the activation of Cdc28p by mitotic cyclins triggers the apical-isotropic switch in bud growth, after which Cdc42p is dispersed until cytokinesis, when it accumulates at the mother-bud neck (Pruyne and Bretscher, 2000; Richman et al., 2002). Like unbudded cells, small-budded cells also lost polarized Cdc42p staining after exposure to Lat B (unpublished data) or prolonged shift of tropomyosin mutants to restrictive temperature (Figure 5A). However, in the tropomyosin mutants this loss was significantly slower than the depolarization of Cdc42p in unbudded cells (compare Figure 5A with Figure 2F): Cdc42p initially depolarized because of temperature shift at the same rate in wild-type and tropomyosin mutant cells and then repolarized 50 min later, but was subsequently dispersed in the tropomyosin mutants. Although some of this dispersal likely reflects progress of the cell cycle to a point where Cdc42p normally becomes dispersed, recent evidence suggests that at least some of the budded tropomyosin mutants arrest the cell cycle before that stage (Pruyne et al., 2004). Thus, loss of actin cables leads to a delayed loss of Cdc42p polarization in budded cells.
Figure 5.
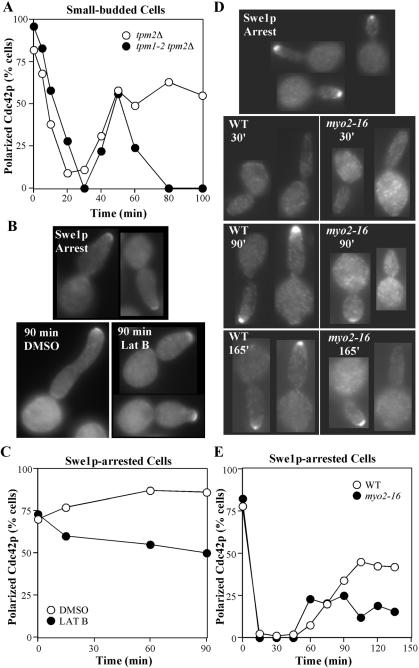
Cdc42p in budded cells is more resistant to dispersal by actin perturbation. (A) Tropomyosin mutant (tpm1-2 tpm2Δ: ABY971) and control (tpm2Δ: ABY973) cells from the same experiment as in Figure 2F above were scored to quantitate the proportion of small-budded cells displaying a tight patch of polarized Cdc42p. (B and C) Wild-type cells containing a GAL1-regulated SWE1 gene (DLY3466) were grown to exponential phase in YEP-sucrose at 30°C and induced to express Swe1p by addition of galactose to 2% final concentration. After 1 h 40 min, many cells displayed large elongated buds characteristic of Swe1p-mediated G2 arrest (Lew and Reed, 1993), and at that time (Swe1p Arrest) either DMSO or 100 μM Lat B was added. After 90 min, control (DMSO) cells continued to elongate their buds, whereas Lat B–treated cells ceased polarized growth and grew uniformly larger, although most did not disperse tip-localized Cdc42p. (B) Representative cells from the indicated samples. (C) Quantitation: >100 cells were scored for the presence or absence of Cdc42p polarization. (D and E) Wild-type and myo2-16 cells containing a GAL1-regulated SWE1 gene (DLY7543 and DLY7544, respectively) were grown to exponential phase in YEP-raffinose at 24°C and induced to express Swe1p by addition of galactose to 2% final concentration. After 2 h (Swe1p Arrest), the cells were shifted to 37°C, samples were fixed at 15-min intervals and processed to visualize Cdc42p. (D) Representative cells from the indicated samples. (E) Quantitation: >100 cells were scored for the presence or absence of Cdc42p polarization.
To directly test whether budded cells at a cell cycle stage before the apical-isotropic switch would disperse Cdc42p upon loss of cable-directed traffic, we arrested cells by overexpressing the mitotic cyclin/Cdc28p inhibitor Swe1p (Booher et al., 1993) and monitored Cdc42p localization after treatment with Lat B or shift-up of myo2-16 mutants. As shown in Figure 5, B and C, most Swe1p-arrested cells treated with Lat B retained polarized Cdc42p staining. In myo2-16 mutants, Cdc42p polarity was initially lost and then recovered after temperature shift as in wild-type cells. However, the degree of recovery was impaired in myo2-16 cells compared with wild-type controls (Figure 5, D and E), indicating that myosin-mediated traffic contributes to Cdc42p polarity in budded cells. In summary, treatments that all caused dispersal of Cdc42p in unbudded cells had differing effects in budded cells, causing complete (tropomyosin mutant), partial (myosin mutant), or little (Lat B) dispersal. Even when dispersal did occur, it was much slower than in unbudded cells. Thus, the polarized Cdc42p in budded cells is significantly more resistant than that in unbudded cells to treatments that selectively disrupt actin cable-mediated traffic.
DISCUSSION
Opposing Effects of Actin Cables and Actin Patches on Cdc42p Polarity
There are two clearly distinct types of F-actin structures in polarized yeast cells. Actin cables are polarized toward the prebud site and the bud tip and serve as tracks for myosin-mediated traffic to those sites (Schott et al., 2002). Actin patches cluster at the prebud site and within the bud (Adams and Pringle, 1984) and are thought to mark sites of endocytosis as well as endocytic vesicles that have just been internalized (Kaksonen et al., 2003). F-actin is essential for both polarized traffic and endocytosis (Ayscough et al., 1997). Previous studies documented the ability of Cdc42p to become polarized and to maintain polarity in the complete absence of F-actin in yeast (Ayscough et al., 1997; Irazoqui et al., 2003). However, we now report that selective impairment of actin cables caused dispersal of Cdc42p from the prebud site and that such dispersal was dependent on actin patch-mediated endocytosis. Our results suggest that actin patches disperse Cdc42p through endocytosis, whereas oriented actin cables help to maintain Cdc42p polarization through myosin-mediated traffic. Thus, after its initial actin-independent polarization, subsequent maintenance of Cdc42p polarization at the prebud site involves an actin-mediated trafficking cycle.
We used three different perturbations that selectively disrupt actin cable function. Tropomyosin mutants eliminate cables without reducing the number or brightness of the patches (Pruyne et al., 1998). myo2-16 mutants affect the major (though not the only) myosin that delivers cargo along actin cables and do not affect actin patches (Schott et al., 1999). Lat B binds to G-actin (Ayscough et al., 1997) and appears to be more effective at sequestering the actin from cable nucleators than from patch nucleators, although it clearly does reduce the amount of F-actin in patches as well. These perturbations all dispersed Cdc42p from the prebud site, although there were differences in the rapidity and effectiveness of the dispersal (fast and complete in tropomyosin mutants, slower and incomplete upon Lat B treatment). Tropomyosin mutants were also more effective than Lat B at dispersing Cdc42p from the bud tip in budded cells, although in all cases dispersal was slower and less complete in budded than in unbudded cells (see below). These differences are consistent with the hypothesis that dispersal is mediated by the actin patches, whose function is partly impaired upon Lat B treatment but not in tropomyosin mutants. Dispersal of Cdc42p from the prebud site was greatly reduced upon inactivation of the actin patch component Sla2p, which is required for endocytosis. In summary, our data indicate that if unopposed, actin-dependent endocytosis disperses Cdc42p. Endocytosed factors are normally recycled to the polarization site by vesicles trafficking on oriented actin cables, thereby maintaining Cdc42p polarity. As both endocytosis and polarized delivery are actin dependent, actin perturbations exhibit different effects depending on the degree to which actin's opposing roles are affected by the perturbation. Thus, maintenance of Cdc42p polarization at the presumptive bud site involves a dynamic and antagonistic interplay between distinct F-actin structures.
We also confirmed previous work (Ho and Bretscher, 2001) showing that mild temperature shift of wild-type cells causes a transient, reversible dispersal of Cdc42p from polarization sites. Interestingly, temperature shift also induces a transient and partial actin depolymerization associated with selective loss of actin cables (Lillie and Brown, 1994), which may contribute to the dispersal of Cdc42p. However, temperature-shift–induced dispersal was also observed in sla2 mutants (unpublished data), indicating that it does not require endocytosis. Actin patches and glucan synthase complexes are also transiently dispersed upon temperature shift and that dispersal has been ascribed to a Pkc1p-dependent stress signaling pathway (Delley and Hall, 1999), which may also act on Cdc42p.
Establishment versus Maintenance of Cdc42p Polarization
Once it is polarized, Cdc42p directs the orientation of actin cables toward the polarization site, most likely through formin proteins that are thought to be Cdc42p effectors as well as actin cable nucleators (Evangelista et al., 1997, 2002; Sagot et al., 2002). Because we found that actin cables, in turn, contribute to maintaining Cdc42p polarity, there is potential for a positive feedback loop in which clustering of Cdc42p promotes actin cable orientation which reinforces Cdc42p clustering. Does this “actin-feedback” pathway contribute to the initial polarization of Cdc42p?
Wedlich-Soldner and colleagues have proposed that delivery of secretory vesicles carrying Cdc42p along actin cables concentrates Cdc42p at the polarization site and that such actin-mediated positive feedback contributes to polarity establishment when bud site selection cues are absent (Wedlich-Soldner et al., 2003, 2004). Initial support for this model came from the demonstration that polarization of an overexpressed GTP-locked mutant form of Cdc42p was absolutely dependent on F-actin, Myo2p, and secretory function and that the GTP-locked Cdc42p was present on secretory vesicles (Wedlich-Soldner et al., 2003). However, wild-type Cdc42p can polarize in the absence of F-actin (Ayscough et al., 1997), even in the absence of bud site selection cues (Irazoqui et al., 2003), indicating that if such a feedback loop does operate, it is not absolutely required for polarization.
In a more recent study monitoring wild-type GFP-Cdc42p localization, Wedlich-Soldner et al. (2004) found that although most cells established and maintained robust Cdc42p polarization upon treatment with Lat A, a subset of cells established a “flickering” Cdc42p polarity that was subsequently lost. This observation suggests, consistent with our results, that actin can help to maintain Cdc42p polarization, making the polarization site more stable. In addition, they showed that in the absence of the scaffold protein Bem1p, F-actin was absolutely essential for any detectable polarization (Wedlich-Soldner et al., 2004). These observations suggested that the initial polarization of Cdc42p could occur either through an actin-independent pathway involving Bem1p (Irazoqui et al., 2003) or through an actin-dependent pathway. Distinguishing whether this actin-dependent pathway involves the proposed actin-Cdc42p feedback loop will require identification of the trafficking cargo relevant to Cdc42p polarization.
How Do Actin Cables Promote Cdc42p Polarity?
Which cargo transported on actin cables is responsible for helping to maintain Cdc42p polarization? The simplest hypothesis would be that trafficking of Cdc42p itself is important: GTP-locked Cdc42p was found in a vesicle fraction (Wedlich-Soldner et al., 2003), and it seems likely that wild-type Cdc42p can also associate with vesicles. However, it appears that wild-type Cdc42p exchanges between membrane and cytosolic pools far more rapidly that GTP-locked Cdc42p (Wedlich-Soldner et al., 2004). In addition, polarization by the “actin-feedback” membrane recycling pathway illustrated in Figure 1C will only be effective if diffusion is slow relative to endocytic recycling, as has been shown for some integral membrane proteins (Valdez-Taubas and Pelham, 2003), but fluorescence recovery after photobleaching experiments suggest that Cdc42p diffuses much more rapidly (Wedlich-Soldner et al., 2004). Thus, it is not clear whether vesicle trafficking of Cdc42p itself would be a major contributor to Cdc42p polarization. Many integral and peripheral membrane proteins colocalize with Cdc42p (Irazoqui and Lew, 2004), and it seems likely that several of these proteins undergo both endocytosis and cable-mediated delivery. Thus, endocytosis may disperse Cdc42p by removing not only Cdc42p itself but several of its interacting factors from the polarization site. But because most known polarized factors are peripheral (not integral) membrane proteins, it is not clear whether they would need to be recycled to the polarization site via actin cables or whether they could return by diffusion through the cytoplasm. A recent study reported that in tropomyosin mutants, budded cells lost not only Cdc42p but also Rho1p polarization (as well as the ability to nucleate cables at the bud tip) after 1 h without cables at the restrictive temperature (Pruyne et al., 2004). However, other peripheral components including Spa2p and the formin Bni1p were still largely polarized under those conditions, suggesting that these components either escape endocytosis or are able to return to the polarization site in a cable-independent manner.
Maturation of the Polarization Site after Bud Emergence
To all appearances, the Cdc42p cap at the presumptive bud site and the cap at the tip of budded cells are very similar and share numerous components. However, we found that the cap in budded cells was considerably more resistant to dispersal upon selective loss of cable function than the cap in unbudded cells. In principle, this could be explained either by a difference in endocytic recycling or a difference in the cap itself. At present we cannot distinguish between these possibilities, but because extensive research into endocytosis has never (to our knowledge) revealed any differences between budded and unbudded cells, we favor the hypothesis that the organization of the Cdc42p cap changes during or shortly after bud emergence. The nature and function of any such change remain to be discovered.
Supplementary Material
Acknowledgments
We thank Patrick Brennwald for anti-Sec4p and anti-Cdc42p antibodies and Tony Bretscher, David Drubin, Raphael Valdivia, Howard Riezman, and David Stone for advice and yeast strains. We also thank Jack McNulty for the halo assays, Lukasz Kozubowski for help with the figures, and Sally Kornbluth, Steve Haase, and Dan Kiehart for comments on the manuscript. J.E.I. was supported by predoctoral fellowship DAMD17-01-0231 from the Department of Defense Breast Cancer Research Program. This work was supported by National Institutes of Health grant GM62300 and a Scholar Award from the Leukemia and Lymphoma Society to D.J.L.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-05-0430) on December 22, 2004.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Adams, A.E.M., Johnson, D. I., Longnecker, R. M., Sloat, B. F., and Pringle, J. R. (1990). CDC42 and CDC43, two additional genes involved in budding and the establishment of cell polarity in the yeast Saccharomyces cerevisiae. J. Cell Biol. 111, 131-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams, A.E.M., and Pringle, J. R. (1984). Relationship of actin and tubulin distribution to bud growth in wild-type and morphogenetic-mutant Saccharomyces cerevisiae. J. Cell Biol. 98, 934-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayscough, K. R., Stryker, J., Pokala, N., Sanders, M., Crews, P., and Drubin, D. G. (1997). High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137, 399-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barral, Y., Mermall, V., Mooseker, M. S., and Snyder, M. (2000). Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol. Cell 5, 841-851. [DOI] [PubMed] [Google Scholar]
- Booher, R. N., Deshaies, R. J., and Kirschner, M. W. (1993). Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 12, 3417-3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellano, F., Montcourrier, P., Guillemot, J. C., Gouin, E., Machesky, L., Cossart, P., and Chavrier, P. (1999). Inducible recruitment of Cdc42 or WASP to a cell-surface receptor triggers actin polymerization and filopodium formation. Curr. Biol. 9, 351-360. [DOI] [PubMed] [Google Scholar]
- Chant, J. (1999). Cell polarity in yeast. Annu. Rev. Cell Dev. Biol. 15, 365-391. [DOI] [PubMed] [Google Scholar]
- Delley, P. A., and Hall, M. N. (1999). Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147, 163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville, S. (2004). Cdc42—the centre of polarity. J. Cell Sci. 117, 1291-1300. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., Blundell, K., Longtine, M. S., Chow, C. J., Adames, N., Pringle, J. R., Peter, M., and Boone, C. (1997). Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276, 118-122. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., Pruyne, D., Amberg, D. C., Boone, C., and Bretscher, A. (2002). Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4, 260-269. [DOI] [PubMed] [Google Scholar]
- Friant, S., Zanolari, B., and Riezman, H. (2000). Increased protein kinase or decreased PP2A activity bypasses sphingoid base requirement in endocytosis. EMBO J. 19, 2834-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G. R. (eds.) (1991). Guide to yeast genetics and molecular biology. San Diego, CA: Academic Press.
- Harkins, H. A., Page, N., Schenkman, L. R., De Virgilio, C., Shaw, S., Bussey, H., and Pringle, J. R. (2001). Bud8p and Bud9p, proteins that may mark the sites for bipolar budding in yeast. Mol. Biol. Cell 12, 2497-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, K. R., D'Hondt, K., Chang, J., Newpher, T., Huang, K., Hudson, R. T., Riezman, H., and Lemmon, S. K. (2002). Scd5p and clathrin function are important for cortical actin organization, endocytosis, and localization of sla2p in yeast. Mol. Biol. Cell 13, 2607-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho, J., and Bretscher, A. (2001). Ras regulates the polarity of the yeast actin cytoskeleton through the stress response pathway. Mol. Biol. Cell 12, 1541-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irazoqui, J. E., Gladfelter, A. S., and Lew, D. J. (2003). Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 5, 1062-1070. [DOI] [PubMed] [Google Scholar]
- Irazoqui, J. E., and Lew, D. J. (2004). Polarity establishment in yeast. J. Cell Sci. 117, 2169-2171. [DOI] [PubMed] [Google Scholar]
- Kaksonen, M., Sun, Y., and Drubin, D. G. (2003). A pathway for association of receptors, adaptors, and actin during endocytic internalization. Cell 115, 475-487. [DOI] [PubMed] [Google Scholar]
- Karpova, T. S., Reck-Peterson, S. L., Elkind, N. B., Mooseker, M. S., Novick, P. J., and Cooper, J. A. (2000). Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell 11, 1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch, G., Tanaka, K., Masuda, T., Yamochi, W., Nonaka, H., and Takai, Y. (1997). Association of the Rho family small GTP-binding proteins with Rho GDP dissociation inhibitor (Rho GDI) in Saccharomyces cerevisiae. Oncogene 15, 417-422. [DOI] [PubMed] [Google Scholar]
- Lehman, K., Rossi, G., Adamo, J. E., and Brennwald, P. (1999). Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J. Cell Biol. 146, 125-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew, D. J., and Reed, S. I. (1993). Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J. Cell Biol. 120, 1305-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie, S. H., and Brown, S. S. (1994). Immunofluorescence localization of the unconventional myosin, Myo2p, and the putative kinesin-related protein, Smy1p, to the same regions of polarized growth in Saccharomyces cerevisiae. J. Cell Biol. 125, 825-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda, T., Tanaka, K., Nonaka, H., Yamochi, W., Maeda, A., and Takai, Y. (1994). Molecular cloning and characterization of yeast rho GDP dissociation inhibitor. J. Biol. Chem. 269, 19713-19718. [PubMed] [Google Scholar]
- McMillan, J. N., Sia, R.A.L., Bardes, E.S.G., and Lew, D. J. (1999). Phosphorylation-independent inhibition of Cdc28p by the tyrosine kinase Swe1p in the morphogenesis checkpoint. Mol. Cell. Biol. 19, 5981-5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan, J. N., Sia, R.A.L., and Lew, D. J. (1998). A morphogenesis checkpoint monitors the actin cytoskeleton in yeast. J. Cell Biol. 142, 1487-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle, J. R., Bi, E., Harkins, H. A., Zahner, J. E., De Virgilio, C., Chant, J., Corrado, K., and Fares, H. (1995). Establishment of cell polarity in yeast. Cold Spring Harbor Symp. Quant. Biol. 60, 729-744. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., and Bretscher, A. (2000). Polarization of cell growth in yeast. J. Cell Sci. 113, 571-585. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., Gao, L., Bi, E., and Bretscher, A. (2004). Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol. Biol. Cell 15, 4971-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne, D. W., Schott, D. H., and Bretscher, A. (1998). Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast [In Process Citation]. J. Cell Biol. 143, 1931-1945. [DOI] [PubMed] [Google Scholar]
- Raths, S., Rohrer, J., Crausaz, F., and Riezman, H. (1993). end3 and end4, two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J. Cell Biol. 120, 55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redding, K., Holcomb, C., and Fuller, R. S. (1991). Immunolocalization of Kex2 protease identifies a putative late Golgi compartment in the yeast Saccharomyces cerevisiae. J. Cell Biol. 113, 527-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman, T. J., Sawyer, M. M., and Johnson, D. I. (2002). Saccharomyces cerevisiae Cdc42p localizes to cellular membranes and clusters at sites of polarized growth. Eukaryot. Cell 1, 458-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot, I., Klee, S. K., and Pellman, D. (2002). Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4, 42-50. [DOI] [PubMed] [Google Scholar]
- Schenkman, L. R., Caruso, C., Page, N., and Pringle, J. R. (2002). The role of cell cycle-regulated expression in the localization of spatial landmark proteins in yeast. J. Cell Biol. 156, 829-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott, D., Ho, J., Pruyne, D., and Bretscher, A. (1999). The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 147, 791-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott, D., Huffaker, T., and Bretscher, A. (2002). Microfilaments and microtubules: the news from yeast. Curr. Opin. Microbiol. 5, 564-574. [DOI] [PubMed] [Google Scholar]
- Spector, I., Shochet, N. R., Kashman, Y., and Groweiss, A. (1983). Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science 219, 493-495. [DOI] [PubMed] [Google Scholar]
- Takizawa, P. A., DeRisi, J. L., Wilhelm, J. E., and Vale, R. D. (2000). Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science 290, 341-344. [DOI] [PubMed] [Google Scholar]
- Valdez-Taubas, J., and Pelham, H. R. (2003). Slow diffusion of proteins in the yeast plasma membrane allows polarity to be maintained by endocytic cycling. Curr. Biol. 13, 1636-1640. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner, R., Altschuler, S., Wu, L., and Li, R. (2003). Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299, 1231-1235. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner, R., Wai, S. C., Schmidt, T., and Li, R. (2004). Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J. Cell Biol. 166, 889-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesp, A., Hicke, L., Palecek, J., Lombardi, R., Aust, T., Munn, A. L., and Riezman, H. (1997). End4p/Sla2p interacts with actin-associated proteins for endocytosis in Saccharomyces cerevisiae. Mol. Biol. Cell 8, 2291-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S., Cope, M. J., and Drubin, D. G. (1999). Sla2p is associated with the yeast cortical actin cytoskeleton via redundant localization signals. Mol. Biol. Cell 10, 2265-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman, M., Preuss, D., Mulholland, J., O'Brien, J. M., Botstein, D., and Johnson, D. I. (1993). Subcellular localization of Cdc42p, a Saccharomyces cerevisiae GTP-binding protein involved in the control of cell polarity. Mol. Biol. Cell 4, 1307-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.