Abstract
This study addressed whether phosphorylation regulates trafficking of yeast membrane proteins that cycle between the trans-Golgi network (TGN) and endosomal system. The TGN membrane proteins A-ALP, a model protein containing the Ste13p cytosolic domain fused to alkaline phosphatase (ALP), and Kex2p were found to be phosphorylated in vivo. Mutation of the S13 residue on the cytosolic domain of A-ALP to Ala was found to block trafficking to the prevacuolar compartment (PVC), whereas a S13D mutation generated to mimic phosphorylation accelerated trafficking into the PVC. The S13 residue was shown by mass spectrometry to be phosphorylated. The rate of endoplasmic reticulum-to-Golgi transport of newly synthesized A(S13A)-ALP was indistinguishable from wild-type, indicating that the lack of transport of A(S13A)-ALP to the PVC was instead due to differences in Golgi/endosomal trafficking. The A(S13A)-ALP protein exhibited a TGN-like localization similar to that of wild-type A-ALP. Similarly, the S13A mutation in endogenous Ste13p did not reduce the extent of or longevity of its localization to the TGN as shown by α-factor processing assays. These results indicate that S13 phosphorylation is required for TGN-to-PVC trafficking of A-ALP and imply that phosphorylation of S13 may regulate recognition of A-ALP by vesicular trafficking machinery.
INTRODUCTION
Resident trans-Golgi network (TGN) membrane proteins of the yeast Saccharomyces cerevisiae frequently cycle between the TGN and endosomal system. Ste13p (dipeptidyl aminopeptidase A) and the endopeptidase Kex2p are TGN resident enzymes with single transmembrane spanning domains that process the mating pheromone α-factor in the TGN. These proteins are delivered to a prevacuolar endosomal compartment (PVC) with a half-time of ∼60 min as determined using the Ste13p-based reporter protein A-ALP (Bryant and Stevens, 1997; Nothwehr et al., 1999). Once at the PVC, Ste13p and Kex2p are packaged into retrograde vesicles for delivery to the TGN. This transport step is dependent on a peripheral membrane complex called the retromer that may function as a vesicle coat (Seaman et al., 1997, 1998; Nothwehr et al., 1999). Retromer-based sorting of Ste13p/A-ALP involves the association of retromer subunit Vps35p with an aromatic amino acid-based sorting signal (FXFXD) in the cytosolic domain of Ste13p (Nothwehr et al., 2000). Kex2p and Vps10p also contain aromatic PVC retrieval signals. Mutation of these signals, or loss of retromer function, causes rapid default transport of these cargo to the vacuole (Wilcox et al., 1992; Nothwehr et al., 1993; Cereghino et al., 1995; Cooper and Stevens, 1996).
In addition to a cycling itinerary that includes the PVC, TGN residents seem to also visit early endosomal compartments as shown by several recent studies. For example, Kex2p and Ste13p seem to localize to a certain degree with early endosomes. This has been shown by colocalization with early endosomal markers Tlg1p, Chs3p, and Snc1p, a secretory v-SNARE that transits through the early endosomal system on its journey from the plasma membrane to the TGN (Ziman et al., 1996; Santos and Snyder, 1997; Holthuis et al., 1998; Lewis et al., 2000). Both Ste13p and Kex2p contain poorly defined cytosolic domain signals that when mutated accelerate trafficking into the PVC (Brickner and Fuller, 1997; Bryant and Stevens, 1997), suggesting that either they function as static retention signals in the TGN or regulate trafficking through the early endosomal system. Mutation of the yeast synaptojanin Inp53p also caused A-ALP (as a model for Ste13p) and Kex2p to be more rapidly delivered to the PVC; however, trafficking of Vps10p was unaffected (Ha et al., 2001). The phenotypes associated with a loss of Inp53p function and a loss of the AP-1 adaptor complex share similarities in that both types of lesions exhibit synthetic growth defects when combined with mutations in GGA1 and GGA2 (Costaguta et al., 2001; Ha et al., 2001, 2003). GGA1 and GGA2 encode adaptor proteins necessary for clathrin-mediated transport from the TGN directly to the PVC (Black and Pelham, 2000; Costaguta et al., 2001; Scott et al., 2004). The synthetic growth defects obtained with mutations in the AP-1 adaptor complex or Inp53p with mutations in the GGAs suggest that AP-1 and Inp53p probably mediate a distinct pathway into the endosomal system, presumably to early endosomes. This assertion also is supported by the observation that trafficking of Chs3p and Tlg1p between the TGN and early endosomes is disrupted by mutations in AP-1 (Valdivia et al., 2002). Finally, mutation of SOI3/RAV1, which is required for efficient transport between the early endosomes and the PVC, reduced the rate of trafficking of Kex2p to the PVC (Sipos et al., 2004). Together, these results suggest that Ste13p and Kex2p cycle between the TGN and early endosome and also reach the PVC via transport from the early endosome.
With yeast TGN resident proteins engaging in such a complex trafficking itinerary, there is clearly more to learn regarding the regulation of trafficking between the different compartments. Phosphorylation of membrane proteins that cycle within the TGN/endosomal system of mammalian cells is known to influence their trafficking. In some cases, phosphorylation of a serine or threonine influences the activity of a nearby sorting signal, whereas in other cases phosphorylation generates a new sorting signal that functions by binding to an accessory protein (Bonifacino and Traub, 2003; Hinners and Tooze, 2003). For example, TGN localization of the Kex2p homologue furin is dependent on an acidic cluster motif in its cytosolic domain (Schäfer et al., 1995; Takahashi et al., 1995; Voorhees et al., 1995). This motif contains a serine that can be phosphorylated by casein kinase II (Jones et al., 1995). In response to phosphorylation, furin is thought to cycle between a TGN/endosomal loop and also between an early endosome/plasma membrane loop (for review, see Molloy et al., 1999). Transport in each cycling loop relies on binding of the PACS-1 adaptor to the phosphorylated form of furin and PACS-1, in turn, associates with the clathrin-associated sorting machinery (Wan et al., 1998; Crump et al., 2001). Dephosphorylation by protein phosphatase 2A of furin causes movement of furin from one loop to another (Molloy et al., 1998).
In yeast, phosphorylation has been shown to be important for routing of cell surface transporters and pheromone receptors into ubiquitin-dependent degradative vacuolar pathways (Hicke et al., 1998; Feng and Davis, 2000; Marchal et al., 2000). However, the role of phosphorylation in sorting of cargo that cycle between the Golgi and endosomes is unknown. In this study, we have investigated the role of potentially phosphorylatable residues in the cytosolic domain of Ste13p, both in the Ste13p and A-ALP contexts. We show for both proteins that mutation of a phosphorylation site prevents delivery into the PVC, indicating that phosphorylation regulates trafficking of A-ALP/Ste13p.
MATERIALS AND METHODS
General Methods and Antibodies
The production of minimal (synthetic dextrose) and rich (YPD) yeast media, the genetic manipulation of yeast strains, and all general molecular biology methods were performed as described previously (Ausebel et al., 2000) or as otherwise noted. Rabbit polyclonal antibodies against alkaline phosphatase (ALP) and Kex2p have been described previously (Nothwehr et al., 1996; Spelbrink and Nothwehr, 1999). Rabbit polyclonal antibodies against rabbit anti-Och1p were a gift from Vladimir Lupashin (University of Arkansas, Fayetteville, AR). Mouse antibodies against Vma2p, Vph1p, and Dpm1p were from Molecular Probes (Eugene, OR) and rabbit anti-hemagglutinin epitope (HA) antibodies were from Covance (Richmond, CA).
Plasmids and Yeast Strains
Plasmids pSN34, pSN55, pSN100, pAH16, and pAH49 have been described previously (Nothwehr et al., 1993, 1999). pSN55-PS1 to pSN55-PS11 were made using site-directed mutagenesis (Kunkel et al., 1987) to introduce point mutations (detailed below) into pSN55, a pRS316 derivative containing a STE13-PHO8 gene fusion. A TRP1-based version of pSN55-PS2, called pSN397, was made by subcloning the 2.3-kbp EagI-EcoRI fragment from pSN55-PS2 into the EagI/EcoRI sites of pRS314. Plasmids pHJ63, pHJ73, pHJ74, and pHJ75, which are derivatives of pSN55 containing various combinations of Δ2-11, S13A, S13D, F85A, and F87A mutations, were made using the “megaprimer method” (Tyagi et al., 2004). Primer sequences are available upon request. The mutations were verified by DNA sequencing. Yeast strains used in this study are listed in Table 1.
Table 1.
Yeast strains used in this study
| Strain/plasmid | Description | Origin or reference |
|---|---|---|
| SHY35 | MATα leu2-3112 ura-52 his3-Δ200 trp1-Δ901 suc2-Δ9 pho8Δ::ADE2 | Ha et al., 2001 |
| SHY38 | SHY35 inp53Δ::LEU2 | Ha et al., 2001 |
| SNY148 | SHY35 vps4Δ::NatR | This study |
| SHY39 | SHY35 vps1Δ::LEU2 | This study |
| SNY36-9A | MATaleu-3112 ura-52 his3-Δ200 trp1-Δ901 suc2-Δ9 pho8Δ::ADE2 | Nothwehr et al., 1995 |
| LSY2 | SNY36—9A pep4Δ::TRP1 | Spelbrink and Nothwehr, 1999 |
| PBY33 | LSY2 vps4Δ::KanR | This study |
| SNY17 | MATα leu-3112 ura3—52 his3-Δ200 trp1-Δ901 lys-801 suc2-Δ9 pho8Δ::LEU2 | Nothwehr et al., 1995 |
| SNY37 | SNY17 chc-521ts | Ha et al., 2003 |
| AHY4-13D | MATα leu-3112 ura-52 his3-Δ200 trp1-Δ901 suc2-Δ9 pho8Δ::ADE2 pep4Δ::LEU2 kex2Δ::LEU2 | This study |
| SNY9-9D | MATα leu-3112 ura-52 his3-Δ200 trp1-Δ901 lys2—801 suc2-Δ9 pho8Δ::ADE2 vps1Δ::LEU2 end3-ts | Nothwehr et al., 1999 |
| W30-1B | MATα leu-3112 ura-1 his-11 trp-1 ade-1 | R. Rothstein, Columbia University, New York, NY |
| HJY3 | W303—1B dap2Δ::HIS3 | This study |
| HJY4 | HJY3 ste13::GAL1-STE13 | This study |
| HJY5 | HJY3 ste13::GAL1-ste13 (F85A; F87A) | This study |
| HJY6 | HJY3 ste13::GAL1-ste13 (S13A) | This study |
| HJY7 | HJY3 ste13::GAL1-ste13 (S13A; F85A; F87A) | This study |
| AHY62 | MATα ura-1 leu-3112 his-11,15 trp-1 ade-1 can-100 | Ha et al., 2001 |
| JHRY2-2Ca | MATa leu-3112, ura-52, his3-Δ200 | T. Stevens, University of Oregon, Eugene, OR |
Plasmid pSN286, used to introduce a GAL1-STE13 allele at the STE13 locus, consists of pRS306 (Sikorski and Hieter, 1989) containing an insert with the following elements in the order given: a 1.9-kbp BamHI-EagI fragment from the 5′ untranslated region of STE13, a 1.1-kbp EagI-BamHI fragment containing the GAL1 promoter, and a 2.7-kbp EcoRI-KpnI fragment containing the STE13 open reading frame and 3′ untranslated region. Three derivatives of pSN286 contain the following point mutations in the STE13 coding region: F85A, F87A (pHJ68), S13A (pHJ69), and S13A, F85A, F87A (pHJ70). These four GAL1-STE13 plasmids were introduced into yeast HJY3 by first linearizing them at the unique SmaI site that lies in the 5′ untranslated region of STE13; transforming them into yeast; selecting transformants on media lacking uracil; and finally, looping out intervening DNA on media containing 5-fluoroorotic acid.
A GAL1 promoter-driven A-ALP construct tagged with two copies of the IgG-binding Z domain was generated by ligating the 2.25-kbp EagI-SalI fragment from pAH98 (Nothwehr et al., 2000) and a 1.2-kbp SalI-XbaI fragment, consisting of a 3′ fragment of the PHO8 open reading frame (ORF) fused in-frame to the green fluorescent protein (GFP) ORF, into the EagI/XbaI sites of pRS316. The resulting plasmid, (pSN332), consisting of a GAL1-STE13-PHO8-GFP construct, was digested with BamHI, blunted with T4 DNA polymerase, and digested with PacI to release the GFP region. Plasmid pFA6a-ZZ (a gift from Per Stromhaug, University of Missouri, Columbia, MO) was digested with PstI, blunted as described above, digested with PacI, and the 0.75-kbp insert was ligated to the pSN332 vector backbone fragment described above, resulting in the GAL1-STE13-PHO8-ZZ construct pCF7.
Radioactive Labeling, Immunoprecipitation, and Western Blot Analysis
The procedure for immunoprecipitation of wild-type and mutant A-ALP from [35S]methionine/cysteine-labeled cells was performed as described previously (Nothwehr et al., 1993). Radioactively labeled proteins were quantified from gels using a PhosphorImager system (Fuji Photo Film, Tokyo, Japan). For calculation of the half-time of A-ALP processing, the log of the percentage of A-ALP that was unprocessed at each time point was plotted as a function of time, and the plots were analyzed by linear regression analysis.
For assessment of in vivo phosphorylation, cultures were grown for several doublings in phosphate-depleted rich (YPD) media (Warner, 1991) to log phase. Fifty to 100 μCi of [32P]Pi was then added to 0.5 OD600 units of cells, and the culture was incubated at 30°C for 45 min. The cells were then spheroplasted, lysed, and subjected to A-ALP immunoprecipitation as described previously (Nothwehr et al., 1993). After separation by SDS-PAGE, the proteins were electroblotted onto nitrocellulose, and the relative extent of radioactive labeling quantified as described above. To quantify the level of recovery of wild-type and mutant A-ALP proteins on the blot, immunoblotting by using a rabbit anti-ALP antibody was carried out followed by incubation with ALP-conjugated anti-rabbit secondary antibodies and chemiluminescent detection using the Lumi-Phos substrate (Pierce Chemical, Rockford, IL). The blots were quantified and imaged using a Fuji LAS-1000 CCD camera and ImageReader LAS-1000 1.2 software (Fuji Photo Film).
Subcellular Fractionation
Subcellular fractionation of [35S]-labeled cells by differential centrifugation was carried out as described previously (Nothwehr et al., 1999), except that the immunoprecipitations were performed using anti-ALP antibody, and the pulse was for 10 min and the chase was for 0, 20, and 40 min.
The nonradioactive subcellular fractionation experiment performed by differential centrifugation was carried out as described previously (Ha et al., 2001), except centrifugation at 13,000 × g was used instead of 15,000 × g. Equivalent percentages of the P13, P200, and S200 fractions were subjected to SDS-PAGE followed by blotting to nitrocellulose. The blots were probed with the indicated primary antibodies followed by incubation with ALP-conjugated anti-rabbit or anti-mouse secondary antibodies and were detected, quantified, and imaged as described above. Images were further adjusted and formatted using Adobe Photoshop 7.0.
To perform the assay for plasma membrane localization, 12 OD600 units of cells labeled with [35S]methionine/cysteine were washed with 10 mM NaN3 and 10 mM NaF and resuspended in 550 μl of ice-cold lysis buffer [5% sucrose (wt/wt), 20 mM triethanolamine, pH 7.2, and 1 mM EDTA] supplemented with protease inhibitors (Roche Diagnostics, Mannheim, Germany). Cells were lysed by vortexing with glass beads, and lysates were centrifuged at 500 × g to pellet unlysed cells. Then, 350 μl of the 500-g supernatant was loaded on top of a sucrose step gradient made in 20 mM triethanolamine, 1 mM EDTA. The percentage of sucrose and volume of each step were as follows from bottom to top: 350 μl of 50%, 875 μl of 45%, and 700 μl of 35%. The gradients were centrifuged at 44,000 rpm in a SW 55 Ti rotor (Beckman Coulter, Fullerton, CA) for 17 h at 4°C. Eight fractions of 284 μl each were removed from the top and were subjected to immunoprecipitation of A-ALP, A(S13A)-ALP, Pma1-HA, and Och1p. Radioactively labeled proteins were quantified from gels using a PhosphorImager system (Fuji Photo Film).
Fluorescence Microscopy
The procedures for preparation of fixed spheroplasted yeast cells and attachment to microscope slides were described previously (Roberts et al., 1991). All secondary antibodies were diluted 1:500 before use. Simultaneous detection of wild-type and mutant A-ALP and Vma2p was performed as described previously (Ha et al., 2003). Yeast cells were photographed using a BX-60 fluorescence microscope (Olympus, Lake Success, NY) equipped with a C4742-95 digital camera (Hamamatsu, Bridgewater, NJ). Images were initially captured using Openlab 3.1.7 software (Improvision, Lexington, MA) and were processed into figures using Adobe Photoshop 7.0.
Mating Assay to Measure the Onset of Sterility
The mating efficiency of MATα yeast strains at various times after terminating expression of STE13 and mutant derivatives under control of the GAL1 promoter was determined using a published assay (Hartwell, 1980; Wilcox et al., 1992). Briefly, cells were grown synthetic galactose media for several generations to log phase and were then switched to synthetic glucose (SD) media. After various times of growth in glucose, 0.25 OD600 units of cells (∼5 × 106 cells) were mixed with 0.75 OD600 units of cells (∼1.5 × 107 cells) of MATa mating partner strain JHRY20-2Ca, and the cells were adhered onto a 2.4-cm HATF filter (Millipore, Billerica, MA). The filters were incubated cell side up on YPD plates at 30°C for 4 h, the cells were resuspended off the filter, and the dilutions of cells were plated on SD-his media to select for MATα and diploid cells and onto SD-his-trp to select for diploids only. Mating efficiency is expressed as the number of diploids divided by the number of diploids plus MATα haploids.
Mass Spectrometry Analysis of Ste13p Cytosolic Domain Phosphorylation
The A-ALP protein fused to two copies of the IgG-binding Z domain was purified from yeast strain AHY62 carrying plasmid pCF7. In total, 100 OD600 units of cells was spheroplasted and lysed by incubating in 2.5 ml of 5% SDS/8 M urea at 100°C for 5 min in the presence of protease inhibitors (see above). The yeast extract was then diluted into 50 ml of immunoprecipitation (IP) buffer lacking SDS (10 mM Tris, pH 8.0, 0.1% Triton X-100, and 2 mM EDTA), and a 0.5-ml bed volume of Sepharose beads was added. After incubating for 1 h at 4°C, the beads were sedimented, and the supernatant was then added to 0.1-ml bed volume of IgG-Sepharose beads. After incubating for 2 h at 4°C, the beads were washed four times with 50-ml volumes of IP buffer containing 0.1% SDS (Nothwehr et al., 1993), incubated with SDS-PAGE sample buffer at 100°C, and the eluted protein separated by SDS-PAGE and stained with Coomassie Brilliant Blue. A gel band corresponding to full-length A-ALP-ZZ was subjected to in-gel trypsin digestion according to (Shevchenko et al., 1996), and peptides were purified and desalted using Zip Tip columns (Millipore) according to the instructions of the manufacturer.
Nanospray quadrapole time of flight (TOF) time mass spectrometry and tandem mass spectrometry (MS/MS) analyses of the obtained peptides were performed with a QSTAR/PULSARi instrument (Applied Biosystems, Foster City, CA) fitted with a nanospray source (Proxeon, Odense, Denmark). A stable spray was achieved at 800 V in the presence of nitrogen curtain gas. Positive ion spectra were acquired in the profile MCA mode at a pulser frequency of 6.99 kHz. Collision-induced dissociation spectra (MS/MS) were acquired for peptides selected by the first quadrupole and fragmented in the collision quadrupole at appropriate nitrogen collision gas pressures and collision energy settings.
RESULTS
Yeast Resident TGN Proteins Are Phosphorylated
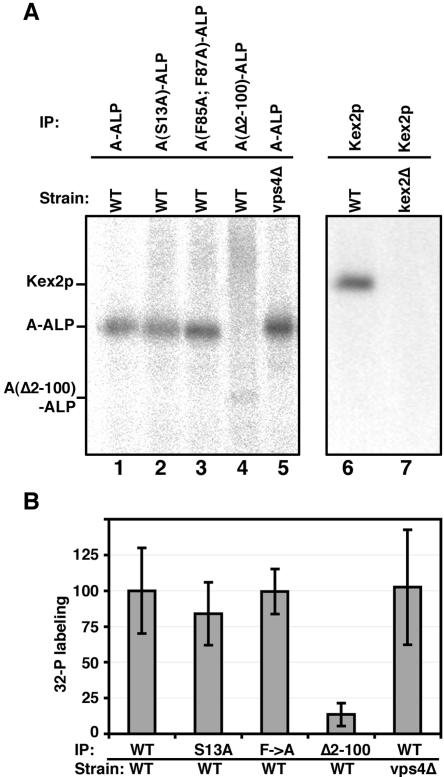
As a first step to investigate whether phosphorylation of TGN resident proteins is important for their trafficking, we assessed whether the model TGN protein A-ALP is phosphorylated. A-ALP is a model TGN-membrane protein consisting of the N-terminal cytosolic domain of Ste13p fused to the transmembrane and lumenal domains of ALP, the PHO8 gene product (Nothwehr et al., 1993). A failure to retain A-ALP in the TGN/endosomal system results in its delivery to the vacuole where its C-terminal propeptide is proteolytically removed, resulting in a mobility shift on SDS-PAGE. However, to simplify the phosphorylation analysis, pep4 yeast strains deficient in vacuolar protease activity were used to prevent any vacuolar proteolytic processing from occurring. A yeast strain expressing A-ALP was grown in phosphate-depleted media for several generations and cells were then incubated with [32P]Pi for 45 min followed by immunoprecipitation of A-ALP. A band of the expected size for A-ALP was obtained, whereas this band was missing in a strain expressing an A-ALP mutant lacking residues 2–100 of the cytosolic domain (Figure 1A, lanes 1 and 4). However, a very faint band at the size expected for A(Δ2-100)-ALP was observed. The extent of 32P incorporation into wild-type and mutant A-ALP was quantified and normalized to the amount of A-ALP protein immunoprecipitated (Figure 1B). These results indicated that phosphorylation of the A(Δ2-100)-ALP mutant was reduced to <20% of that of A-ALP. We thus conclude that A-ALP is phosphorylated and that most, if not all, of the phosphorylation occurs on the cytosolic domain. In this regard it is worth noting that the truncated cytosolic domain in A(Δ2-100)-ALP contains two Ser residues (Figure 2A). Kex2p (Figure 1A) and Vps10p (our unpublished data) also were demonstrated to be phosphoproteins.
Figure 1.
The cytosolic domains of both A-ALP and Kex2p are phosphorylated in vivo. (A) Yeast strains were grown for several doublings in phosphate depleted media and were then continuously labeled with [32P]Pi for 45 min. In lanes 1–7 strains LSY2/pSN55, LSY2/pSN55-PS2, LSY2/pSN100, LSY2/pSN34, PBY33/pSN55, LSY2, and AHY48-13D were analyzed, respectively, by immunoprecipitation with anti-ALP antibodies (lanes 1–5) or with anti-Kex2p antibodies (lanes 6 and 7). (B) The strains analyzed in lanes 1–5 of A were labeled with 32P and subjected to immunoprecipitation of wild-type and mutant A-ALP proteins as described above. After separation by SDS-PAGE, the relative amounts of purified wild-type and mutant A-ALP proteins was determined by immunoblotting, and the relative amounts of 32P incorporated was determined by PhosphorImager analysis as described under Materials and Methods. Relative phosphorylation is the extent of 32P incorporation divided by the level of wild-type or mutant A-ALP protein on the blot. Phosphorylation of wild-type A-ALP expressed in a wild-type strain was arbitrarily set at 100%. The average and SD of three independent data points for each strain are shown.
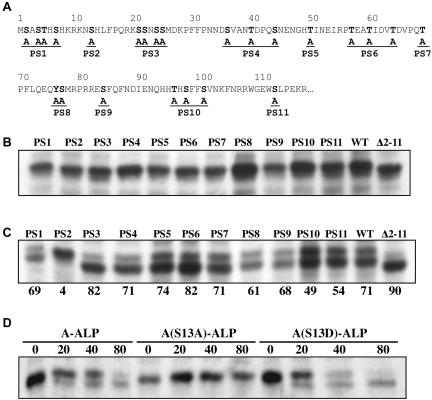
Figure 2.
Mutation of the S13 residue of A-ALP blocks its processing in class E vps cells. (A) The sequence of the cytosolic domain of Ste13p is shown. All potentially phosphorylatable residues (bold) in the cytosolic domain were mutated in the context of A-ALP carried in pSN55 (in groups of 1–4 mutations) by site-directed mutagenesis resulting in 11 mutants named PS1 to PS11. (B–D) Wild-type and mutant A-ALP proteins were immunoprecipitated from 35S-labeled cell extracts and were analyzed by SDS-PAGE. (B) Wild-type strains (SHY35) carrying plasmids pSN55-PS1-pSN55-PS11, pSN55 (WT; A-ALP) and pAH49 [Δ2-11; A(Δ2-11)-ALP] were pulsed for 10 min with [35S]methionine/cysteine and chased for 150 min. (C) The vps4Δ mutant SNY148 carrying the same plasmids as in B was pulsed for 10 min and chased for 60 min. The percentage of A-ALP processing is indicated below each lane. (D) SNY148 cells carrying pSN55, pSN55-PS2, and pHJ56 [A(S13D)-ALP] were pulsed for 10 min and chased for 0, 20, 40, and 80 min.
We next addressed whether trafficking defects would affect phosphorylation of A-ALP. A mutant form of A-ALP, A(F85A; F87A)-ALP, defective for retrieval from the PVC (Nothwehr et al., 1993; Bryant and Stevens, 1997) was phosphorylated to a similar degree as A-ALP (Figure 1). Phosphorylation of A-ALP expressed in the class E vps mutant vps4Δ also was analyzed. In class E mutants, transport into the PVC occurs normally but both retrograde and anterograde traffic out of the PVC is blocked (Piper et al., 1995; Babst et al., 1997; Finkeneigen et al., 1997). Thus, in class E cells A-ALP rapidly becomes trapped in the PVC (Bryant and Stevens, 1997). However, loss of Vps4p function did not seem the affect the extent of phosphorylation of A-ALP (Figure 1B).
A S13A Mutation in the Cytosolic Domain of A-ALP Blocks Its Delivery to the PVC
The 24 potentially phosphorylatable Ser, Thr, and Tyr residues in the cytosolic domain of Ste13p/A-ALP were systematically mutated to determine whether they have a role in trafficking of A-ALP. In total, 11 mutant forms of A-ALP were generated by site-directed mutagenesis of one to four residues at a time (Figure 2A). These mutants, called PS1–PS11, and controls were expressed in a wild-type strain, cells were pulsed for 10 min, chased for 150 min, lysed, and then immunoprecipitated using anti-ALP antibody. Little or no vacuolar processing was observed, with the exception of PS3 that exhibited weak processing (Figure 2B). A defect in retrieval of A-ALP from the PVC results in processing with a half-time of ∼60 min (Nothwehr et al., 1993; Bryant and Stevens, 1997); thus, the mutations apparently do not have a marked affect on PVC-to-TGN retrieval.
To determine whether the mutations affect the rate of transport into the PVC, we expressed wild-type and mutant A-ALP proteins in the class E vps mutant vps4Δ that trap TGN resident proteins in the PVC. In contrast to wild-type cells, the PVC in such strains contains substantial vacuolar protease activity. Therefore, the rate of processing of newly synthesized A-ALP in class E mutants reflects the rate of transport from its site of synthesis at the endoplasmic reticulum (ER) to the PVC (Bryant and Stevens, 1997; Ha et al., 2001). vps4Δ cells carrying wild-type and mutant A-ALP proteins were pulsed for 10 min and chased for 60 min. Under these conditions, 71% of wild-type A-ALP was processed (Figure 2C). A mutant form of A-ALP lacking amino acids 2–11, A(Δ2-11)-ALP, was more extensively processed (90%), consistent with previous work showing that this deletion accelerates trafficking into the PVC (Bryant and Stevens, 1997; Ha et al., 2001). Most of the PS mutants were processed to a similar degree as wild-type, suggesting that the mutated residues play little or no role in trafficking. Notable exceptions were the PS2 mutant (S13A) that exhibited little or no processing, and the PS3 (S21A, S22A, S24A, S25A) and PS6 (T57A, T60A, T64A) mutants whose processing was somewhat accelerated (82% processing). Because the S13A mutation caused such a striking affect on A-ALP trafficking, we compared A-ALP and A(S13A)-ALP expressed in a vps4Δ strain in a more extensive pulse-chase time course (Figure 2D). No processing of A(S13A)-ALP was observed in the 80-min time course, whereas wild-type was processed with a half-time of 46 min (Table 2).
Table 2.
Rate of processing of wild-type and mutant A-ALP proteins in various strain backgrounds
| Strain | Form of A-ALP | Half-time of processing | |
|---|---|---|---|
| Wild type | Wild type | ≫80 | |
| Wild type | F85A; F87A | 59 ± 4 | n = 2 |
| Wild type | S13A | ≫80 | |
| Wild type | S13A; F85A; F87A | ≫80 | n = 2 |
| Wild type | S13D; F85A; F87A | 48 | |
| inp53Δ | F85A; F87A | 35 | |
| inp53Δ | S13A; F85A; F87A | ≫80 | |
| inp53Δ | S13D; F85A; F87A | 28 ± 3 | n = 2 |
| vps1Δ | wild type | 62 ± 3 | n = 2 |
| vps1Δ | S13A | ≫80 | n = 2 |
| vps4Δ | Wild type | 46 ± 4 | n = 5 |
| vps4Δ | S13A | ≫80 | n = 5 |
| vps4Δ | S13D | 32 ± 1 | n = 5 |
| vps4Δ | Δ2-11 | 22 ± 8 | n = 2 |
| vps4Δ | Δ2-11; S13A | 23 ± 6 | n = 2 |
| vps4Δ | Δ2-11; S13D | 19 ± 3 | n = 2 |
Strains of the indicated genotype (SHY35, SHY38, SHY39, and SNY148) carrying plasmids for expression of wild-type (pSN55), F85A; F87A (pSN100), S13A (pSN55-PS2), S13A; F85A; F87A (pHJ63), S13D; F85A; F87A (pHJ75), Δ2-11 (pAH49), Δ2-11; S13A (pHJ73), and Δ2-11; S13D (pHJ74) forms of A-ALP were analyzed. Strains were pulsed with [35S]methionine/cysteine for 10 min and chased for a total of four time points at 30°C. After each time point, wild-type or mutant A-ALP proteins were immunoprecipitated, separated by SDS-PAGE, the percentage of processing quantified by PhosphorImager analysis, and the processing half-time calculated (see Materials and Methods). For data points derived from multiple experiments (n > 1), the average and SD are indicated.
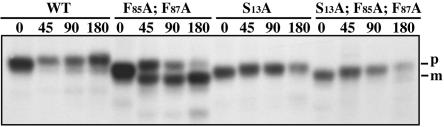
To determine whether the S13A mutation blocks delivery of A-ALP to the PVC in wild-type cells, we introduced this mutation into the A(F85A; F87A)-ALP construct that is incapable of being retrieved from the PVC. The rate of processing of A-ALP, A(F85A; F87A)-ALP, A(S13A)-ALP, and A(S13A; F85A; F87A)-ALP in wild-type cells was determined. Whereas retrieval-defective A(F85A; F87A)-ALP was processed with a half-time of 59 min, introduction of the S13A mutation into this protein dramatically delayed its processing with just a hint of processing at the 180-min time point (Figure 3 and Table 2). Consistent with the results of Figure 2B, no processing of the A(S13A)-ALP single mutant was observed.
Figure 3.
The S13A mutation blocks delivery of A-ALP to the PVC in wild-type cells. Strain SHY35 carrying pSN55 (A-ALP), pSN100 [A(F85A; F87A)-ALP], pSN55-PS2 (A(S13A)-ALP), and pHJ63 [A-(S13A; F85A; F87A)-ALP] were pulsed for 10 min and chased for 0, 45, 90, and 180 min. Little or no processing of the double mutant is seen, even after 180 min, suggesting that the S13A mutation blocks delivery into the PVC in wild-type cells.
The S13 Residue of A-ALP Is Phosphorylated
Given that A-ALP is phosphorylated and that the S13A mutation severely retards delivery to the PVC, we next attempted to assess whether the S13 residue is phosphorylated. If S13 is phosphorylated, then mutating it to a D residue to mimic the phosphorylated state would be expected to give a much different phenotype than the S13A mutation, which would mimic the nonphosphorylated state. Consistent with S13 phosphorylation, the A(S13D)-ALP mutant was indeed found to be processed significantly faster than A-ALP in vps4Δ cells (Figure 2D and Table 2). As another approach, we directly assessed the extent of in vivo phosphorylation of A(S13A)-ALP compared with wild-type. We have repeatedly observed a modest decrease in the extent of phosphorylation due to the S13A mutation although the extent of this decrease varies from experiment to experiment. In the experiment shown in Figure 1B, this difference fell within the SD due to quantitative limitations in the assay. If S13 is phosphorylated it is clearly not the only phosphorylation site in A-ALP, although the other unidentified site(s) do not seem to dramatically influence trafficking.
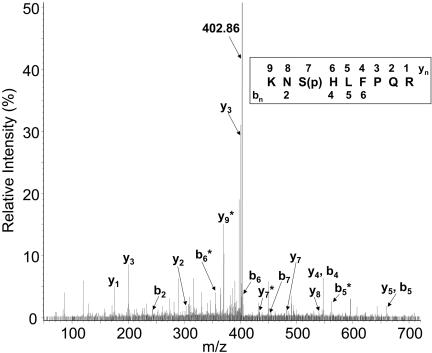
Given this uncertainty, mass spectrometry was used to address whether the S13 residue was phosphorylated. A-ALP fused to two copies of the IgG-binding Z domain was expressed in yeast, purified, and the protein contained within a one-dimensional gel band was trypsinized. Single-charge positive ions corresponding to the phosphorylated residue 12–19 peptide (at 1078.5 Da) and phosphorylated 11–19 or 12–20 peptides (at 1206.6 Da) were observed in the matrix-assisted laser desorption ionization/TOF MS analysis of both the nonbound and bound peptide fractions from a C18 matrix used for desalting the in-gel tryptic digest (our unpublished data). These ions were observed at significant signal-to-noise ratios only in the presence of the matrix 2,5-dihydroxybenzoic acid [at 10 mg/ml in 500:500:10 (vol/vol/vol) acetonitrile/water/o-phosphoric acid] and not with the matrix alpha-cyano-4-hydroxycinnamic acid. Subsequent nanospray quadrupole time of flight mass spectrometry analysis revealed a triple-charge ion at a mass/charge ratio (m/z) of 402.9 that corresponded well to the expected m/z for a phosphorylated peptide corresponding to residues 11–19. The fragmentation spectrum of this peptide showed a complete y-ion series consistent with phosphorylation at S13 that included both phosphorylated fragments and the respective fragment ions resulting from the loss of phosphoric acid (Figure 4). For example, the triple-charge y9* ion at m/z = 370.2 corresponds to the fragment with the loss of phosphoric acid (402.9 × 3–370.2 × 3 = 98, the mass of H3PO4). Thus, we conclude that the S13 residue of A-ALP is phosphorylated as well as one or more unidentified sites within the cytosolic domain. Together, these results strongly suggest that phosphorylation at S13 controls delivery of A-ALP/Ste13p to the PVC.
Figure 4.
The S13 residue in the Ste13p cytosolic domain is phosphorylated. The MS/MS fragmentation spectra and amino acid sequence of a trypsin-miscleaved peptide from the Ste13p cytosolic domain beginning with Lys11 is shown. The intact 3+ charged parent ion (y9) had a mass/charge ratio (m/z) of 402.86. Peaks corresponding to the y (C-terminal) and b (N-terminal) fragment ions (inset) are labeled on the plot. An exception is the relatively small peak for the y6 ion with an m/z of 399.22 that could only be discerned on an expanded version of the plot. The ion fragments with an expected mass resulting from neutral loss of H3PO4 are marked with asterisks. The y3 fragment was identified in two distinct peaks with one corresponding to a 1+ charged ion (m/z = 400.24) and the other to a 2+ charged ion (m/z = 200.62) species.
The A(S13A)-Alkaline Phosphatase Mutant Exhibits a TGN/Endosomal-like Distribution
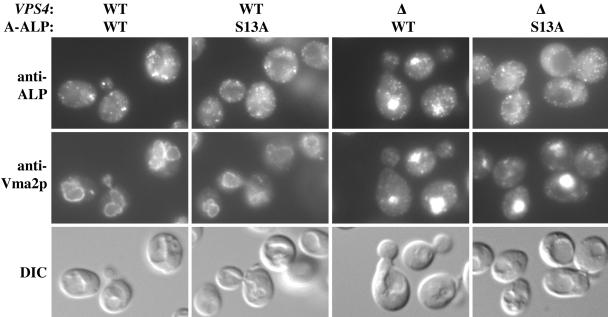
The results mentioned above suggested that the S13 residue is required for trafficking of A-ALP into the PVC. To explore the alternative possibility that the S13A mutation converts A-ALP into an unsuitable substrate for vacuolar processing, we assessed the localization of A(S13A)-ALP in wild-type and vps4Δ cells by immunofluorescence microscopy (Figure 5). In wild-type cells, both A-ALP and A(S13A)-ALP exhibited punctate staining patterns typical of localization to the yeast Golgi/endosomal system. However, extensive analysis of many fields of cells indicated that the staining pattern of A(S13A)-ALP was subtly different in that it decorated structures that seemed slightly smaller and more numerous than A-ALP (our unpublished data). The exaggerated PVC (class E compartment) in vps4Δ cells is marked by Vma2p, a component of the vacuolar ATPase (Raymond et al., 1992). Although wild-type A-ALP clearly colocalizes with Vma2p in vps4Δ cells, indicating that it is trapped in the exaggerated PVC, A(S13A)-ALP remains punctate in the vps4Δ cells. Thus, the lack of processing of A(S13A)-ALP in vps4Δ cells is due to its lack of transport to the PVC.
Figure 5.
A(S13A)-ALP mutant seems to localize to the TGN/early endosome with little or no cycling through the late endosome. Strains LSY2/pSN55, LSY2/pSN55-PS2, PBY33/A-ALP and PBY33/A(S13A)-ALP (shown in columns from left to right) were fixed and spheroplasted. The cells were then costained for A-ALP/Vma2p or A(S13A)-ALP/Vma2p as described under Materials and Methods. The cells were viewed by differential interference contrast optics (bottom row) and by epifluorescence with filters specific for each fluorochrome (top and center rows).
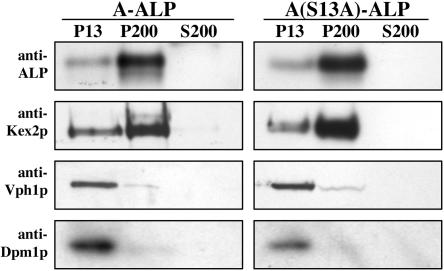
The punctate staining pattern suggested that A(S13A)-ALP had reached the TGN/endosomal system; however, an alternative possibility was that this mutant protein was trapped in the ER. To explore this issue, we used a differential centrifugation approach to fractionate organelles from lysates derived from wild-type cells expressing A-ALP and cells expressing A(S13A)-ALP. Lysates were centrifuged at 13,000 × g to generate a pellet (P13) and supernatant (S13) fraction. The S13 fraction was then centrifuged at 200,000 × g to generate P200 and S200 fractions. Under these conditions A-ALP, A(S13A)-ALP, and Kex2p were mainly found in the P200 fraction with a minor amount in the P13 fraction (Figure 6). In contrast, the ER marker Dpm1p and vacuolar marker Vph1p were clearly enriched in the P13 fraction. These results indicated that A(S13A)-ALP was not localized to the ER in the steady state and were consistent with a Golgi/endosomal localization.
Figure 6.
A(S13A)-ALP fractionates similarly to wild-type A-ALP by differential centrifugation of cell lysates. Strains SHY35/pSN55 and SHY35/pSN55-PS2 were spheroplasted, lysed, and lysates were subjected to centrifugation at 13,000 × g to generate pellet (P13) and supernatant (S13) fractions. The S13 fraction was the centrifuged at 200,000 × g to generate P200 and S200 fractions. Equivalent percentages of the three fractions from each strain were run on SDS-PAGE, gels were transferred to nitrocellulose, and blots were probed using antibodies to detect the indicated proteins.
The S13A Mutation Does Not Slow the Rate of ER-to-Golgi Transport of A-ALP
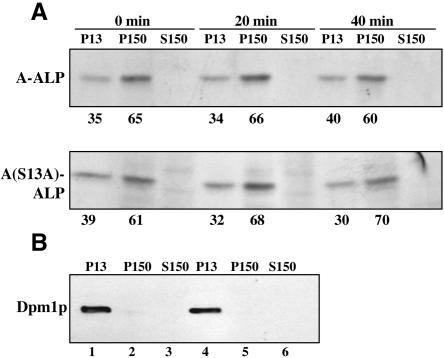
Although A(S13A)-ALP was not localized to the ER under steady-state conditions, it was possible that newly synthesized A(S13A)-ALP transited slowly through the early part of the secretory pathway. If so, this could partially account for the slow rate of transport into the PVC. To investigate this issue, we performed subcellular fractionation on strains expressing A-ALP and A(S13A)-ALP that had been pulse labeled for 10 min and chased for 0, 20, and 40 min (Figure 7). By the earliest time point measured (0 min), the majority of A-ALP and A(S13A)-ALP were already found in the P150 fraction with only minor amounts in the P13 fraction. As in Figure 6, the ER marker Dpm1p in this experiment was confined almost completely to the P13 fraction, with little or no Dpm1p in the P150 and S150 fractions (Figure 7B). Proceeding through the 0- to 40-min time course, little or no additional shift of A-ALP and A(S13A)-ALP from the P13 to the P150 fraction was observed. Although this type of experiment is not capable of ruling out very minor differences in ER-to-Golgi trafficking rates, it is clear that both proteins were transported from the ER to the Golgi in <10 min after being synthesized. Together, these results indicate that the block into transport of A(S13A)-ALP into the PVC must be due to an effect on intra-Golgi or post-Golgi trafficking.
Figure 7.
The S13A mutation does not alter the kinetics of A-ALP trafficking from the ER to the Golgi. (A) Strains SHY35/pSN55 (A-ALP) and SHY35/pSN55-PS2 [A(S13A)-ALP] were spheroplasted and pulse labeled for 10 min and were chased for 0, 20, and 40 min. After each chase period, the cells were lysed and lysates were centrifuged at 13,000 × g to generate pellet (P13) and supernatant (S13) fractions. The S13 fraction was then centrifuged at 150,000 × g to generate P150 and S150 fractions. A-ALP or A(S13A)-ALP were immunoprecipitated from each fraction, analyzed by SDS-PAGE, and quantified by PhosphorImager analysis. The numbers below each panel represent the amount of the A-ALP or A(S13A)-ALP present in the P13 and P150 fractions as a percentage of the sum total present in the P13 and P150 fractions. At each time point, the amount of A-ALP or A(S13A)-ALP found in the S150 was <5% of the total found in the P13, P150, and S150 fractions combined. (B) Equivalent percentages of the 0 min P13, P150, and S150 fractions (nonimmunoprecipitated) were run on SDS-PAGE and were Western-blotted for Dpm1p. Fractions from strain SHY35/pSN55 and strain SHY35/pSN55-PS2 are shown in lanes 1–3 and 4–6, respectively.
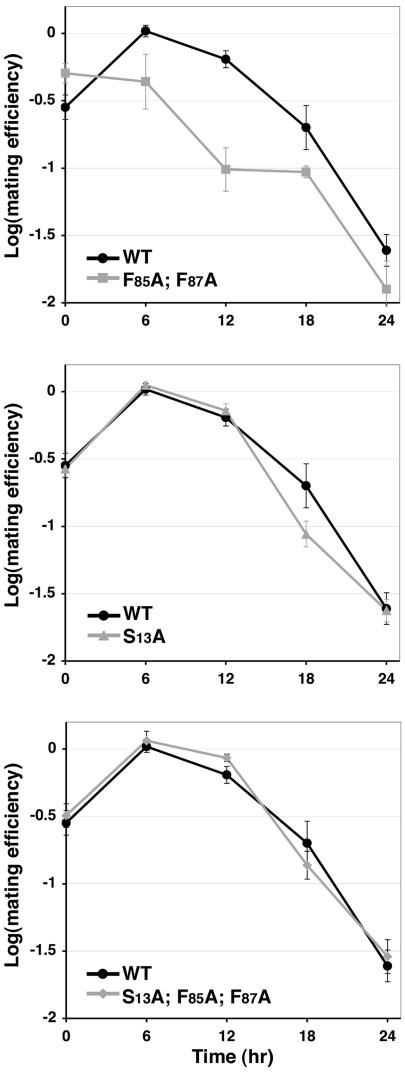
The S13A Mutation Suppresses the Accelerated Depletion of PVC Retrieval-Defective Ste13p from the α-Factor Processing Compartment
The S13A mutation could cause A-ALP to limit its cycling to a TGN/early endosome itinerary. Alternatively, A(S13A)-ALP could be localized to a post-TGN non-PVC endosomal organelle distinct from the α-factor processing compartment. To investigate this issue, and to extend our analysis to endogenous Ste13p, we used a functional assay that measures the persistence of wild-type and mutant forms of Ste13p in the α-factor processing compartment (the TGN and/or early endosome). We hypothesized that retrieval-defective Ste13p (F85A; F87A) would be depleted from the TGN/early endosome more rapidly after its synthesis was shut-off than wild-type Ste13p but that the S13A mutation would block entry into the PVC and thus suppress this effect. Strains were constructed in which the endogenous STE13 gene was replaced with constructs containing the galactose-inducible GAL1 promoter fused to the following alleles of the STE13 gene: 1) wild-type, 2) F85A; F87A, 3) S13A, and 4) S13A; F85A; F87A. These strains were grown on galactose media to induce expression of wild-type and mutant Ste13p, switched to glucose media for various times, and then were tested for their ability to mate with a MATa strain (Figure 8). The efficiency of mating of the F85A; F87A mutant did indeed drop off more quickly than wild type after the shift to glucose, as can be seen at the 6- and 12-h time points. The Ste13 (S13A) single mutant activity persisted in the TGN/early endosome to a similar degree as wild type over the time course. In addition, the S13A mutation suppressed the effect of the F85A, F87A mutations with the triple mutant persisting similarly to wild-type Ste13p. These data indicate that Ste13 (S13A) is localized to the TGN/early endosomal compartment with similar efficiency to that of wild-type Ste13p where it can participate in α-factor processing. Furthermore, the suppression of the F85A and F87A mutations by S13A indicates that, like A-ALP, the delivery of Ste13p to the PVC relies on phosphorylation of S13.
Figure 8.
The S13A mutation suppresses the accelerated loss of retention in the α-factor processing compartment observed for Ste13p carrying the F85A; F87A mutations. Strains HJY4, HJY5, HJY6, and HJY7 carrying wild-type and mutant forms of the STE13 under control of the GAL1 promoter were grown on galactose for several generations. The strains were then shifted to glucose-containing media to turn off expression of the wild-type and mutant STE13 constructs. At the indicated time points after shifting to glucose the ability of the cells to mate was measured to generate an index of mating efficiency (for details, see Materials and Methods). The data shown are the average of three independent data points and the error bars indicate the SD.
The S13 Phosphorylation Site Is Necessary for Plasma Membrane-mislocalized A-ALP to Reach the PVC
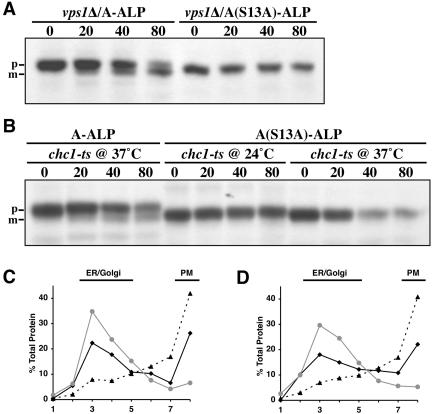
Loss of function of clathrin heavy chain, Chc1p, and the dynamin homolog Vps1p block direct Golgi-to-endosome trafficking of A-ALP. In chc1-521ts and vps1Δ mutants, A-ALP is initially mislocalized to the plasma membrane and is then delivered to the vacuole in a manner dependent on the End3 and Sla2/End4 proteins (Nothwehr et al., 1995; Ha et al., 2003). End3p and Sla2p are required for the internalization step of endocytosis (Raths et al., 1993; Bénédetti et al., 1994). Presumably in vps1 and chc1 mutants A-ALP vacuolar delivery from the plasma membrane occurs via the early endosome and endosome-to-TGN retrieval is compromised in these mutants. We assessed whether A(S13A)-ALP could access the PVC/vacuole after it was mislocalized to the plasma membrane in chc1-521ts and vps1Δ mutants. Wild-type A-ALP was clearly processed in vps1Δ cells and in chc1-521ts mutants at the nonpermissive temperature within the 80-min time course (Figure 9, A and B) as has been observed previously (Nothwehr et al., 1995; Ha et al., 2003). However, processing of A(S13A)-ALP was blocked in these mutants.
Figure 9.
Unlike A-ALP, A(S13A)-ALP is not mislocalized to the vacuole in vps1Δ and chc1-521ts cells. (A and B) Cells were pulsed with [35S]methionine/cysteine for 10 min and chased for 0, 20, 40, and 80 min before being lysed and subjected to immunoprecipitation by using an anti-ALP antibody to analyze the indicated proteins. (A) Strains SHY39/pSN55 and SHY39/pSN55-PS2 were propagated at 30°C for the entire experiment. (B) Strains SNY37/pSN55 and SNY37/pSN55-PS2 were propagated for several generations at 24°C. Ten minutes before the pulse and chase, the strains were shifted to 37°C or were allowed to continue growth at 24°C, as indicated. Analysis of vps1Δ end3-ts strains SNY96–9D/pWQ13/pAH16 (C) and SNY96–9D/pWQ13/pSN397 (D) expressing HA-Pma1p and either A-ALP (C) or A(S13A)-ALP (D) is shown. The strains were propagated for several generations at permissive temperature (24°C), shifted to nonpermissive temperature (37°C) for 15 min, pulsed for 10 min, and chased for 60 min. Cell lysates were fractionated on sucrose equilibrium gradients and fractions were analyzed by SDS-PAGE and quantitative immunoblotting for the following proteins: A-ALP or A(S13A)-ALP (black solid line), the Golgi marker Och1p (gray line), and the plasma membrane marker HA-Pma1p (dotted line).
A pool of A-ALP is trapped on the plasma membrane in vps1Δ cells with a loss of End3p or Sla2p function (Nothwehr et al., 1995, 1999). If A(S13A)-ALP also is mislocalized to the plasma membrane in vps1Δ cells, it should be trapped there in vps1Δ end3-ts cells. To investigate this, we pulse labeled a vps1Δ end3-ts strain at the nonpermissive temperature for 10 min and chased for 60 min. Cell lysates were loaded at the top of a sucrose equilibrium gradient designed to separate plasma membrane from internal membranes. Both A-ALP and A(S13A)-ALP exhibited a bimodal distribution with peaks around fractions 3 and 8 (Figure 9, C and D). The Golgi marker Och1p peaked around fraction 3, whereas the plasma membrane marker Pma1p peaked in fraction 8. These data indicate that pools of both A-ALP and A(S13A)-ALP are localized to the plasma membrane in the vps1Δ end3-ts strain. These results suggest that A-ALP lacking the S13 phosphorylation site is unable to traffic to the PVC once it has been mislocalized to the plasma membrane.
Accelerated Transport of A-ALP to the PVC in inp53/sjl3 Mutants Is Blocked by the S13A Mutation
Loss of function of the synaptojanin homolog Inp53/Sjl3p causes A-ALP transport into the PVC to be accelerated (Ha et al., 2001, 2003). To investigate the epistatic relationship between INP53 and the S13 phosphoserine, we analyzed the rate of processing of retrieval defective A(F85A; F87A)-ALP in inp53Δ cells with or without the S13A mutation. As observed previously, A(F85A; F87A)-ALP was processed faster in inp53Δ cells (35 min) than in wild-type cells (59 min; Table 2). However, processing of the F(S13A; F85A; F87A)-ALP mutant was blocked in inp53Δ cells as well as in wild-type cells. Interestingly, the acceleration of trafficking into the PVC caused by the S13D mutation was additive to that caused by the inp53Δ mutation with the processing half-time for the S13D; F85A; F87A triple mutant in wild-type cells (48 min) decreasing in inp53Δ cells (28 min). These data indicate that Inp53p mediates trafficking of A-ALP in a manner dependent on the phosphoserine.
The Block in A-ALP Trafficking Caused by the S13A Mutation Requires the 2–11 Region
Deletion of residues 2–11 of A-ALP accelerates its transport into the PVC (Bryant and Stevens, 1997; Ha et al., 2001); thus, this region may contain a signal to slow transport into the PVC. The S13D mutation constructed to mimic hyperphosphorylation accelerates A-ALP trafficking to the PVC, whereas the S13A mutation that mimics unphosphorylated serine blocks this trafficking step. Given the contrast in phenotype between the Δ2-11 and S13A mutants, we tested the possibility that the phosphoserine and 2–11 motifs antagonize each other.
The rate of processing of single and double mutant A-ALP constructs was assessed in the vps4Δ mutant that provides a measure of the rate of trafficking into the PVC. Interestingly, in vps4Δ cells the S13A; Δ2-11 double mutant was processed more rapidly than wild-type, with kinetics similar to the Δ2-11 single mutant (Table 2). Similar results were obtained for the Δ2-11; S13D double mutant. Therefore, these double mutant experiments indicate that the Δ2-11 mutation neutralizes the effect that mutating S13 has on the rate of trafficking into the PVC, consistent with the idea that the phosphoserine may act by antagonizing the 2-11 signal.
DISCUSSION
This study demonstrates for the first time that phosphorylation influences trafficking of a yeast resident TGN membrane protein. Mutation of S13 to A in the cytosolic domain of A-ALP did not slow trafficking to the Golgi. However, this mutation dramatically inhibited the normal delivery of A-ALP to the PVC under several different conditions. The notion that the S13A mutation simply converts A-ALP to an unsuitable proteolytic substrate can be ruled out because the A(S13A)-ALP was clearly shown to exhibit a non-PVC, Golgi-like localization pattern in class E vps4 cells, whereas wild-type A-ALP was predominantly associated with the PVC in vps4 mutants. In addition, we demonstrate that the phosphorylation state of S13 regulates A-ALP trafficking. First, analysis of peptides containing S13 by mass spectrometry demonstrated that this serine residue is indeed phosphorylated. Second, mutation of S13 to D to mimic phosphorylation accelerated trafficking into the PVC, whereas the S13A mutation to mimic unphosphorylated serine retarded trafficking into the PVC.
New insights into how yeast TGN membrane proteins reach the PVC have been gained in recent years, and these are relevant to understanding the role of S13 phosphorylation. A variety of evidence suggests that TGN membrane proteins reach the PVC via an early endosomal compartment (see Introduction). Whether A-ALP and Kex2p also use the GGA-dependent direct TGN-to-PVC pathway is less clear. A loss of function of the GGA proteins clearly delays vacuolar processing of Cps1p (Costaguta et al., 2001) consistent with the idea that Cps1p relies on the direct TGN-to-PVC pathway. However, the loss of GGA protein function does not affect the rate of trafficking of A(F85A; F87A)-ALP trafficking into the PVC/vacuole (Ha et al., 2003). Trafficking of A(F85A; F87A)-ALP into the PVC/vacuole in gga1,2Δ mutants does not occur via the plasma membrane because it is not blocked by an end3-ts mutation (Foote and Nothwehr, unpublished data). Together, these results suggest either that A-ALP and Kex2p do not normally use the GGA-dependent direct TGN-to-PVC pathway or they do use this pathway to a limited degree but alternatively use the TGN-to-early endosome pathway if the GGA pathway is blocked. A definitive resolution to this issue will require thorough analysis of the transport vesicles from these pathways and their contents.
To gain clues about the function of the S13 phosphoserine, we attempted to determine where the A(S13A)-ALP protein was localized. A(S13A)-ALP exhibited a punctate staining appearance typical of Golgi/endosomal proteins. However, the structures containing A(S13A)-ALP seemed slightly smaller and more numerous that those containing A-ALP. Nevertheless, equilibrium density gradient analysis demonstrated that A(S13A)-ALP associated with membranes having a density indistinguishable from that of A-ALP (Johnston and Nothwehr, unpublished data). Thus, the basis for the slightly different staining pattern of A-ALP and A(S13A)-ALP is presently unclear. In this regard, the S13A mutation does not preclude localization to the compartment(s) where α-factor maturation occurs because Ste13p carrying the S13A mutation exhibited α-factor processing efficiency that was similar to wild-type Ste13p. Furthermore, the rates of depletion of wild-type and S13A mutant Ste13p out of the processing compartment after their synthesis was stopped were very similar. Consistent with the A-ALP processing results, the S13A mutation in Ste13p suppressed the effect of the F85A; F87A mutations, indicating that the S13A mutation inhibited delivery of Ste13p into the PVC. Together, the results imply for A-ALP/Ste13p that both the wild-type and S13A mutants populate the TGN/early endosome. Rather, the difference between the wild-type and mutant proteins seems to involve the wild-type protein frequently cycling via the PVC, whereas the S13A mutant does not.
A loss in Inp53p function causes accelerated transport of A-ALP into the PVC (Ha et al., 2001). Epistasis analysis between the S13 mutations and the inp53Δ mutation was carried out to gain clues about the relative order of function of the phosphoserine and Inp53p. Like wild-type cells, inp53Δ cells exhibited a block in delivery of A(S13A)-ALP to the PVC. Interestingly, a small additive effect was observed between S13D and inp53Δ. These data suggest that the acceleration of A-ALP through the pathway that occurs due to loss of Inp53p function occurs before (or at) the step in which the phosphoserine is required. A previous study showed that processing of retrieval-defective A-ALP was severely retarded in an inp53Δ gga1Δ gga2Δ triple mutant (Ha et al., 2003). A model proposed in that study was that A-ALP reached the PVC more rapidly in inp53Δ single mutants because it used the direct TGN-to-PVC pathway by default. The implication was that Inp53p was needed to restrict trafficking to the TGN-to-early endosome pathway. However, the present study would seem to rule out such a model. The S13A mutation seems to affect trafficking at the early endosome (see below). Therefore, if A-ALP were using the direct TGN-to-PVC pathway in inp53Δ mutants then the S13A mutation would not be expected to have an effect on A-ALP trafficking in inp53Δ cells. The data suggest instead that loss of Inp53p function could reduce static retention in the TGN/early endosome so that A-ALP rapidly enters the PVC in a phosphoserine-dependent manner. The reason for the delay in retrieval defective A-ALP processing in the inp53Δ gga1Δ gga2Δ triple mutant is not known but these mutations also caused a severe growth defect and the slow trafficking could be due to more broad-ranged defects in the TGN/endosomal system.
To learn more about the trafficking step affected by the S13A mutation, we tested whether trafficking of A(S13A)-ALP from the plasma membrane to the PVC could occur. This was accomplished using the vps1Δ and chc1-521ts mutations that mislocalize A-ALP to the plasma membrane whereupon A-ALP is then delivered to the vacuole, presumably via the PVC. The A(S13A)-ALP was shown to mislocalize to the plasma membrane, but unlike A-ALP, it was not subsequently delivered to the vacuole. Together with the other results of this study, the most straightforward interpretation of this result is that the early endosome-to-PVC transport step is abolished for A(S13A)-ALP. These results imply that the phosphoserine is needed at the level of an early endosomal compartment for subsequent delivery to the PVC.
Two sorting signals have previously been characterized in the A-ALP cytosolic domain. The FXFXD motif associates with the retromer to mediate PVC-to-TGN retrieval (Nothwehr et al., 2000), whereas a poorly characterized signal in the 2–11 region somehow regulates entry into the PVC. The 2–11 signal was identified based on the observation that deletion of this region accelerated A-ALP transport into the PVC (Bryant and Stevens, 1997). A similar region in Kex2p (TLS2) has been proposed to function by regulating the partitioning of Kex2p between the TGN-to-early endosome (EE) pathway and the direct TGN-to-PVC pathway (Sipos et al., 2004). According to this model, mutation of TLS2 causes Kex2p to predominantly use the direct TGN-to-PVC pathway. Recent data seem to argue against this type of role for the 2–11 signal of A-ALP. Analysis of trafficking of A-ALP and A(Δ2-11)-ALP into the PVC in a gga1,2Δ end3-ts strain, which should limit trafficking to the TGN/early endosome/PVC route, revealed that deletion of 2–11 accelerated trafficking into the PVC (Nothwehr, unpublished data). Thus, it is plausible that the 2–11 signal could function in static retention at the TGN/early endosome or in retrieval from the early endosome back to the TGN (but see below).
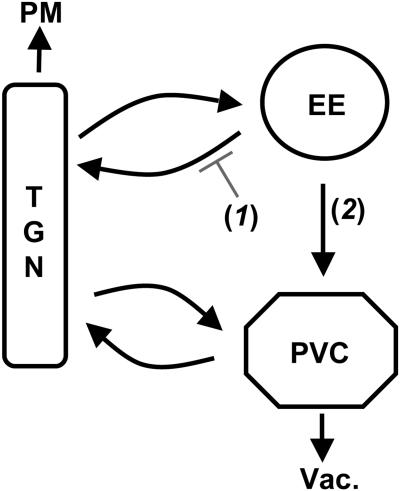
Although the 2–11 region is necessary to slow delivery into the PVC, it is not known whether this region is sufficient for this function or whether other motifs may regulate its function. Thus, it is possible that a true signal slowing transport into the PVC might lie outside of this region. One interesting possibility is that the 2–11 region and phosphorylated S13 might play antagonistic roles. To test this, we analyzed the phenotype of double mutant combinations of S13A, S13D, and Δ2-11 mutations. Interestingly, we found in a vps4Δ background that the S13 mutations had no effect in the context of A(Δ2-11)-ALP. The finding that the 2–11 region is needed for the S13 mutations to have an impact on trafficking suggests that the phosphoserine might antagonize the 2–11 signal. Thus, it seems likely that S13 and Δ2-11 mutations affect the same transport step. A role for the phosphoserine at an early endosome to encourage transport to the PVC, as the vps1Δ results imply (Figure 9), would be consistent with a model in which the 2–11 region mediates phosphoserine-regulated retrieval from an early endosome. In this case, the extent of wild-type A-ALP flux through the PVC could be increased by S13 phosphorylation that would make retrieval less efficient (Figure 10, step 1). Thus, the lack of transport of A(S13A)-ALP into the PVC could be a reflection of enhanced early endosome-to-TGN retrieval compared with wild-type A-ALP. A variation of this basic model is that the 2–11 signal could be a static retention signal for the early endosome and thus would tend to inhibit early endosome-to-PVC transport. Antagonism of the 2–11 signal by the phosphoserine would promote early endosome-to-PVC transport (Figure 10, step 2). Deletion of 2–11 or phosphorylation of S13 would thus enhance the rate of transport into the PVC.
Figure 10.
Potential membrane trafficking steps affected by the phosphorylated S13 residue. Data presented here are consistent with a model in which the phosphorylated S13 residue of A-ALP/Ste13p functions at the early endosome to either reduce the efficiency of early endosome-to-TGN retrieval (1) or enhance transport to the PVC (2).
Several animal cell cargo proteins contain phosphorylation sites embedded with an acidic cluster motif that, when phosphorylated, enhance binding of adaptors and other sorting machinery. In addition to the furin example (see Introduction), phosphorylation of the mannose 6-phosphate receptor increases affinity for the GGA proteins by approximately threefold (Kato et al., 2002). Similarly, AP-1 and AP-2 binding to the T-cell coreceptor CD4 via a dileucine motif is dramatically enhanced by phosphorylation at nearby Ser residues (Pitcher et al., 1999). However, models discussed here for the S13 residue of Ste13p/A-ALP instead posit that phosphorylation may decrease affinity for a sorting factor. In this regard it is worth noting that the 2–11 region is highly basic. Thus, the efficiency of interaction between a sorting factor and the basic 2–11 region might rely on electrostatic interactions could be reduced by the presence of a negatively charged phosphoserine at position 13.
What purpose might be served by using phosphorylation as way of regulating trafficking of a TGN membrane protein? An interesting possibility is that this may allow the cell to alter trafficking patterns of certain proteins as a function of environmental or intrinsic conditions e.g., stress, nutrient availability, pheromone response, cell cycle etc. A few examples of this type of regulation are known in yeast. For example, cell surface receptors such the uracil permease and a-factor receptor also are phosphorylated which positively regulates their ubiquitin-mediated internalization (Hicke et al., 1998; Marchal et al., 2000, 2002). The localization of the yeast glycosyltransferase Mnn1p to early Golgi compartments is controlled by the high osmolarity glycerol mitogen-activated protein kinase pathway (Reynolds et al., 1998), presumably to integrate osmotic stress responses with localization of enzymes such as Mnn1p that are important for cell wall biosynthesis. Moreover, osmotic stress also causes yeast chitin synthase Chs3p to be transported from an early endosomal compartment to the plasma membrane, an event regulated by a protein kinase C pathway (Valdivia and Schekman, 2003). Thus, it is possible that the pheromone processing enzymes Ste13p and Kex2p also might be regulated by cell signaling pathways that could affect the production of mature pheromone or other proteins that are dependent on processing by these enzymes. Testing of this idea awaits identification of the kinase and phosphatase for the S13 residue of Ste13p/A-ALP and characterization of the upstream regulatory machinery.
Acknowledgments
The excellent technical expertise of Beverly DaGue with mass spectroscopy is greatly appreciated. We also thank Vladimir Lupashin for providing antibodies. This work was supported by a grant from the National Institutes of Health (GM-53449) awarded to S.F.N.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-07-0642) on January 12, 2005.
Abbreviations used: ALP, alkaline phosphatase; ER, endoplasmic reticulum; PVC, prevacuolar/endosomal compartment; TGN, trans-Golgi network.
References
- Ausebel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Struhl, K., and Smith, J. A. (2000). Current Protocols in Molecular Biology, New York, John Wiley & Sons.
- Babst, M., Sato, T. K., Banta, L. M., and Emr, S. D. (1997). Endosomal transport function in yeast requires a novel AAA-type ATPase, Vps4p. EMBO J. 16, 1820-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bénédetti, H., Raths, S., Crausaz, F., and Riezman, H. (1994). The END3 gene encodes a protein that is required for the internalization step of endocytosis and for actin cytoskeleton organization in yeast. Mol. Biol. Cell 5, 1023-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black, M. W., and Pelham, H.R.B. (2000). A selective transport route from Golgi to late endosomes that requires the yeast GGA proteins. J. Cell Biol. 151, 587-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J. S., and Traub, L. M. (2003). Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395-447. [DOI] [PubMed] [Google Scholar]
- Brickner, J. H., and Fuller, R. S. (1997). SOI1 encodes a novel, conserved protein that promotes TGN-endosomal cycling of Kex2p and other membrane proteins by modulating the function of two TGN localization signals. J. Cell Biol. 139, 23-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant, N. J., and Stevens, T. H. (1997). Two separate signals act independently to localize a yeast late Golgi membrane protein through a combination of retrieval and retention. J. Cell Biol. 136, 287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghino, J. L., Marcusson, E. G., and Emr, S. D. (1995). The cytoplasmic tail domain of the vacuolar protein sorting receptor Vps10p and a subset of VPS gene products regulate receptor stability, function, and localization. Mol. Biol. Cell 6, 1089-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, A. A., and Stevens, T. H. (1996). Vps10p cycles between the late-Golgi and prevacuolar compartments in its function as the sorting receptor for multiple yeast vacuolar hydrolases. J. Cell Biol. 133, 529-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costaguta, G., Stefan, C. J., Bensen, E. S., Emr, S. D., and Payne, G. S. (2001). Yeast Gga coat proteins function with clathrin in Golgi to endosome transport. Mol. Biol. Cell 12, 1885-1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump, C. M., Xiang, Y., Thomas, L., Gu, F., Austin, C., Tooze, S. A., and Thomas, G. (2001). PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20, 2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y., and Davis, N. G. (2000). Akr1p and the type I casein kinases act prior to the ubiquitination step of yeast endocytosis: Akr1p is required for kinase localization to the plasma membrane. Mol. Cell. Biol. 20, 5350-5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkeneigen, M., Rohricht, R. A., and Kohrer, K. (1997). The VPS4 gene is involved in protein transport out of a yeast prevacuolar endosome-like compartment. Curr. Genet. 31, 469-480. [DOI] [PubMed] [Google Scholar]
- Ha, S.-H., Bunch, J. T., Hama, H., DeWald, D. B., and Nothwehr, S. F. (2001). A novel mechanism for localizing membrane proteins to the yeast trans-Golgi network requires function of a synaptojanin-like protein. Mol. Biol. Cell 12, 3175-3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha, S. A., Torabinejad, J., DeWald, D. B., Wenk, M. R., Lucast, L., De Camilli, P., Newitt, R. A., Aebersold, R., and Nothwehr, S. F. (2003). The synaptojanin-like protein Inp53/Sjl3 functions with clathrin in a yeast TGN-to-endosome pathway distinct from the GGA protein-dependent pathway. Mol. Biol. Cell 14, 1319-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell, L. H. (1980). Mutants of Saccharomyces cerevisiae unresponsive to cell division control by polypeptide mating pheromone. J. Cell Biol. 85, 811-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke, L., Zanolari, B., and Riezman, H. (1998). Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. J. Cell Biol. 141, 349-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinners, I., and Tooze, S. A. (2003). Changing directions: clathrin-mediated transport between the Golgi and endosomes. J. Cell Sci. 116, 763-771. [DOI] [PubMed] [Google Scholar]
- Holthuis, J.C.M., Nichols, B. J., Dhruvakumar, S., and Pelham, H.R.B. (1998). Two syntaxin homologues in the TGN/endosomal system of yeast. EMBO J. 17, 113-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, B. G., Thomas, L., Molloy, S. S., Thulin, C. D., Fry, M. D., Walsh, K. A., and Thomas, G. (1995). Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 14, 5869-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato, Y., Misra, S., Puertollano, R., Hurley, J. H., and Bonifacino, J. S. (2002). Phosphoregulation of sorting signal-VHS domain interactions by a direct electrostatic mechanism. Nat. Struct. Biol. 9, 532-536. [DOI] [PubMed] [Google Scholar]
- Kunkel, T. A., Roberts, J. D., and Zakour, R. A. (1987). Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 154, 367-382. [DOI] [PubMed] [Google Scholar]
- Lewis, M. J., Nichols, B. J., Prescianotto-Baschong, C., Riezman, H., and Pelham, H.R.B. (2000). Specific retrieval of the exocytic SNARE Snc1p from early yeast endosomes. Mol. Biol. Cell 11, 23-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchal, C., Dupre, S., and Urban-Grimal, D. (2002). Casein kinase I controls a late step in the endocytic trafficking of yeast uracil permease. J. Cell Sci. 115, 217-226. [DOI] [PubMed] [Google Scholar]
- Marchal, C., Haguenauer-Tsapis, R., and Urban-Grimal, D. (2000). Casein kinase I-dependent phosphorylation within a PEST sequence and ubiquitination at nearby lysines signal endocytosis of yeast uracil permease. J. Biol. Chem. 275, 23608-23614. [DOI] [PubMed] [Google Scholar]
- Molloy, S. S., Anderson, E. D., Jean, F., and Thomas, G. (1999). Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 9, 28-35. [DOI] [PubMed] [Google Scholar]
- Molloy, S. S., Thomas, L., Kamibayashi, C., Mumby, M. C., and Thomas, G. (1998). Regulation of endosome sorting by a specific pp2a isoform. J. Cell Biol. 142, 1399-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S. F., Bruinsma, P., and Strawn, L. S. (1999). Distinct domains within Vps35p mediate the retrieval of two different cargo proteins from the yeast prevacuolar/endosomal compartment. Mol. Biol. Cell 10, 875-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S. F., Bryant, N. J., and Stevens, T. H. (1996). The newly identified yeast GRD genes are required for retention of late-Golgi membrane proteins. Mol. Cell. Biol. 16, 2700-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S. F., Conibear, E., and Stevens, T. H. (1995). Golgi and vacuolar membrane proteins reach the vacuole in vps1 mutant yeast cells via the plasma membrane. J. Cell Biol. 129, 35-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S. F., Ha, S.-A., and Bruinsma, P. (2000). Sorting of yeast membrane proteins into an endosome-to-Golgi pathway involves direct interaction of their cytosolic domains with Vps35p. J. Cell Biol. 151, 297-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nothwehr, S. F., Roberts, C. J., and Stevens, T. H. (1993). Membrane protein retention in the yeast Golgi apparatus: dipeptidyl aminopeptidase A is retained by a cytoplasmic signal containing aromatic residues. J. Cell Biol. 121, 1197-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper, R. C., Cooper, A. A., Yang, H., and Stevens, T. H. (1995). VPS27 controls vacuolar and endocytic traffic through a prevacuolar compartment in Saccharomyces cerevisiae. J. Cell Biol. 131, 603-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher, C., Honing, S., Fingerhut, A., Bowers, K., and Marsh, M. (1999). Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation. Mol. Biol. Cell 10, 677-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raths, S., Rohrer, J., Crausaz, F., and Riezman, H. (1993). end3 and end4: two mutants defective in receptor-mediated and fluid-phase endocytosis in Saccharomyces cerevisiae. J. Cell Biol. 120, 55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond, C. K., Howald-Stevenson, I., Vater, C. A., and Stevens, T. H. (1992). Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell 3, 1389-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, T. B., Hopkins, B. D., Lyons, M. R., and Graham, T. R. (1998). The high osmolarity glycerol response (hog) map kinase pathway controls localization of a yeast Golgi glycosyltransferase. J. Cell Biol. 143, 935-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, C. J., Raymond, C. K., Yamashiro, C. T., and Stevens, T. H. (1991). Methods for studying the yeast vacuole. Methods Enzymol. 194, 644-661. [DOI] [PubMed] [Google Scholar]
- Santos, B., and Snyder, M. (1997). Targeting of chitin synthase 3 to polarized growth sites in yeast requires Chs5p and Myo2p. J. Cell Biol. 136, 95-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer, W., Stroh, A., Berghofer, S., Seiler, J., Vey, M., Kruse, M. L., Kern, H. F., Klenk, H. D., and Garten, W. (1995). Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO J. 14, 2424-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, P. M., Bilodeau, P. S., Zhdankina, O., Winistorfer, S. C., Hauglund, M. J., Allaman, M. M., Kearney, W. R., Robertson, A. D., Boman, A. L., and Piper, R. C. (2004). GGA proteins bind ubiquitin to facilitate sorting at the trans-Golgi network. Nat. Cell Biol. 6, 252-259. [DOI] [PubMed] [Google Scholar]
- Seaman, M.N.J., Marcusson, E. G., Cereghino, J. L., and Emr, S. D. (1997). Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the Vps29, Vps30, and Vps35 gene products. J. Cell Biol. 137, 79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman, M.N.J., McCaffery, J. M., and Emr, S. D. (1998). A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J. Cell Biol. 142, 665-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996). Mass spectrometric sequencing of proteins from sliver-stained polyacrylamide gels. Anal. Chem. 68, 850-858. [DOI] [PubMed] [Google Scholar]
- Sikorski, R. S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos, G., Brickner, J. H., Brace, E. J., Chen, L., Rambourg, A., Kepes, F., and Fuller, R. S. (2004). Soi3p/Rav1p functions at the early endosome to regulate endocytic trafficking to the vacuole and localization of TGN transmembrane proteins. Mol. Biol. Cell 15, 3196-3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spelbrink, R. G., and Nothwehr, S. F. (1999). The yeast GRD20 gene is required for protein sorting in the trans-Golgi network/endosomal system and for polarization of the actin cytoskeleton. Mol. Biol. Cell 10, 4263-4281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, S., Nakagawa, T., Banno, T., Watanabe, T., Murakami, K., and Nakayama, K. (1995). Localization of furin to the trans-Golgi network and recycling from the cell surface involves Ser and Tyr residues in the cytoplasmic domain. J. Biol. Chem. 270, 28397-28401. [DOI] [PubMed] [Google Scholar]
- Tyagi, R., Lai, R., and Duggeleby, R. G. (2004). A new approach to `megaprimer' polymerase chain reaction mutagenesis without an intermediate gel purification step. BMC Biotechnol. 4, 2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia, R. H., Baggot, D., Chuang, J. S., and Schekman, R. (2002). The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell 2, 283-294. [DOI] [PubMed] [Google Scholar]
- Valdivia, R. H., and Schekman, R. (2003). The yeasts Rho1p and Pkc1p regulate the transport of chitin synthase III (Chs3p) from internal stores to the plasma membrane. Proc. Natl. Acad. Sci. USA 100, 10287-10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees, P., Deignan, E., van Donselaar, E., Humphrey, J., Marks, M. S., Peters, P. J., and Bonifacino, J. S. (1995). An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 14, 4961-4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, L., Molloy, S. S., Thomas, L., Liu, G. P., Xiang, Y., Rybak, S. L., and Thomas, G. (1998). PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94, 205-216. [DOI] [PubMed] [Google Scholar]
- Warner, J. R. (1991). Labeling of RNA and phosphoproteins in Saccharomyces cerevisiae. Methods Enzymol. 194, 423-424. [DOI] [PubMed] [Google Scholar]
- Wilcox, C. A., Redding, K., Wright, R., and Fuller, R. S. (1992). Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts Golgi retention and results in default transport to the vacuole. Mol. Biol. Cell 3, 1353-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman, M., Chuang, J. S., and Schekman, R. W. (1996). Chs1p and Chs3p, two proteins involved in chitin synthesis, populate a compartment of the Saccharomyces cerevisiae endocytic pathway. Mol. Biol. Cell 7, 1909-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]