Abstract
Polarized exocytosis is important for morphogenesis and cell growth. The exocyst is a multiprotein complex implicated in tethering secretory vesicles at specific sites of the plasma membrane for exocytosis. In the budding yeast, the exocyst is localized to sites of bud emergence or the tips of small daughter cells, where it mediates secretion and cell surface expansion. To understand how exocytosis is spatially controlled, we systematically analyzed the localization of Sec15p, a member of the exocyst complex and downstream effector of the rab protein Sec4p, in various mutants. We found that the polarized localization of Sec15p relies on functional upstream membrane traffic, activated rab protein Sec4p, and its guanine exchange factor Sec2p. The initial targeting of both Sec4p and Sec15p to the bud tip depends on polarized actin cable. However, different recycling mechanisms for rab and Sec15p may account for the different kinetics of polarization for these two proteins. We also found that Sec3p and Sec15p, though both members of the exocyst complex, rely on distinctive targeting mechanisms for their localization. The assembly of the exocyst may integrate various cellular signals to ensure that exocytosis is tightly controlled. Key regulators of cell polarity such as Cdc42p are important for the recruitment of the exocyst to the budding site. Conversely, we found that the proper localization of these cell polarity regulators themselves also requires a functional exocytosis pathway. We further report that Bem1p, a protein essential for the recruitment of signaling molecules for the establishment of cell polarity, interacts with the exocyst complex. We propose that a cyclical regulatory network contributes to the establishment and maintenance of polarized cell growth in yeast.
INTRODUCTION
Exocytosis is a basic membrane traffic event mediated by transport, docking, and fusion of secretory vesicles carrying proteins and lipids to defined areas of the plasma membrane. For most eukaryotic cells, exocytosis is spatially controlled by signaling molecules and the cytoskeleton. On the other hand, polarized exocytosis is important for the delivery of polarity regulators. The simple eukaryote Saccharomyces cerevisiae grows asymmetrically by “budding,” a seemingly simple process that requires sophisticated mechanisms that couple exocytosis to cell polarization and cell cycle progression. At the early stages of the cell cycle, polarized delivery of lipid and membrane proteins to the bud is important for daughter cell growth. In addition, the polarized secretion of cell surface glucanases is needed to modify the rigid yeast cell wall so that the specific areas of the daughter cell surface can expand. The distinctive growth characteristics, in combination with the facile genetics and well-studied genomics, make the budding yeast an excellent model system to study spatial regulation of exocytosis.
The late stage of exocytosis in yeast consists of at least three steps. First, post-Golgi secretory vesicles are targeted to designated areas of plasma membrane via actin cables. The class V myosin, Myo2p, serves as a motor for this transport process (Govindan et al., 1995; Pruyne et al., 1998; Schott et al., 1999; Karpova et al., 2000). Second, the vesicles are tethered to specific plasma membrane domains mediated by a multiprotein complex, the exocyst (Guo et al., 2000; Whyte and Munro, 2002; Hsu et al., 2004; Chu and Guo, 2004). Finally, interactions between vesicle and plasma membrane integral membrane proteins, termed v-SNAREs and t-SNAREs, respectively (SNARE, soluble N-ethylmaleimide-sensitive fusion attachment protein receptors), lead to the fusion of the bilayers of the secretory vesicle and the plasma membrane (Rothman, 1994). This fusion event allows the secretion of vesicle contents and the incorporation of membrane proteins at specific plasma membrane domains. The rab family of small GTPases are master regulators of exocytosis (Novick and Zerial, 1997; Lazar et al., 1997).
The exocyst is an evolutionarily conserved multiprotein complex implicated in tethering secretory vesicles at specific sites of the plasma membrane preceding SNARE assembly and membrane fusion (Novick and Guo, 2002; Lipschutz and Mostov, 2002; Hsu et al., 2004). It consists of 8 components: Sec3p, Sec5p, Sec6p, Sec8p, Sec10p, Sec15p, Exo70p, and Exo84p. All are hydrophilic proteins that exist in the cytosol and associate with the plasma membrane (TerBush and Novick, 1995; TerBush et al., 1996; Guo et al., 1999a). In budding yeast, the exocyst proteins are specifically localized to regions of active exocytosis and cell surface expansion: the sites of bud emergence, the tips of small daughter cells, and the mother/daughter junction of dividing cells (TerBush and Novick, 1996; Finger et al., 1998; Guo et al., 1999b). This pattern of localization is in contrast to that of the t-SNARE proteins, which are evenly distributed along the entire yeast plasma membrane (Brenn-wald et al., 1994).
One component of the exocyst complex, Sec3p, is localized to sites of exocytosis independent of ongoing secretion and the actin cytoskeleton (Finger et al., 1998). In a variety of secretion mutants blocked at or before the Golgi apparatus, Sec3-GFP is still localized at bud tips and mother/daughter cell connections. Furthermore, the localization of Sec3p appears to be independent of the other subunits of the exocyst. Based on these observations, it was proposed that Sec3p represents a spatial landmark for polarized secretion (Finger et al., 1998). Recent studies demonstrated that Sec3p directly interacts with Rho1p and Cdc42p, members of the Rho family of small GTPases through its N-terminus (Guo et al., 2001; Zhang et al., 2001). Cdc42p and Rho1p compete for their binding to this region and disruption of the binding results in mislocalization of Sec3p (Guo et al., 2001; Zhang et al., 2001).
While Sec3p is thought to be the exocyst subunit most proximal to the plasma membrane, another subunit, Sec15p, directly interacts with the GTP-bound form of the rab protein, Sec4p, on the secretory vesicles (Guo et al., 1999b). This interaction is specific for Sec4p but not for other rab proteins such as Ypt1p and Ypt51p. Replacement of four amino acids in the “effector loop” of Sec4p with the corresponding residues of Ypt1p completely abolished the interaction of the Rab protein with Sec15p. Sec4p promotes the protein-protein interactions among the exocyst components, which eventually link Sec15p to Sec3p at the target membrane (Guo et al. 1999a).
To better understand how exocytosis is spatially controlled, we have systematically examined the localization of the exocyst in yeast cells. Here we focus on Sec15p, the direct downstream effector of Sec4p (Guo et al., 1999b). We found that upstream traffic and the rab GTPase controls the localization of Sec15p to site of secretion. The targeting of Sec15p is also under the control of cell polarity determinants and requires polarized actin cables. Sec3p and Sec15p, though both members of the exocyst complex, are spatially controlled by different mechanisms. In addition to examining how the exocyst is controlled by polarity regulators, we also examined the role of exocytosis on cell polarity by using different alleles of the secretory mutants. We found that the proper localization of these cell polarity regulators themselves also requires a functional exocytosis pathway. We further report that Bem1p, a protein essential for the establishment of cell polarity, interacts with the exocyst. We propose that a cyclical regulatory mechanism may contribute to the establishment of cell polarity and maintenance of polarized cell growth in yeast.
MATERIALS AND METHODS
Strains and Growth Conditions
The genotypes of the yeast strains used in this study are listed in Table 1. Standard culture media and genetic techniques were used (Guthrie and Fink, 1991).
Table 1.
Parental strains used in this study
| Strainsa | Genotype |
|---|---|
| ABY971 | Mat a / α tmp1-2::LEU2/tmp1-2::LEU2, tmp2::HIS3/tmp2::HIS3, his3Δ-200/his3Δ-200, leu2-3, 112/leu2-3, 112, lys2-801/lys2-801, trp1-1/trp1-1, ura3-52/ura3-52 |
| ABY973 | Mat a / α tmp2::HIS3/tmp2::HIS3, his3Δ-200/his3Δ-200, leu2-3, 112/leu2-3, 112, lys2-801/lys2-801, trp1-1/trp1-1, ura3-52/ura3-52 |
| NY179 | Mat aura3-52, leu2-3, 112 |
| NY27 | Mat α ura3-52, sec2-59 |
| NY774 | Mat α ura3-52, leu2-3, 112, sec4-8 |
| NY776 | Mat α ura3-52, leu2-3, 112, sec5-24 |
| NY778 | Mat α ura3-52, leu2-3, 112, sec6-4 |
| NY760 | Mat α ura3-52, leu2-3, 112, sec7-1 |
| NY1218 | Mat α ura3-52, leu2-3, 112, sec18-1 |
| NY1213 | Mat α ura3-52, leu2-3, 112, sec19-1 |
| NY1426 | Mat α ura3-52, leu2-3, 112, sec22-3 |
| YEF473A | Mat aCDC42, trp1-1, leu2-3, his3Δ200, ura3-52, lys2-801 |
| YEF1961 | Mat acdc42-201, trp1-1, leu2-3, his3Δ200, ura3-52, lys2-801 |
| NY1500 | Mat α, ura3-52, leu2-3, 112, cdc24-4 |
| GY1456 | Mat α, ura3-52, leu2-3, 112, his3Δ200, trp1-1, Gal+, LA+, bem1Δ::HIS3 |
ABY from A. Bretscher's laboratory, NY from P. Novick's laboratory, and YEF from E. Bi's laboratory
Observation of GFP-tagged Proteins in Yeast Cells
C-terminal GFP tagging of the exocyst components and Bem1p at their chromosomes in wild-type and mutant yeast strains was performed as previously described (Finger et al., 1998; Guo et al., 1999a). Cells were grown to early log phase in SCD media (synthetic complete plus 2% dextrose) lacking uracil. Yeast cells were fixed as follows: cells were pelleted 1 min at 4°C at 3000 rpm in an Eppendorf 5415R bench top centrifuge, washed once in ice-cold phosphate-buffered saline (PBS), and then fixed at 4°C for 10 min in -20°C methanol. The cells were washed once in -20°C acetone and rehydrated by three washes in PBS for viewing. GFP was scored as mislocalized when it appeared diffused or in patches in the mother cells. Protein localization in yeast released from G0 was examined as previously described (Ayscough et al., 1997).
Immunofluorescence
Immunofluorescence staining of yeast cells was performed as previously described (Walch-Solimena et al., 1997). Briefly, the cells were fixed and transferred to isotonic media containing 0.1 M KPi, pH 7.4, and 1.2 M sorbitol. After cell wall removal by zymolase, the spheroplasts were attached to eight-well microslides and then treated with 0.5% SDS in 0.1 M KPi, pH 7.4, and 1.2 M sorbitol for 5 min. The cells were then washed 10 times with 20 μl per well of PBS containing 1 mg/ml bovine serum albumin, and nonspecific binding was blocked for 30 min in the same buffer. The incubation with primary antibodies was performed at room temperature for 2 h. After 10 washes in PBS-bovine serum albumin buffer, the cells were incubated for 1 h with Alexa-fluor 594 secondary antibody. The wells were washed with PBS-bovine serum albumin buffer and then mounted for observation. The immunofluorescence was visualized with a microscope equipped with epifluorescence using a 100× objective. The anti-Sec4p polyclonal antibody was used at 1:1000 dilution. Immunostaining of myc-tagged Bem1p was performed with a mouse anti-myc monoclonal antibody (9E10) at 1:500 dilution.
Coprecipitation Assays
The in vivo interaction between Sec15p and Bem1p was performed using coprecipitation assays. Yeast cells expressing GST or GST-Bem1p in a CEN plasmid under the TEF promoter were grown to early log phase, and cell lysates were prepared by disrupting the cell wall using glass beads in the lysis buffer (20 mM HEPES-NaOH, pH 7.5, 100 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol, 1% Sigma protease inhibitor cocktail). The lysates were incubated with glutathione Sepharose 4B for 1 h at 4°C. The Sepharose were washed five times and the proteins were separated using 10% SDS-PAGE. Proteins coprecipitated with the GST fusion proteins from the lysates were analyzed by Western blot.
RESULTS
Sec15p Polarization Is Dependent on Upstream Traffic and Active Sec4p
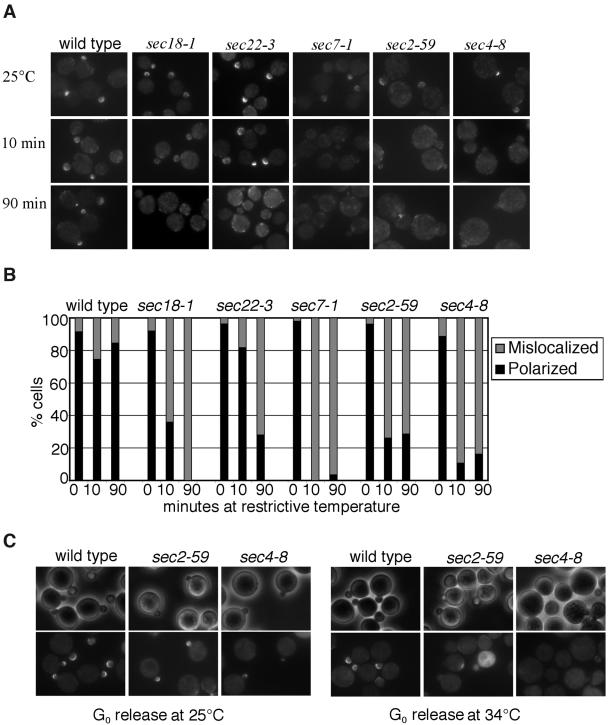
Sec15p was previously shown to interact with the GTP-bound form of the rab protein Sec4p. Furthermore, biochemical analysis suggests that the assembly of the exocyst complex is defective in the sec4 mutant (Guo et al., 1999b). It is possible that Sec15p travels to sites of polarized secretion on vesicles and Sec4p regulates this process. If so, mutations affecting the activity of Sec4p would block the ability of Sec15p to polarize in yeast cells. In addition, defects in upstream trafficking (e.g., ER to Golgi, intra-Golgi transport, etc.) will also affect Sec15p delivery as the flow of membranes is blocked. To test this hypothesis, we examined the localization of Sec15-GFP in various mutants. These include sec18-1 (defective in SNARE disassembly and ER-to-Golgi traffic), sec22-3 (defective in ER-to-Golgi traffic), sec7-1 (defective in Golgi traffic), sec4-8 (mutation in the rab protein responsible for post-Golgi exocytosis; Novick et al., 1980). In addition, to further test whether the activated form of Sec4p is required, we examined the localization of Sec15-GFP in the sec2-59 strain, in which the guanine nucleotide exchange factor (GEF) of Sec4p is mutated (Walch-Solimena et al., 1997). The yeast cells were grown to early log phase and then shifted to the restrictive temperature for periods of 10 and 90 min. Cells were then fixed in methanol/acetone and examined for Sec15-GFP localization. In wild-type cells, Sec15-GFP was concentrated as a crescent at the bud tip at 25°C, 10 min at 34°C, and 90 min at 34°C (Figure 1A). In contrast, in the mutant cells defective in various stages of traffic or defective in Sec4p, Sec15-GFP signals were diffused from the bud after 10 min at 34°C. At 90 min, Sec15p remained mislocalized in these cells. A quantification of the observation is presented in Figure 1B.
Figure 1.
Sec15-GFP polarization is dependent on upstream traffic and functional Sec4p. (A) Sec15-GFP was expressed in wild-type, sec18-1 (defective in SNARE disassembly and ER-to-Golgi traffic), sec22-3 (defective in ER-to-Golgi traffic), sec7-1 (defective in Golgi traffic), sec4-8 (mutation in the post-Golgi rab protein), and sec2-59 (a mutant allele of the GEF for Sec4p). Cells were grown in SC-Ura media at 25°C to log phase and then shifted to 34°C for 10 and 90 min. Cells were fixed in methanol for microscopy. (B) Percentage of cells with polarized Sec15-GFP. Small-budded cells from the above experiments were scored for the presence of Sec15-GFP at the bud tip (polarized) or lack of Sec15-GFP in the bud tip (mislocalized). (C) The initial targeting of Sec15-GFP to the bud tip is dependent on activated Sec4p. Wild-type, sec2-59, and sec4-8 cells expressing Sec15-GFP were arrested at G0 and then released in fresh media for 90 min at 25°C (left) or 34°C (right). Cells were then fixed for microscopy. Bottom: Sec15-GFP; top: phase contrast images of the cells.
To further investigate the role of Sec4p in the initial targeting of Sec15p to the bud tip, we examined Sec15-GFP localization in cells released from G0. The yeast cells were isolated as a homogenous population arrested at G0 (Ayscough et al., 1997). Releasing the cells from G0 allowed us to examine the initial recruitment of secretory machinery to the emerging bud for polarized cell growth. We looked for polarization of Sec15-GFP in the tips of small buds or patches of Sec15-GFP at the cortexes representing incipient buds. As shown in Figure 1C, Sec15-GFP was localized to small buds or incipient buds in wild-type cells released from G0 at both 25°C and 34°C. In sec2-59 and sec4-8 cells, Sec15-GFP formed patches in small buds and incipient buds at 25°C. However, at 34°C many of the mutant cells remain unbudded. Even in cells with small buds, Sec15-GFP was not concentrated to the bud. The lack of Sec15-GFP at buds in G0 released mutant cells indicates that Sec4p and Sec2p functions are required for the initial delivery of Sec15p to sites of polarized exocytosis.
The Localization of Sec15p in Cells Treated with Latrunculin
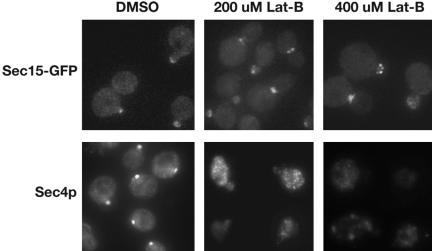
As shown above, the polarized localization of Sec15p requires functional rab protein Sec4p. Because Sec4p associates with vesicle and is transported to the bud tip along actin cables (Walch-Solimena et al., 1997), we hypothesized that the ability of Sec15p to polarize in yeast cells also requires polarized actin organization. To test this hypothesis, we examined the localization of Sec15-GFP in cells treated with latrunculin-B (Lat-B), which disrupts the F-actin in yeast cells. Wild-type yeast cells were grown to early log phase and then treated with Lat-B to a final concentration of 200 or 400 μM. The yeast cells were cultured for an additional 30 min. A portion of the cells was collected for immunofluorescence staining of Sec4p. The remaining cells were fixed in methanol/acetone and examined for Sec15-GFP localization. As shown in Figure 2, Sec4p was completely mislocalized after Lat-B treatment. However, to our surprise, Sec15-GFP was well polarized after treatment with 200 or 400 μM Lat-B.
Figure 2.
The localization of Sec15p and Sec4p in cells treated with latrunculin. The localization of Sec4p, but not Sec15p, is disrupted by Lat-B treatment. Fluorescence images of Sec15-GFP (top panel) and immunofluorescence image of Sec4p (bottom panel) are shown for wild-type cells treated with DMSO (left), 200 μM Lat-B (middle), and 400 μM Lat-B (right) for 30 min.
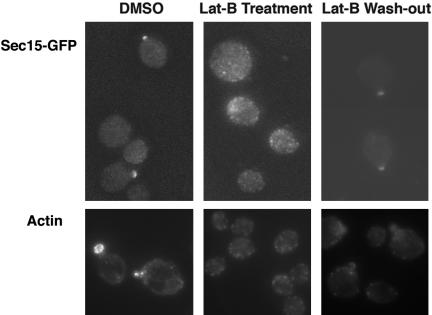
A previous study showed that targeting of Sec3p to emerging buds is independent of actin, as Sec3p remained a single “patch” at the plasma membrane even in cells released from G0 under latrunculin treatment (Finger et al., 1998). Here, we examined the localization of Sec15-GFP in yeast cells under the same conditions (Figure 3). Wild-type yeast cells were isolated as a homogenous population arrested at G0. An aliquot of cells was released into fresh media in the presence of 200 μM Lat-B or dimethyl sulfoxide (DMSO), as a control. As shown in Figure 3, actin cables were intact in cells treated with DMSO, and Sec15-GFP was localized to small buds in these cells. However, in the cells treated with Lat-B, most of the cells remained unbudded. Actin cables were completely disrupted and Sec15-GFP could not form any patch structures in the cells. After we recovered the cells in fresh media in the absence of Lat-B for 15 min, the cells began to form buds, and Sec15-GFP became polarized in these cells. These results suggest that, unlike Sec3p, the initial delivery of Sec15p to the site of polarized exocytosis requires actin cables. It was shown previously that most of endogenous Sec15p is distributed at the plasma membrane in yeast cells (Salminen and Novick, 1989; Bowser and Novick, 1991), whereas most of Sec4p is associated with secretory vesicles (Goud et al., 1988). The Sec15-GFP remaining at the bud tips after Lat-B treatment may reflect the plasma membrane pool of Sec15p, which is less sensitive to the disruption of F-actin.
Figure 3.
The initial targeting of Sec15p to the bud tip depends on actin cables. The initial targeting of Sec15p to the bud tips is disrupted by Lat-B. The Left and middle: the fluorescence images of Sec15-GFP in cells released from G0 in the presence of DMSO and 200 μM Lat-B, respectively; right: the cells 15 min after recovery from Lat-B treatment with fresh medium. Bottom: the actin staining with phalloidin in these cells.
The Localization of Sec15p in Tropomyosin Mutant Cells
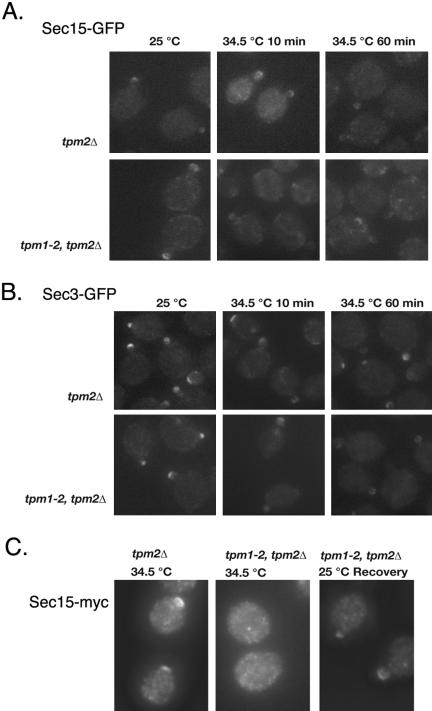
Lat-B treatment disrupts both actin cables and actin patches in yeast cells. To better examine the role of actin cables in the localization of Sec15p, we examined the localization of Sec15p in tpm1-2, tpm2Δ cells, which rapidly lose actin cables upon shifting to restrictive temperature (Pruyne et al., 1998). The yeast cells were grown to early log phase and then shifted to 34.5°C for periods of 10 and 60 min. Cells were fixed in methanol/acetone and examined for Sec15-GFP localization. In wild-type cells, Sec15-GFP was concentrated as a crescent at the tips of small buds at 25°C. It remained mostly polarized after shifting to 34.5°C for 10 and 60 min (Figure 4A). In contrast, Sec15-GFP was mislocalized from the bud tips in the mutant cells after 10 min at 34.5°C. After 60 min, Sec15-GFP was almost completely mislocalized. We also examined the localization of Sec3-GFP under the same conditions (Figure 4B) and found that Sec3-GFP was only moderately depolarized after 10 min at 34.5°C. After 60 min, Sec3-GFP remained mostly polarized in the cells, consistent with the previous proposal that Sec3p localization is independent of actin (Finger et al., 1998).
Figure 4.
The localization of Sec15p, Sec3p, and Sec4p to the bud tip in the tpm mutants. (A) Fluorescence image of Sec15-GFP in tpm2Δ (top) and tpm1-2, tpm2Δ (bottom) cells grown at 25°C, and the restrictive temperature 34.5°C for 10 and 60 min. (B) Fluorescence image of Sec3-GFP in tpm2Δ (top) and tpm1-2, tpm2Δ (bottom) cells grown at 25°C, and the restrictive temperature 34.5°C for 10 and 60 min. (C) Fluorescence image of Sec15-myc are shown for tpm2Δ (left) and tpm1-2, tpm2Δ (middle and right) cells. Cells were released from G0 and grown for 2 h at 34.5°C (left and middle). A portion of tpm1-2, tpm2Δ cells was then shifted back to 25°C and recovered for 10 min (right).
To examine whether the initial targeting of Sec15p needs actin cables, we examined Sec15p localization in tpm mutant cells released from G0 using immunofluorescence. As shown in Figure 4C, Sec15p was localized to small buds or incipient buds in wild-type cells released from G0 at both 25°C and 34.5°C. In tpm1-2, tpm2Δ cells, Sec15 was polarized to the small buds and incipient buds at 25°C, but was not polarized at 34.5°C. Upon shifting the mutant cells back to 25°C for 10 min, Sec15p was repolarized to the small buds and incipient buds. These results suggest that actin cables are required for the initial delivery of Sec15p to sites of polarized exocytosis.
The Rab GDI Mediates the Rapid Recycling of Sec4p from the Bud Tip
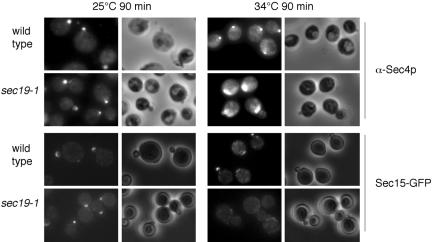
Although both Sec15p and Sec4p need polarized actin for their delivery to the daughter cell membrane, what accounts for their different responses to the latrunculin treatment (Figure 2)? One possibility is that the diffusion or recycling of Sec4p and Sec15p from the bud tip membrane is regulated differently. The recycling of the rab proteins from their target membranes is mediated by the GDI protein Sec19p (Garrett et al., 1994). We examined the localization of Sec4p and Sec15p in the sec19-1 mutant. As shown in Figure 5, after shifting the sec19-1 mutant to the restrictive temperature (34°C) for 90 min, Sec4p remained polarized at the buds. However, the polarization pattern slightly changed. Instead of being concentrated at the bud tip as in the wild-type cells, Sec4p spread slightly toward the inner region. In some cases, the staining of Sec4p is even brighter. Sec19p is the GDI for all the rab proteins in yeast including Ypt1p that functions in the ER-to-Golgi stage of transport. Like the NSF protein Sec18p, Sec19p functions in every stage of traffic and mutations in Sec19p first block upstream ER-to-Golgi transport. It was shown previously that the localization of Sec4p relies on upstream traffic (Walch-Solimena et al., 1997). Mutations affecting early stages of traffic block the membrane flow to the post-Golgi stage, therefore affecting the transport of Sec4p by the secretory vesicles to its target membrane. Although Sec4p is diffused in all the early stage mutants, we show here that it accumulated in the bud membrane in the sec19-1 mutant. Clearly, recycling of the GDP-bound form of Sec4p from the plasma membrane depends on Sec19p.
Figure 5.
Effects of the rab GDI mutant (sec19-1) on the localization of Sec4p and Sec15p. Wild-type and sec19-1 cells were grown in SC-Ura media at 25°C and then shifted to 34°C for 90 min and fixed for microscopy. Sec15-GFP was mislocalized in the mutant cells (the bottom right panel). However, Sec4p accumulated near the bud tips in a “polarized” manner, although less concentrated comparing with those in the wild-type cells (the top right panel). Cells were then fixed for microscopy.
In contrast to Sec4p, Sec15p completely diffused in the sec19-1 mutant at the restrictive temperature (Figure 5). The diffusion may be attributed to the block of membrane traffic from early stages or the failure of the recycling of GDP-Sec4p to GTP-Sec4p. We do not know what accounts for the diffusion or recycling of Sec15p and the exocyst complex from the plasma membrane in wild-type cells.
The Localization of Sec15p and Sec4p Depends on Cdc42p
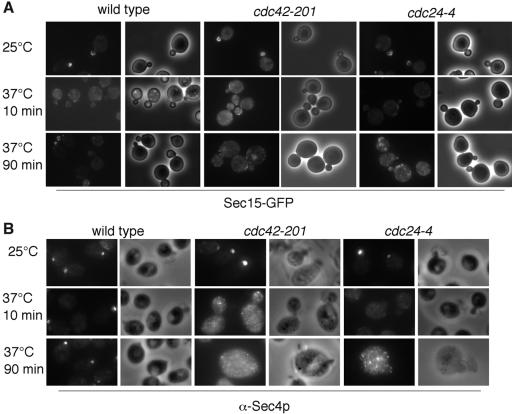
The Rho protein Cdc42p plays an essential role in the establishment of cell polarity in yeast. Cdc42p controls the polarized organization of actin cables, on which secretory vesicles are transported to the bud (Dong et al., 2003). In addition, Cdc42p directly interacts with the exocyst protein Sec3p and deletion of the N-terminus Cdc42p binding domain (sec3ΔN) resulted in mislocalization of Sec3p (Guo et al., 2001; Zhang et al., 2001). However, we also noticed in our experiments that several exocyst proteins, including Sec15p, remain polarized in the sec3ΔN mutant (Guo et al., 2001; Guo laboratory, unpublished results). Here, we examine the role of Cdc42p on the polarization of Sec15p and Sec4p. We used Sec15-GFP and Sec4p immunofluorescence staining to examine the localization of these two proteins in cdc42-201 mutants. Because Cdc42 is activated in the cells by its GEF Cdc24p, we also examined the localization of Sec15p and Sec4p in the cdc24-4 mutant. Yeast cells were grown to early log phase and then shifted to the restrictive temperature of 37°C for 10 or 90 min before fixation. Sec15-GFP was polarized to the bud tips of wild-type cells at both 25 and 37°C (Figure 6). In cdc42-201 and cdc24-4 mutant cells, Sec15-GFP was polarized at the permissive temperature of 25°C but became diffused after 10 or 90 min at 37°C. Similarly, Sec4p was mislocalized in cdc42-201 and cdc24-4 cells at 37°C. The depolarization of these two proteins in both cdc42-201 and cdc24-4 suggests that active Cdc42p is necessary for the recruitment of Sec15p and Sec4p to the bud tip. Why Sec15p is depolarized in these cells is unclear. However, it is unlikely that the depolarization of Sec15p is a consequence of Sec3p mislocalization.
Figure 6.
Sec15p and Sec4p require functional Cdc42p for their polarized localization to the bud tip. Sec15-GFP (A) and Sec4p (B) cannot polarize to bud tips in cdc42-201 and cdc24-4 mutants. Sec15-GFP and Sec4p immunofluorescence images are paired with the phase contract images of the cells to the right.
The Localization of Sec15p and Sec4p in bem1Δ Cells
To further study the role of polarity determinants on the polarization of Sec15p and Sec4p, we examined cells lacking Bem1p (bem1Δ). Bem1p binds to a number of proteins such as Cdc42p, Cdc24p, Rsr1p/Bud1p, the PAK-like kinases Ste20p and Cla4p and therefore is important for organizing the signaling molecules for the establishment of cell polarity (Bender and Pringle, 1991, Chenevert et al., 1992; Leeuw et al., 1995; Park et al., 1997; Butty et al., 1998; Gulli et al., 2000; Bose et al., 2001; Butty et al., 2002; Irazoqui et al., 2003). Yeast cells with Bem1 deletion are viable but exhibit defects in actin organization and cell polarity (Bender and Pringle, 1991, Chenevert et al., 1992). We used bem1Δ yeast cells to examine the role of this polarity protein in Sec15p localization. The bem1Δ cells were grown to early log phase at 25°C. These cells exhibited heterogeneous morphological defects such as multibudded cells and extremely large cells, consistent with previous observations (Bender et al., 1991). In most of the cells, Sec15-GFP was mislocalized (unpublished data). In multi-budded cells, Sec15-GFP tended to be preferentially concentrated in one or several of the buds (Figure 7A). In single budded cells, Sec15-GFP was localized to the tip of the bud. (Figure 7A).
Figure 7.
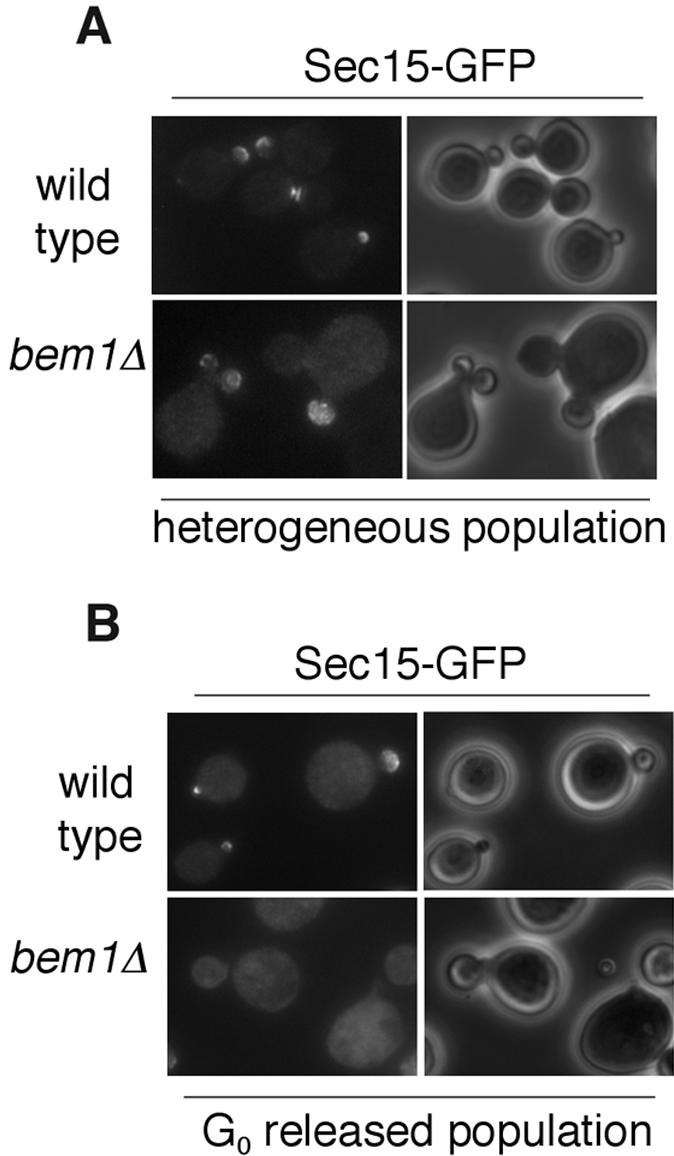
Localization of Sec15-GFP and Sec4p in bem1Δ cells. (A) Localization of Sec15-GFP in bem1Δ cells in a heterogeneous population. (B) Bem1p is required for the initial targeting of Sec15-GFP to the bud tip. bem1Δ cells expressing Sec15-GFP were arrested at G0. A homogeneous population of unbudded cells were released in fresh media at 25°C and fixed in methanol/acetone for microscopy. Sec15-GFP fluorescence images are paired with the phase contract images of the cells to the right.
It is possible that some bem1Δ cells may be sufficiently able to initiate polarized growth while other cells are delayed or fail in this process, which leads to the observed heterogeneity in morphology. We reasoned that if we isolated a population at the G0 stage and examined the initial budding in the synchronized cells, we might be able to see the effect of Bem1p more clearly. After isolating a G0 population, we released the cells at 25°C for 2 h and then fixed them in methanol/acetone for microscopy. We found that, in the majority of the bem1Δ cells released from G0, Sec15-GFP was not polarized. Even in small budded cells, a large portion of Sec15p was not enriched in the buds (Figure 7B). On the basis of these results, we propose that Sec15p polarization is facilitated by Bem1p during budding.
Localization of Cdc42p to the Bud Tip Requires Polarized Actin Cables and a Functional Secretory Pathway
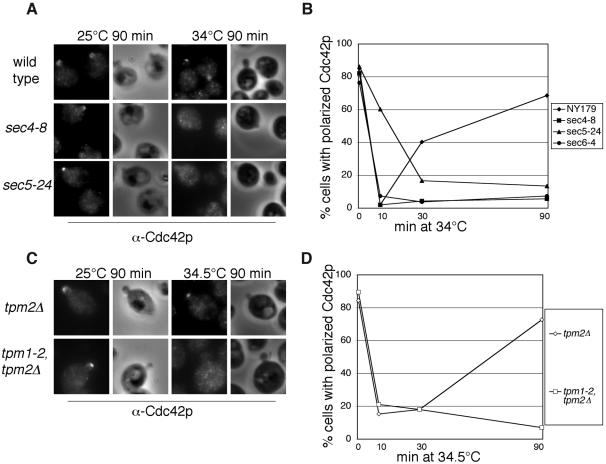
Spatial regulation of exocytosis is mediated by the Rho family of small GTPases and polarized cytoskeleton organization. On the other hand, membrane traffic may mediate the transport of cell polarity regulators to the site of cell growth. Recent works using GFP-tagged Cdc42p demonstrated that vesicle delivery along actin cables is important for symmetry breaking in yeast (Wedlich-Soldner et al., 2003, 2004). Here, we examined the localization of endogenous Cdc42p in yeast mutants defective in polarized exocytosis. We first examined the localization of Cdc42p in the sec mutants (sec4-8, sec5-24, and sec6-4). Yeast cells were grown to early log phase and then shifted to 34°C for 10, 30, or 90 min before staining with affinity-purified anti-Cdc42p polyclonal antibodies. We especially focused on the early stage budding cells (e.g., cells with tiny or small buds), where Cdc42p should be concentrated in the tip in wild-type cells. As shown in Figure 8, A and B, shifting the temperature for 10 min caused a loss of Cdc42p staining at the bud tip in the wild-type as well as all the mutant cells. With a longer period of incubation at the restrictive temperature, the wild-type cells recovered Cdc42p polarization, appearing 40.3% polarized after 30 min at 34°C and near normal polarization (70%) after 90 min. In contrast, Cdc42p remained mislocalized in sec4-8, sec5-24, and sec6-4 cells after 30 and 90 min at the restrictive temperature. The loss of Cdc42p localization 10 min after the temperature shift probably resulted from the heat-shock response as commonly observed in yeast cells (Delley and Hall, 1999). These localization studies suggest that Cdc42p, although itself the master regulator of cell polarity, requires the functional exocytosis pathway for the maintenance of its polarization at the bud tips. To further investigate the role of vesicle delivery in Cdc42p localization, we also examined Cdc42p polarization in the tpm1-2, tpm2Δ mutant (Figure 8, C and D), which rapidly loses actin cables when shifted to 34.5°C (Pruyne et al., 1998). We found that Cdc42p was significantly depolarized after 10 min (21% polarized) in the tpm1-2, tpm2Δ strain and gradually became even less polarized after 30 min (18% polarized) and 90 min (7% polarized). We propose that Cdc42p requires a functional secretory pathway and polarized actin cables for the maintenance of its localization at the buds.
Figure 8.
Polarization of endogenous Cdc42p requires functional secretory pathway and actin cable. (A) sec mutants disrupt Cdc42p polarization. Wild-type yeast (NY179), sec4-8, sec5-24, and sec6-4 (unpublished data) were grown in YPD to log phase and then shifted to 34°C for 10, 30, and 90 min. Cells were stained with affinity-purified α-Cdc42p polyclonal antibodies. The Cdc42p immunofluorescence images are paired with the phase contract images of the cells to the right. Cells from the experiment were scored for polarization of Cdc42p to the bud tip (B). (C) Cdc42p is mislocalized in tpm1-2, tpm2Δ cells at the restrictive temperature of 34.5°C. Cells from C were scored for polarization of Cdc42p to the bud tip (D).
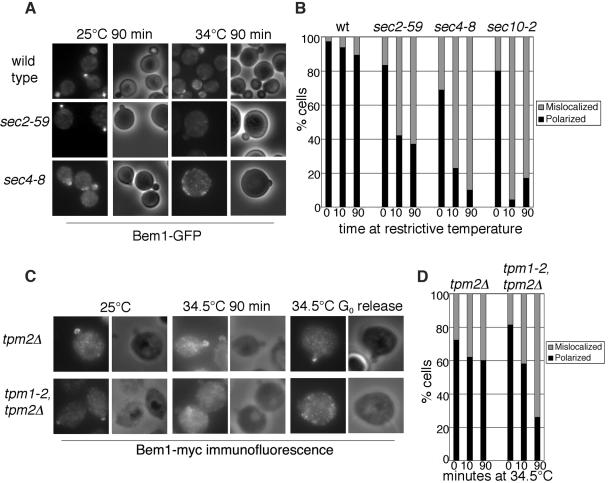
Bem1p Polarization Is Decreased in sec and tpm Mutants
Bem1p is important for the organization and assembly of a number of signal proteins for bud emergence (Bender and Pringle, 1991, Chenevert et al., 1992; Gulli et al., 2000; Bose et al., 2001; Butty et al., 2002; Irazoqui et al., 2003). We expressed Bem1-GFP in the sec mutants to study the effect of exocytosis on the polarization of Bem1p. The mutant yeast were grown to log phase and then shifted to the restrictive temperature for 10 or 90 min. We found that the number of cells with polarized Bem1-GFP decreased over time (Figure 9A). Because Bem1-GFP fluorescence signal was very weak in the tpm1-2, tpm2Δ mutant strain, we expressed Bem1–12xmyc in this mutant for immunofluorescence staining. In a heterogeneous population shifted to 34.5°C for 10 min, Bem1p polarization decreased modestly to 60%. After 90 min the percentage of polarized cells decreased further to 26%. This is different from that of Cdc42p, which decreased to 21% after a 10-min shift and to <10% at a 90-min shift (Figure 8). So far, we were not able to perform double-labeling of the same cells to compare the localization of Bem1p and Cdc42p. However, the difference in the time course of depolarization for the two proteins is obvious.
Figure 9.
Bem1p polarization is affected in mutants defective in polarized exocytosis. (A) sec mutants affect Bem1p polarization. Wild-type, sec4-8, and sec2-59 strains expressing Bem1-GFP were grown in SC-Ura media to log phase and then shifted to the restrictive temperature for 10 min and 90 min. Cells were fixed in methanol/acetone for microscopy. (B) Cells from A were scored for polarization of Bem1-GFP to the bud tip. (C) Actin cables are necessary for Bem1p polarization. tpm2Δ and tpm1-2, tpm2Δ cells expressing Bem1–12xmyc were grown to log phase in YPD and then shifted to 34.5°C for 10 and 90 min. The cells were fixed and stained with α-myc antibody to view the localization of Bem1p. Scoring of small budded cells with polarized Bem1p is shown in D. In addition, the localization of Bem1p in cells released from G0 was shown in the right panel of C.
To investigate the effect of actin cables on the initial delivery of Bem1p to the bud tip, we examined Bem1-myc in tpm mutants released from G0 at the restrictive temperature. We found that the G0 arrested cells were not able to polarize Bem1p when released at the restrictive temperature, although they could at the permissive temperature. This result suggests that localization of Bem1p at the onset of the cell cycle needs the availability of functional actin cable construction machinery.
The Exocyst Interacts with Bem1p
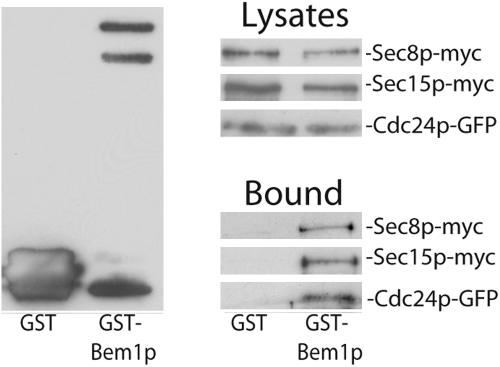
A number of yeast two-hybrid screenings revealed potential interaction between Sec15p and Bem1p (Drees et al., 2001; also see SGD for more references). In the screening performed by the yeast polarity consortium, positive interaction was found between Sec15p and Cdc24p in addition to Bem1p (Drees et al., 2001). Cdc24p was shown to bind the PB1 domain of Bem1p (Gulli et al., 2000; Butty et al., 2002; Yoshinaga et al., 2003). The two-hybrid result may reflect an indirect interaction between Sec15p with Cdc24p through Bem1p. To test whether Sec15p interacts with Bem1p in vivo, GST-Bem1p was expressed in a yeast strain with its endogenous Sec15 (the chromosomal copy) tagged by the myc epitope (Sec15p-myc). Pull-down experiments were performed using glutathione-Sepharose. As shown in Figure 10, Sec15p coprecipitates with GST-Bem1p. In addition, Sec8p, another exocyst component, also coprecipitates, suggesting that the exocyst complex interacts with Bem1p in the cell. As a control, GST does not bind to Sec15p or Sec8p. Using this assay, we also found that Cdc24p coprecipitates with Bem1p, consistent with previous observations. Although detailed protein-protein interactions of the exocyst with Bem1p and Cdc24p are unclear, our result suggests that the exocyst interacts with Bem1p in vivo.
Figure 10.
Bem1p interacts with Sec15p and Sec8p in vivo. GST-Bem1p or GST (as control) was coexpressed with myc-tagged Sec15p, Sec8p, or GFP-tagged Cdc24p in yeast cells. The same amounts of lysates from these strains were used for coprecipitation with glutathione-Sepharose 4B to test the interaction between Bem1p and the exocyst proteins. The precipitates were separated by SDS-PAGE and subjected to Western blot analyses using anti-GST antibody to detect GST and GST-Bem1p (left panel), anti-myc antibody to detect Sec15-myc and Sec8-myc, and anti-GFP antibody to detect Cdc24-GFP (Bound, bottom right panel).
DISCUSSION
Localization of a protein to the bud tip represents a balance between targeting of the protein to this region, and diffusion or recycling of the protein from this site. The exocyst protein Sec15p is a direct downstream effector of Sec4p (Guo et al., 1999b). We show here that the localization of Sec15p to the bud tip requires active Sec4p, suggesting that Sec4p controls the delivery and/or assembly of the exocyst complex at the bud. But in contrast to Sec4p, treatment of cells with latrunculin does not affect Sec15p localization. The initial targeting of Sec15p needs actin cables as Sec15p could not be found in the emerging buds in the tpm mutant cells released from G0. However, once Sec15p reaches its destination, its association with the plasma membrane seems to be quite stable. This hypothesis is consistent with the previous biochemical fractionation data that the majority of the endogenous Sec15p is associated with the plasma membrane (Bowser and Novick, 1991) even though Sec15p clearly has the ability to associate with the vesicles (Salminen and Novick, 1989; Guo et al., 1999b). The rab protein Sec4p is also targeted to the bud along actin cables (Walch-Solimena et al., 1997; Pruyne et al., 1998). However, unlike Sec15p, Sec4p is rapidly depolarized in the presence of latrunculin (Figure 2; also see Ayscough et al., 1997) or in the tpm mutant shifted to the restrictive temperature (Pruyne et al., 1998). GTP-loaded Sec4p is transported via post-Golgi vesicles to the plasma membrane. Once reaching the bud tip membrane, its GAPs, Msb3p and Msb4p, promote GTP hydrolysis and Sec4p becomes GDP-bound (Gao et al., 2003) at the bud. The GDP-bound form of Sec4p is then extracted from the lipids by Sec19p, the GDI for the rab GTPases, for subsequent recycling (Garrett et al., 1994). Sec19p not only regulates Sec4p, its GDI function is toward all the rab proteins at different stages of membrane traffic. Therefore, in sec19 mutants, early stage trafficking defects were also detected (Novick et al., 1980). It has been shown that polarized Sec4p localization needs functional upstream traffic (Walch-Solimena et al., 1997). However, in the sec19 mutant, Sec4p accumulates in the bud (Figure 5). This result suggests that the recycling of Sec4-GDP was blocked in the GDI mutant; therefore the remaining Sec4p was accumulated in the bud in a seemingly “polarized” manner. We have also treated the sec19-1 mutant cells by latrunculin and examined the localization of Sec4p in these cells. We expect that Sec4p will not be accumulated in the buds in sec19-1 cells as both the targeting and recycling of Sec4p are blocked. We indeed observed the loss of Sec4p in the bud (unpublished data). However, we also found that DMSO (used to dissolve latrunculin, as a control) treatment alone led to Sec4p delocalization in ∼40% of the sec19-1 cells. One possibility is that DMSO, as an organic solvent, may modify the lipids in the plasma membrane, therefore complicating our interpretation of the GDI function at the plasma membrane.
The recycling of Sec4p in the presence of functional GDI is a fast process compared with that of Sec15p, as disruption of the actin cytoskeleton by latrunculin leads to rapid depolarization of Sec4p but not Sec15p. Sec15p, though controlled by Sec4p, is mislocalized in the GDI mutant. This mislocalization probably resulted from disruption of upstream trafficking in the sec19 mutant. The mechanisms for the recycling or diffusion of Sec15p and other exocyst proteins are unknown. Future experiments are needed to address this question.
We have also compared the localization of Sec15p with another exocyst protein, Sec3p, in the cells. The relationship between Sec3p and actin was rigorously tested previously (Finger et al., 1998). Like Sec15p, the maintenance of Sec3p at the bud tip is not sensitive to latrunculin treatment. However, the targeting of Sec3p was also shown to be independent of actin as Sec3-GFP can be targeted as a patch-like structure even in G0 released cells in the presence of latrunculin (Finger et al., 1998). Also in the tpm mutant cells, Sec3p is much more stable than Sec15p at the bud tip, remaining mostly polarized after a 90-min shift to the restrictive temperature (Figure 4). The time course of Sec15p localization closely resembles that of Sec8p in the tpm mutant previously reported (Pruyne et al., 1998; Guo laboratory, unpublished results). These results revealed that different mechanisms direct the targeting of Sec3p versus other members of the exocyst complex. We previously found that Cdc42p and Rho1p control the localization of Sec3p through their interaction with the N-terminus of Sec3p (Guo et al., 2001; Zhang et al., 2001). Rho1p and Cdc42p compete with each other in their binding to this region of Sec3p (Zhang et al., 2001). This interaction is necessary for the polarized localization of Sec3p, as deletion of the N-terminus of Sec3p (Sec3ΔN) leads to mislocalization of Sec3p without affecting the assembly of the exocyst complex (Guo et al., 2001). Interestingly, in the sec3ΔN mutant strain, Sec15p is still polarized (Guo laboratory, unpublished results). Therefore, there must be parallel pathways that target different exocyst components to sites of active secretion. Here we demonstrate that Cdc42p controls Sec15p localization in the cells (Figure 6) similar to Sec3p. Although Cdc42p controls Sec3p localization through its direct binding with Sec3p N-terminus, its role for Sec15p and other exocyst components must be mediated through a Sec3p-independent pathway. Future works are needed to further compare these proteins in the same cells using time-lapse microscopy so that their kinetics differences can be better studied. These results revealed the diversity of exocyst targeting in yeast cells. As the exocyst functions in a step proceeding SNARE-mediated membrane fusion, the eight proteins are targets for cellular regulators (Lipschutz and Mostov, 2002; Novick and Guo, 2002; Hsu et al., 2004). The assembly of the exocyst may integrate various sources of cellular information to ensure that exocytosis occurs at the right time and place.
Although our studies demonstrate that the post-Golgi secretory machinery is under the control of the actin cytoskeleton and polarity regulators, we also examined the localization of the polarity determinants in cells defective in exocytosis. We hypothesize that membrane traffic may be important for the delivery of polarity regulators to specific domains of the plasma membrane. We first examined Cdc42p, the “master” regulator for the establishment of polarity in yeast. Using affinity-purified antibodies, we found that the localization of endogenous Cdc42p at sites of polarized cell growth relies on a functional secretory pathway, as the sec mutant cells lose Cdc42p staining at the bud tip over time. Cdc42p localization also needs actin cables, which are essential for polarized transport of secretory vesicles. Disruption of actin cables in the tpm mutant results in loss of polarized localization of Cdc42p, consistent with the observation by Pruyne et al. (2004). Recently, Wedlich-Soldner et al. (2003, 2004) demonstrated that polarized localization of GFP-tagged Cdc42p required targeted secretion directed by the actin cytoskeleton. It was proposed that the F-actin–dependent transport of Cdc42p to the plasma membrane, which in turn is controlled by Cdc42p, provides a positive feedback loop that amplifies initial small signals for symmetry breaking in the early stages of yeast budding. Our study demonstrates that Cdc42p loses its localization in the established buds in exocytosis mutants. Our results suggest that a functional secretory pathway and actin cables are not only needed for bud emergence, but are also important for maintaining the localization of Cdc42p in the growing bud. Biochemical experiments (Wedlich-Soldner et al., 2003, 2004) suggest that Cdc42p associates with secretory vesicles. It is possible that continued replenishment of Cdc42p to the bud to counter the endocytosis of bud membrane is important for the maintenance of Cdc42p at sites of cell polarization.
The function of Cdc42p is carried out in conjunction with Bem1p, which interacts with Cdc42p, its GEF Cdc24p, and other signaling molecules involved in cell polarity establishment. Here we found that Bem1p also loses its polarized localization in the sec and tpm mutants, suggesting that the maintenance of Bem1p requires polarized transport. However, the time course of Bem1p depolarization is slower than that of Cdc42p. Especially, the slow depolarization of Bem1p in the tpm mutant, which is known to rapidly lose cables after 1 min (Pruyne et al., 1998), suggests that loss of actin cables affects Bem1p polarization indirectly over time. It is possible that the diffusion or recycling of Bem1p from the bud takes a time course different from Cdc42p. A recent finding suggests that the self-assembly of Bem1p leads to scaffold-mediated symmetry breaking in yeast (Irazoqui et al., 2003). It is possible that the mislocalization of Bem1p observed in our study resulted indirectly from Cdc42p mislocalization in the mutants. Besides Bem1p, it was reported that proteins regulating actin polymerization, the motor protein Myo2p, and actin itself are disrupted in secretory mutants (Jin and Amberg, 2000; Gao et al., 2003; Aronov and Gerst, 2004; Pruyne et al., 2004). Overall these studies present a cyclical regulatory mechanism that may be important for the establishment and maintenance of polarized cell growth. In this regard, it is interesting to note that several yeast two-hybrid screenings from different sources revealed potential interactions between Sec15p and Bem1p (Drees et al., 2001; also see SGD for more references). Here, we demonstrated that Sec15p indeed interacts with Bem1p in yeast cells. We have previously shown that Cdc42p directly interacts with Sec3p (Zhang et al., 2001). Stringent cytological experiments suggest that Sec3p, unlike the other exocyst components, is not dependent on actin for its polarized localization (Finger et al., 1998). Sec3p may associate with the plasma membrane and then assemble with other exocyst proteins, including Sec15p, that arrive at the bud tip via secretory vesicles along the actin cables (Finger and Novick, 1998). The interaction of Sec15p with Bem1p and its assembly into the exocyst complex bring Cdc42p, its exchange factor Cdc24p, and the Cdc42p effector Sec3p into close proximity at the bud tip. These molecular interactions may represent a positive feedback loop coupling vesicle delivery and cell polarization. However, to test this hypothesis, the molecular details of the interactions clearly need to be elucidated first.
If secretion is important for the polarized localization of cell polarity regulators, why were cell morphology defects not observed in sec mutants? Most of the sec mutants previously isolated were “tight” alleles. On shifting to the restrictive temperature, bud emergence stopped quickly, as indicated by the nearly constant number of cells and buds (Novick et al., 1980). In a heterogeneous population, cells were found to arrest at all stages of cell cycle, and no significant increase in cell size was noted (Novick et al., 1980; Guo laboratory, unpublished observation). These characteristics of these sec alleles are results of the screening strategy: only “dense” cells in which net cell surface growth stopped while cell mass increased, were selected (Novick et al., 1980). Identification of new alleles of exocyst mutants may help reveal defects in both secretion and morphogenesis.
This cyclical regulatory mechanism between secretion and cell polarity has been observed in mammals. One recent example was presented by O'Brien et al. (2001). The authors demonstrated an autocrine loop, in which epithelial cells create their own extracellular polarity cue through exocytosis during cystogenesis. The secretion and assembly of extracellular laminin acts back on the cells to direct membrane traffic. Blocking the activity of the small GTPase Rac1 disrupts the loop, which leads to an inversion of the apical pole. Future studies using a variety of model systems will help us better understand the molecular basis and biological consequences underlying this cyclical regulatory mechanism.
Acknowledgments
We are grateful to Drs. Erfei Bi, Tony Bretscher, and Allan Bender for valuable reagents. Especially, we are grateful to Dr. Keith Kozminski for the affinity-purified anti-Cdc42p antibodies. We thank Dr. Erfei Bi for many helpful discussions and Dr. Peter Novick for the communications of data before publication. This work is supported by grants from the National Institutes of Health, American Cancer Society, and Pew Scholar Program in Biomedical Sciences to W.G.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E04-10-0896) on January 12, 2005.
The authors declare that they have no competing financial interests.
References
- Aronov, S., and Gerst, J. E. (2004). Involvement of the late secretory pathway in actin regulation and mRNA transport in yeast. J. Biol. Chem. 279(35), 36962-36971. [DOI] [PubMed] [Google Scholar]
- Ayscough, K. R., Stryker, J., Pokala, N., Sanders, M., Crews, P., and Drubin, D. G. (1997). High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137, 399-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender, A., and Pringle, J. R. (1991). Use of a screen for synthetic lethal and multicopy suppressing mutants to identify two new genes involved in morphogenesis in Saccharomyces cerevisiae. Mol. Cell. Biol. 11, 1295-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose, I., Irazoqui, J. E., Moskow, J. J., Bardes, E. S., Zyla, T. R., and Lew, D. J. (2001). Assembly of scaffold-mediated complexes containing Cdc42p, the exchange factor Cdc24p, and the effector Cla4p required for cell cycle-regulated phosphorylation of Cdc24p. J. Biol. Chem. 276, 7176-7186. [DOI] [PubMed] [Google Scholar]
- Bowser, R., and Novick, P. (1991). Sec15 protein, an essential component of the exocytotic apparatus, is associated with the plasma membrane and with a soluble 19.5S particle. J. Cell Biol. 112, 1117-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennwald, P., Kearns, B., Champion, K., Keranen, S., Bankaitis, V., and Novick, P. (1994). Sec9 is a SNAP-25-like component of a yeast SNARE complex that may be the effector of Sec4 function in exocytosis. Cell 79, 245-258. [DOI] [PubMed] [Google Scholar]
- Butty, A. C., Pryciak, P. M., Huang, L. S., Herskowitz, I., and Peter, M. (1998). The role of Far1p in linking the heterotrimeric G protein to polarity establishment proteins during yeast mating. Science 282, 1511-1516. [DOI] [PubMed] [Google Scholar]
- Butty, A. C., Perrinjaquet, N., Petit, A., Jaquenoud, M., Segall, J. E., Hofmann, K., Zwahlen, C., and Peter, M. (2002). A positive feedback loop stabilizes the guanine-nucleotide exchange factor Cdc24 at sites of polarization. EMBO J. 21, 1565-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenevert, J., Corrado, K., Bender, A., Pringle, J., and Herskowitz, I. (1992). A yeast gene (BEM1) necessary for cell polarization whose product contains two SH3 domains. Nature 356, 77-79. [DOI] [PubMed] [Google Scholar]
- Chu, S., and Guo, W. (2004). Secretory vesicle tethering complexes. Topics Curr. Genet. 10, 89-115. [Google Scholar]
- Delley, P. A., and Hall, M. N. (1999). Cell wall stress depolarizes cell growth via hyperactivation of RHO1. J. Cell Biol. 147, 163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y., Pruyne, D., and Bretscher, A. (2003). Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J. Cell Biol. 161(6), 1081-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drees, B. L. et al. (2001). A protein interaction map for cell polarity development. J. Cell Biol. 154, 549-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger, F., Hughes, T., and Novick, P. (1998). Sec3p is a spatial landmark for polarized secretion in budding yeast. Cell 92, 559-571. [DOI] [PubMed] [Google Scholar]
- Gao, X., Albert, S., Tcheperegine, S. E., Burd, C. G., Gallwitz, D., and Bi, E. (2003). The GAP activity of Msb3p and Msb4p for the Rab GTPase Sec4p is required for efficient exocytosis and actin organization. J. Cell Biol. 162, 635-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett, M. D., Zahner, J. E., Cheney, C. M., and Novick, P. J. (1994). GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 13, 1718-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goud, B., Salminen, A., Walworth, N. C., and Novick, P. J. (1988). A GTP-binding protein required for secretion rapidly associates with secretory vesicles and the plasma membrane in yeast. Cell 53, 753-768. [DOI] [PubMed] [Google Scholar]
- Govindan, B., Bowser, R., and Novick, P. (1995). The role of Myo2, a yeast class V myosin, in vesicular transport. J. Cell Biol. 128, 1055-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulli, M. P., Jaquenoud, M., Shimada, Y., Niederhauser, G., Wiget, P., and Peter, M. (2000). Phosphorylation of the Cdc42 exchange factor Cdc24 by the PAK-like kinase Cla4 may regulate polarized growth in yeast. Mol. Cell 6, 1155-1167. [DOI] [PubMed] [Google Scholar]
- Guo, W., Grant, A., and Novick, P. (1999a). Exo84p is an exocyst protein essential for secretion. J. Biol. Chem. 274, 23558-23564. [DOI] [PubMed] [Google Scholar]
- Guo, W., Roth, D., Walch-Solimena, C., and Novick, P. (1999b). The exocyst is an effecter for Sec4p, targeting secretory vesicles to sites of exocytosis. EMBO J. 18, 1071-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, W., Sacher, M., Barrowman, J., Ferro-Novick, S., and Novick, P. (2000). Protein complexes in transport vesicle targeting. Trends Cell Biol. 10, 251-255. [DOI] [PubMed] [Google Scholar]
- Guo, W., Tamanoi, F., and Novick, P. (2001). Spatial regulation of the exocyst complex by Rho1 GTPase. Nat. Cell Biol. 3, 353-360. [DOI] [PubMed] [Google Scholar]
- Guthrie, C., and Fink, G. R. (1991). Guide to Yeast Genetics and Molecular Biology, Methods in Enzymology, Vol. 194, San Diego, CA: Academic Press. [PubMed]
- Hsu, S., TerBush, D., Abraham, M., and Guo, W. (2004). The exocyst complex in polarized exocytosis. Int. Rev. Cytol. 233, 243-265. [DOI] [PubMed] [Google Scholar]
- Irazoqui, J., Gladfelter, A., and Lew, D. (2003). Scaffold-mediated symmetry breaking by Cdc42p. Nat. Cell Biol. 5, 1062-1070. [DOI] [PubMed] [Google Scholar]
- Jin, H., and Amberg, D. C. (2000). The secretory pathway mediates localization of the cell polarity regulator Aip3p/Bud6p. Mol. Biol. Cell 11, 647-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpova, T. S., Reck-Peterson, S. L., Elkind, N. B., Mooseker, M. S., Novick, P. J., and Cooper, J. A. (2000). Role of actin and Myo2p in polarized secretion and growth of Saccharomyces cerevisiae. Mol. Biol. Cell 11, 1727-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazar, T., Gotte, M., and Gallwitz, D. (1997). Vesicular transport: how many Ypt/Rab-GTPases make a eukaryotic cell? Trends Biochem. Sci. 22, 468-472. [DOI] [PubMed] [Google Scholar]
- Leeuw, T., Fourest-Lieuvin, A., Wu, C., Chenevert, J., Clark, K., Whiteway, M., Thomas, D. Y., and Leberer, E. (1995). Pheromone response in yeast: association of Bem1p with proteins of the MAP kinase cascade and actin. Science 270, 1210-1213. [DOI] [PubMed] [Google Scholar]
- Lipschutz, J., and Mostov, K. (2002). Exocytosis: the many masters of the exocyst. Curr. Biol. 12, R212-R214. [DOI] [PubMed] [Google Scholar]
- Novick, P., Field, C., and Schekman, R. (1980). Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21, 205-215. [DOI] [PubMed] [Google Scholar]
- Novick, P., and Zerial, M. (1997). The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol. 9, 496-504. [DOI] [PubMed] [Google Scholar]
- Novick, P., and Guo, W. (2002). Ras family therapy: Rab, Rho and Ral talk to the exocyst. Trends Cell Biol. 12, 247-249. [DOI] [PubMed] [Google Scholar]
- O'Brien, L. E., Jou, T. S., Pollack, A. L., Zhang, Q., Hansen, S. H., Yurchenco, P., and Mostov, K. E. (2001). Rac1 orientates epithelial apical polarity through effects on basolateral laminin assembly. Nat. Cell Biol. 3, 831-838. [DOI] [PubMed] [Google Scholar]
- Park, H. O., Bi, E., Pringle, J. R., and Herskowitz, I. (1997). Two active states of the Ras-related Bud1/Rsr1 protein bind to different effectors to determine yeast cell polarity. Proc. Natl. Acad. Sci. USA 94, 4463-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruyne, D. W., Schott, D. H., and Bretscher, A. (1998). Tropomyosin-containing actin cables direct the Myo2p-dependent polarized delivery of secretory vesicles in budding yeast. J. Cell Biol. 143, 1931-1945. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., Gao, L., Bi, E., and Bretscher, A. (2004). Stable and dynamic axes of polarity use distinct formin isoforms in budding yeast. Mol. Biol. Cell 15(11), 4971-4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J. E. (1994). Mechanisms of intracellular protein transport. Nature 372(6501), 55-63. [DOI] [PubMed] [Google Scholar]
- Salminen, A., and Novick, P. J. (1989). The Sec15 protein responds to the function of the GTP binding protein, Sec4, to control vesicular traffic in yeast. J. Cell Biol. 109, 1023-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott, D., Ho, J., Pruyne, D., and Bretscher, A. (1999). The COOH-terminal domain of Myo2p, a yeast myosin V, has a direct role in secretory vesicle targeting. J. Cell Biol. 147, 791-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush, D. R., and Novick, P. (1995). Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. J. Cell Biol. 130, 299-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush, D. R., Maurice, T., Roth, D., and Novick, P. (1996). The Exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. EMBO J. 15, 6483-6494. [PMC free article] [PubMed] [Google Scholar]
- Walch-Solimena, C., Collins, R. N., and Novick, P. J. (1997). Sec2p mediates nucleotide exchange on Sec4p and is involved in polarized delivery of post-Golgi vesicles. J. Cell. Biol. 137, 1495-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedlich-Soldner, R., Altschuler, S., Wu, L., and Li, R. (2003). Spontaneous cell polarization through actomyosin-based delivery of the Cdc42 GTPase. Science 299, 1231-1235. [DOI] [PubMed] [Google Scholar]
- Wedlich-Soldner, R., Wai, S. C., Schmidt, T., and Li, R. (2004). Robust cell polarity is a dynamic state established by coupling transport and GTPase signaling. J. Cell Biol. 166, 889-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte, J. R., and Munro, S. (2002). Vesicle tethering complexes in membrane traffic. J. Cell Sci. 115, 2627-2637. [DOI] [PubMed] [Google Scholar]
- Yoshinaga, S., Kohjima, M., Ogura, K., Yokochi, M., Takeya, R., Ito, T., Sumimoto, H., and Inagaki, F. (2003). The PB1 domain and the PC motif-containing region are structurally similar protein binding modules. EMBO J. 22, 4888-4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., Bi, E., Novick, P., Du, L., Kozminski, K., Lipschutz, J., and Guo, W. (2001). Cdc42 interacts with the exocyst and regulates polarized secretion. J. Biol. Chem. 276, 46745–46750. [DOI] [PubMed]