Abstract
Purpose of review
Chronic rejection is associated with persistent mononuclear cell recruitment, endothelial activation and proliferation, local tissue hypoxia and related biology that enhance effector immune responses. Despite similarities, the tumor microenvironment elicits signals/factors that inhibit effector T cell responses and promote local immunoregulation. The identification of immunoregulatory check points and/or secreted factors that are deficient within allografts is of great importance in the understanding and prevention of chronic rejection.
Recent findings
The relative expression of immunomodulatory molecules (cell surface and secreted) on microvascular endothelial cells are deficient within rejecting allografts, and this deficiency is of functional importance in shaping the phenotype of rejection. Furthermore, the expression of co-inhibitory molecules/factors that enhance local immunoregulation are modulated, and the identification of intracellular regulatory signals or secreted factors are the subject of ongoing research. For example, semaphorins may interact with endothelial cells and regulatory T cells to promote local tolerance. Additionally, metabolites and electrolytes within the allograft microenvironment may regulate local effector and regulatory cell responses.
Summary
Multiple factors within allografts shape the microenvironment either towards local immunoregulation or proinflammation. Promoting the expression of intragraft cell surface or secreted molecules that support immunoregulation will be critical for long-term graft survival and/or alloimmune tolerance.
Keywords: alloimmune tolerance, intragraft microenvironment, endothelial cells, T cells, vascular endothelial growth factor
Introduction
In this review, we discuss new findings indicating that events within the allograft itself shape a microenvironment that promotes either proinflammation or local immunoregulation/tolerance. It is increasingly appreciated that select families of immunoregulatory molecules (i.e., immune checkpoints) within tissues may function to elicit a local pro-tolerogenic state within the tumor microenvironment. Thus, the presence or absence of ‘checkpoint’ molecules within the allograft itself, and/or local intragraft events have potential to support systemic alloimmune tolerance. Although a detailed description of potential local immune checkpoints is beyond the scope of this review, we will focus our opinion on how the intragraft microenvironment may be shaped by the integrity and phenotype of the microvascular endothelium to support either pro-inflammatory or pro-tolerogenic events.
Role of the microvasculature in shaping the intragraft microenvironment: A paradigm for understanding local immunoregulation and tolerance
The graft vascular endothelial cell (EC) is uniquely located to serve as an interface between the allograft and the recipient’s immune system. To this end, it is not surprising that microvascular injury occurs as a consequence of all forms of rejection [1–4]. Indeed, in an endothelial-based model, initial injury results from ischemia-reperfusion, leukocytic infiltration and subclinical rejection, and the EC response is characteristically associated with activation as well as proliferation [5–9]. Furthermore, an increasing body of evidence indicates that the response of the microvasculature to injury (e.g. activation, protection and/or repair vs. dysfunctional angiogenesis) is critical in both the initiation as well as the progression of chronic rejection [4,5,8,10]. EC injury also results in cell death and microvascular loss, which in turn results in impaired delivery of oxygen and nutrients within the graft parenchyma/tissue. The resultant (and inevitable) local tissue hypoxia is likely a driving force that regulates local immunity in part through direct effects on infiltrates [4,11,12]. For example, hypoxia may elicit its effect on infiltrating T cells via hypoxia-inducible factor (HIF)-1α regulated expression of proinflammatory cytokines and intracellular signals associated with the dedifferentiation of T regulatory cells (Treg) and the enhanced differentiation of effector T cells [11]. These collective events within the microenvironment are most predictive of the initiation of chronic rejection [4,6,7,9]. Also, cell-mediated and humoral alloimmune responses and hypoxia/HIF-1α lead to a characteristic EC activation response that includes the expression of MHC class I and II molecules, pro-inflammatory cytokines, chemokines and growth factors that collectively serve to modify the phenotype of both acute and chronic rejection [3,13]. Thus, the degree of initial microvascular injury, local tissue hypoxia and the phenotypic response of the EC to inflammation (including pathological leukocyte-induced angiogenesis) shape the intragraft microenvironment for subsequent events (illustrated in the cartoon in Figure 1).
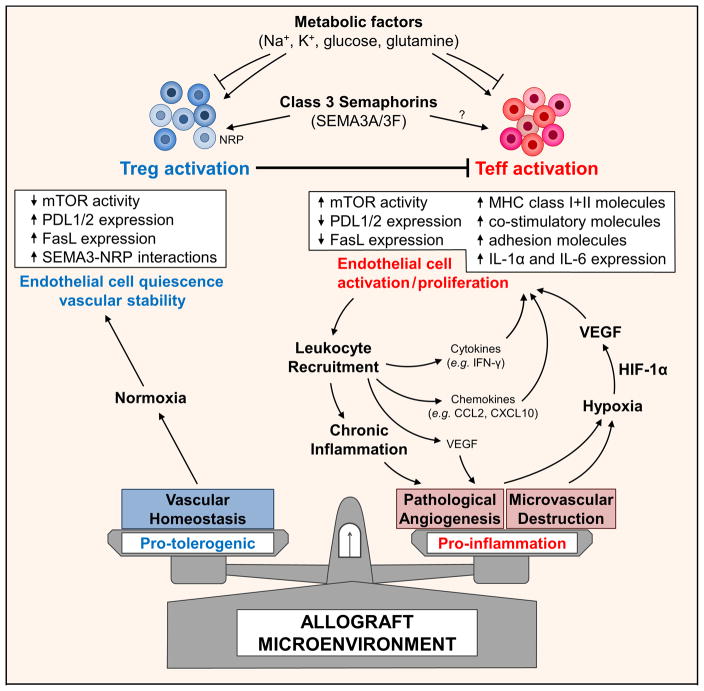
Figure 1. Cartoon illustration of a working model of how the allograft microenvironment may be shaped to support alloimmune tolerance.
Initial inflammatory infiltration into a graft target the microvasculature and may either result in injury or pathological leukocyte-induced angiogenesis. Early injury and microvascular loss results in local tissue ischemia. Uncontrolled pathological angiogenesis also leads to an abnormal microvascular bed, sluggish/chaotic blood flow patterns and may result in tissue hypoxia. Local tissue hypoxia drives HIF-1α-dependent activation of growth factors, cytokines and chemokines (including VEGF-A) but also facilitates the dedifferentiation of T regulatory cells (Teffs) into effectors (Teffs). Within the tumor microenvironment, immune checkpoints expressed on EC, and secreted factors within the microenvironment promote Treg activation, expansion and function, and inhibit T effector cell activity. Also electrolytes and metabolic substrates within the microenvironment regulate local immunity including T regulatory cell responses. Thus, similar to tumors, novel cell surface molecules (e.g. neuropilins (NRP) and secreted molecules, e.g. class 3 semaphorins that are likely to be deficient within allografts have potential to shape the environment and local immunoregulation.
Leukocyte-induced angiogenesis is a pathological reaction that was initially identified following the local injection of allogeneic splenocytes into the skin of nude mice [14,15]. Following injection, the angiogenesis reaction was dose and cell number-dependent and occurred within a period of 3 to 6 days. Interestingly, it failed to occur following the injection of syngeneic cells and was found to be dependent on the alloactivation of effector CD4+ T lymphocytes [16]. Monocyte/macrophages were subsequently identified to produce and deliver angiogenesis factors into tissues, and pathological angiogenesis is generally considered a somewhat characteristic response in association with cell-mediated immunity and chronic inflammatory disease [17,18]. The local expression of Vascular Endothelial Growth Factor (VEGF) is central to all these effects, and may be produced locally and/or by mononuclear cell infiltrates including CD4+ T cells in the course of chronic inflammation [18–22]. Consistent with these observations, we have found that the early expression of VEGF is a feature of rejection [21,23], and it has high potential as a biomarker of evolving disease [21,24,25].
Unexpectedly, it was reported that the leukocyte-induced angiogenesis reaction that occurs at early times in the rejection process is associated with local hypoxia [5,7,10], likely due to sluggish and chaotic blood flow patterns, as observed in tumors [4,6,12,26,27]. This is most suggestive that dynamic changes within the EC of an allograft (destruction and/or proliferation) have potential to create abnormal microvascular networks and local areas of tissue hypoxia. These events serve as a stimulus for HIF-1α-mediated signaling in multiple cell types (including leukocytic infiltrates), the inducible expression of growth factors (such as VEGF), leukocyte chemoattraction and modified local CD4+ T cell activation responses [9,11,28,29]. Remarkably, all these events (angiogenesis, local hypoxia, mononuclear cell infiltrates) are described in tumors and are integral to the formation of the tumor microenvironment [12,26,27], yet this microenvironment supports immunoregulation and not effector T cell activation. We thus hypothesize that select immunoregulatory molecules that are produced within the microenvironment of tumors are deficient within allografts, and further, that their induced expression may target effector alloimmunity and promote local immunoregulation. For example, as will be discussed below, co-inhibitory programmed death ligand (PDL)1/2 expression on microvascular EC and/or the expression of the class 3 semaphorin family of neuronal guidance molecules have great potential to enhance tolerance. Thus, we propose a new paradigm in which chronic rejection evolves from a deficiency and/or lack of production of local immunoregulatory checkpoints within the graft microenvironment. Additionally, we speculate that forced expression of families of tumor associated ‘immune checkpoints’ within an allograft will promote local immunoregulation and augment systemic tolerance.
Immunogenic function of microvascular endothelial cells as a determinant of the phenotype of rejection
In a seminal study, Chalasani et al. challenged existing dogma that the nature and magnitude of an effector immune response alone determines the phenotype of rejection [30]. They used an adoptive model in which fully MHC mismatched cardiac allografts were exposed to a constant number of effector T cells. Adoptive transfer into newly transplanted recipients resulted in acute rejection, whereas transfer of the identical effector T cell population into recipients of healed-in allografts (day 50–70 post-transplantation) resulted in a chronic rejection response. Importantly, they also observed that ischemia-reperfusion injury/retransplantation of healed-in allografts recapitulated the acute rejection response. These observations are most suggestive that the state of the local tissue microenvironment within the allograft, and not only the effector T cell population are determinants of the phenotype of rejection (acute vs. chronic). While these authors did not address the mechanistic basis for this response, it is most intriguing to consider the identity of select factors/cell surface molecules present or deficient within each type of microenvironment that shape these different outcomes.
An explanation supported by a long-standing literature [31–33] is that the state of activation of intragraft microvascular EC regulates the immunogenicity of the local microenvironment and is a central determinant of the phenotype of rejection (acute vs. chronic). Indeed, several studies have demonstrated that the expression of multiple cell surface and secreted molecules on EC regulate local interactions with infiltrating T effector cell and/or T regulatory cells [34–38]. For example, there is a substantial body of evidence demonstrating that microvascular EC express MHC class I and II and costimulatory molecules that function in the reactivation of both CD4+ and CD8+ T cells through both direct and indirect pathways [31,38–42]. Abrahimi et al. found that blocking MHC class II on the graft EC was of critical importance in both CD4+ and CD8+ T cell-mediated rejection in vivo [43]. Costimulatory molecules on EC, including LFA-3 and ICOSL [39,44,45], also support T cell reactivation responses, and the expression of adhesion molecules, e.g. E-selectin, ICAM-1 and VCAM-1 [33,46] facilitate the recruitment and infiltration of T cells into the allograft. Pober’s groups proposed a model (that has stood the test of time) whereby EC recruit and reactivate antigen-specific effector memory T cells during the early stages of inflammation [32,47]. These alloactivated T cells release effector cytokines, e.g. IFN-γ, within the graft that is central to all subsequent EC-dependent mechanisms of rejection. In multiple studies they used a human artery interposition model in humanized mice to further study the role of allograft EC in chronic rejection [48–50]. Systemic administration of human IFN-γ, which only interacted with the donor human vessel and not with mouse cells, sustained endothelial MHC-antigen expression in healed-in allografts up to 30 days post-transplant and resulted in the development of allograft vasculopathy. Pro-inflammatory leukocytes, including innate immune cells follow IFN-γ-inducible gradients into the vessel wall and stimulate the recruitment and proliferation of vascular smooth muscle cells within the intima [8,48,50]. Moreover, using this model, they showed that human allograft EC produce the proinflammatory cytokines IL-1α and IL-6 respectively [51,52], which promote the recruitment and differentiation of Th17 cells within the graft. Neutralizing of IL-1α or IL-6 respectively resulted in an expansion of Tregs and reduced effector T cell-mediated injury. Lion et al. also demonstrated that the local production of IL-6 by EC serves as a key factor to influence CD4+ differentiation [53] suggesting that allograft EC-derived cytokines can shift the microenvironment towards a proinflammatory phenotype.
An increasing body of literature has emphasized the importance of the expression and function of co-inhibitory molecules by EC as a component of local tissue immunoregulation, most notably within tumors [35,54–61] Some additional studies are also most suggestive that the expression of co-inhibitory molecules on intragraft EC may be of great importance in the expansion of Tregs and the prevention of chronic allograft rejection [58,62]. For example, blockade or knockout of PDL1 within donor cardiac allografts results in accelerated chronic rejection and graft loss [58,62]. Using bone marrow chimeras, it was concluded that this accelerated rejection effect is likely related to its expression and function on graft EC [58,62]. CD95/FasL is another EC cell surface molecule that regulates CD4+ and CD8+ effector immunity, but has limited effects on the killing of Treg cells [63,64]. In this manner, its expression on EC has been found to be of functional importance in local immunoregulatory responses within tumors [63] as well as within allografts [64]. Tumor EC expression of CD95/FasL is induced by several secreted factors known to be expressed within allografts [63], and importantly, the inability of FasL to elicit killing of Tregs is due to their high endogenous level of expression of anti-apoptotic c-FLIP [63]. Collectively, these novel observations are suggestive that the state of activation and phenotype of the intragraft EC may play a dominant role to shape effector/regulatory interactions within a graft.
The identity of intragraft cell surface molecules and/or soluble factors and signals that function as ‘alloimmune check points’ (similar to those within tumors [61]) will have high relevance for the development of therapeutics to manipulate the immunogenicity of the graft. To this end, Pober’s group demonstrated that intracellular mTOR-induced signaling functions to inhibit the immunoregulatory phenotype of EC. Specifically, the treatment of EC with rapamycin (an mTOR inhibitor that is used clinically) augmented the inducible level of expression of co-inhibitory PDL1/2 [37], which functions to augment the expansion of FoxP3+ Tregs following co-culture in vitro and following transplantation in vivo. In another report, Wang et al. determined that the inhibition of mTOR reduces the ability of EC to interact with effector T cells and is associated with reduced T cell recruitment [56]. Thus, intracellular signals, molecular events and/or soluble factors regulate the immunogenicity of EC and have potential to shape the intragraft microenvironment to promote local immunoregulation and sustain alloimmune tolerance [65].
Class 3 semaphorins as novel intragraft factors with potential to shape the immunogenicity of endothelial cells and the intragraft microenvironment towards immunoregulation
Axonal/neuronal guidance molecules belong to at least four families, namely the Netrins, Semaphorins, Ephrins and Slits, and all families are implicated in lymphocyte activation and in immunomodulation [66–69]. Furthermore, members of these families are increasingly being appreciated as modulators of cell mediated immune responses, vascular disease and alloimmunity due to high expression levels of their receptors on leukocyte subsets [70–73] and EC [74–78]. Below, we wish to briefly discuss the class 3 semaphorin family (SEMA3) that have potential to regulate the alloimmune response [72,73,79].
Semaphorins were originally described as chemorepellent factors due to their primary function to elicit cytoskeletal collapse in neurons [69,76,77,80]. More recent studies have demonstrated that this family, including SEMA3 family members, regulate a wider array of cellular activation responses, most notably PI-3K/Akt/mTOR intracellular signaling, migration and proliferation [81–83]. Over the past 10 years, several studies have identified SEMA3 receptors, including neuropilin (NRP)-1, NRP-2 and Plexin A family molecules on tumor cells suggesting that they function within the tumor microenvironment to limit cellular migration and growth [80,81,84,85]. Three members of the Class 3 family (SEMA3A, -3C and -3F) also bind NRP’s that are expressed on immune cell types including EC [75–78,81], antigen presenting cells [86,87] and CD4+ T cells [70]. In general, ligation of the NRP receptor alone is not sufficient to elicit a signal, but serves as co-receptor to activate Plexin A family receptors that in turn elicit potent inhibitory signaling responses [88,89]. Indeed, deletion of Plexin A on CD4+ cells is associated with profound effector T cell activation and autoimmunity [88] Both NRPs also bind additional molecules including VEGF, Placental Growth Factor and Sema4A and thus may serve to augment binding/crosslinking of their classical receptors. In this manner, these molecules may compete with SEMA3’s for their NRP binding domain and regulate SEMA-NRP-elicited inhibitory signaling responses [81,90]. As will be discussed below, two members, namely SEMA3A and SEMA3F have high potential to interact with NRP-1 and NRP-2, respectively, on T cells and EC; thus, it is not surprising that they have potential to inhibit T cell activation and migration and to serve as regulators of the immune responses.
NRP-1 is expressed on CD4+ Tregs and APCs [70,91,92] and is reported to function in T cell/APC interactions [93] and in the regulation of cellular migration [91] and suppression [82]. Battaglia et al. compared NRP-1+ and NRP-1− Tregs and found that NRP-1+ Tregs had higher levels of expression of FoxP3 and Treg activation markers [70], and were more suppressive in vitro. In cervical cancer patients, a lower frequency of NRP-1+ within the Treg population was associated with reduced tumor mass following chemoradiotherapy. Yadav et al. identified NRP-1 as a marker of thymic-derived Tregs [92] and demonstrated that NRP-1low Tregs were insufficient to control autoimmunity. Furthermore they identified an association between NRP-1 expression and levels of Foxp3 and Treg function in an experimental autoimmune encephalomyelitis model. Consistent with these reports, Delgoffe et al. observed that signaling via NRP-1 in Tregs inhibits Akt activity, promotes Treg survival and enhances their immunoregulatory function in vitro and in vivo [82]. Hansen et al. demonstrated that NRP-1 is critical for the migration of Tregs into tumors via its binding to tumor-derived VEGF, and they observed that their local accumulation results in suppression of anti-tumor immune responses [91]. Thus, there is substantial data to suggest that NRP-1 and perhaps its SEMA ligands are of great importance in the local pro-tolerogenic microenvironment but additional studies will be required to identify their respectively roles within allografts in the augmentation of alloimmune tolerance.
We recently evaluated the biological effect of SEMA3F on T cells, EC and several tumor cell lines [81]. Similar to Delgoffe [82], we found that it is potent to inhibit PI-3K and Akt activity in part through disruption in the formation of the mTORC2 complex [81]. We also found that this regulatory effect required both NRP-2 and Plexin A1 expression on each cell type. Consistent with our findings, another group reported that semaphorin-plexin interactions regulate TOR signaling (in C. elegans) [94] and are potent to disrupt actin cytoskeleton and morphological changes in various cell types. These findings are most suggestive that SEMA3F-NRP interactions also serve to enhance immunoregulation locally within tissues.
Furthermore, in our studies [81], we also noted that SEMA3F-NRP-2 signals suppressed hypoxia-induced VEGF expression, which, as discussed above is characteristic of the chronic rejection process [4]. Within EC, SEMA3-NRP-2 interactions are anti-angiogenic, and stabilize the microvasculature and inhibit vascular leak in association with cell mediated immunity [77,78,84]. Mucka et al. observed massive tissue swelling and edema in NRP-2 knockout mice compared with wild-type littermates in association with delayed-type hypersensitivity [78]. They also demonstrated that the injection of SEMA3F into skin was potent to inhibit vascular leak induced by VEGF. Collectively, these studies are most suggestive that SEMA3F functions to stabilize the phenotype and integrity of microvascular EC during inflammation, which, as discussed above, is of relevance to chronic rejection (also illustrated in the cartoon in Figure 1). Since the SEMA3 family molecules are well established to be expressed within tumors, we postulate that they are deficient within the allograft microenvironment, and thus their local regulatory effects are lacking. It will be most intriguing to assess if forced expression of regulatory semaphorins within a graft results in enhanced immunomodulation and vascular stability and thus support the hypothesis that they are functional in the pro-tolerogenic allograft microenvironment.
The microenvironment can regulate the immune response by targeting immune metabolism
A final consideration is that the metabolic state of the local microenvironment also regulates T effector and Treg cells. Eil et al. reported that elevated potassium levels inhibit T cell effector function, and that augmenting potassium efflux enhances activation responses in vitro and in vivo [95]. Kleinewietfeld et al. demonstrated that high sodium levels facilitate the differentiation of Th17 effector cells and accelerates the onset and severity of experimental autoimmune encephalomyelitis [96]. Furthermore, Hernandez et al. demonstrated that high sodium diet/levels impair immunoregulation via the induction of IFN-γ in Tregs in vitro and in vivo [97]. Knock-down/neutralization of IFN-γ restored their immunosuppressive function even in the presence of high sodium levels. Safa et al. translated these observations within transplantation models and demonstrated that a high sodium diet accelerates allograft rejection in part via impaired alloimmune Treg function [98].
It is also becoming increasingly appreciated that Tregs favor select substrates to meet their metabolic needs and that their metabolic requirements subsequently influence the immune response [99]. Two major integrated pathways are utilized by T effector and Tregs: one that involves glucose metabolism/glycolysis, and another that involves oxidative phosphorylation. This latter metabolic pathway requires either pyruvate, fatty acids or glutamine to generate ATP within mitochondria. Chang et al. demonstrated that oxidative phosphorylation is required for the initial activation of naive CD4+ T cells [100], whereas T effector cells generally use glycolysis to meet their energy requirements. Interestingly, the consumption of glucose by tumor cells within a tumor microenvironment reduces available substrate and thus restricts glycolysis, which in turn translates into impaired local T effector function [101]. Also, as discussed above, local hypoxia may additionally modulate T cell activation through effects on metabolism, and notably glycolysis [12]. Furthermore, Beier et al. demonstrated that the inhibition of oxidative phosphorylation impairs both T effector and Treg function [102]. However, Treg function is disproportionally altered and restricts long-term allograft survival in a transplant model. Lee et al. systemically inhibited glycolysis and glutamate metabolism (alone or in combination) and observed prolonged survival in fully MHC mismatched cardiac and skin allograft transplantation models [103]. Together, these findings indicate that the balance of electrolytes and availability of metabolic substrates within the intragraft microenvironment may also shape the local pro-tolerogenic immune response.
Conclusion
In this review, we discuss a working model whereby the relative lack of expression of cell surface and/or secreted immunoregulatory molecules within allografts are a determinant of the phenotype of rejection. We speculate that the induced expression of EC cell surface co-inhibitory molecules and secreted immunoregulatory cytokines/factors such as the semaphorins will support local immunoregulation as evidenced within the microenvironment of tumors. The class 3 semaphorin family members and their interactions with neuropilin receptors are intriguing as potential local factors that modulate both the local microenvironment (via interactions with EC) and also local immune responses (via interactions with T regulatory and other immune cells). There is also high potential for local metabolic changes in the graft to shape the local immune response towards tolerance. Collectively, these findings are most suggestive that there is a great need to focus future research on the intragraft microenvironment as a determinant of chronic rejection or local immunoregulation/ tolerance.
Key points.
Distinct cell surface and secreted molecules within the intragraft microenvironment shape either a pro-inflammatory or a pro-tolerogenic immune response.
Dynamic changes in the endothelium influence the phenotype of the microenvironment.
Underappreciated factors within the microenvironment such as semaphorins, electrolytes and metabolites, can influence the behavior of effector and immunoregulatory T cells and thus the phenotype of rejection and/or graft survival.
Acknowledgments
The authors wish to acknowledge Drs. Soumitro Pal and David Sabatini for ongoing scientific collaborations. We also thank members of the laboratory and the Transplant Research Program for ongoing discussions and technical assistance and Nicole Mitton for editorial assistance.
Financial support and sponsorship
The work cited in this review was supported by NIH Grants R01AI046756, R01AI92305 and R21AI114223, and by the Casey Lee Ball Foundation. JW was supported by a fellowship grant from German Research Foundation (DFG).
Abbreviations
- EC
endothelial cell
- HIF
hypoxia-inducible factor
- NRP
neuropilin
- PDL
programmed death ligand
- Sema
semaphorin
- Treg
regulatory T cell
- VEGF
vascular endothelial growth factor
Footnotes
Conflicts of interest
David M. Briscoe has received grants from Astellas, Pfizer, Sanofi and Veloxis and has served as a consultant to Pfizer and Compass Therapeutics. He does not hold stock in any health related company and his interactions with industry are in accordance with the conflicts of interests policies at Boston Children’s Hospital.
Disclosure of funding:
The work cited in this review was supported by NIH Grants R01AI046756, R01AI92305 and R21AI114223, and by the Casey Lee Ball Foundation. JW was supported by a fellowship grant from German Research Foundation (DFG).
References
- 1.Rabelink TJ, Wijewickrama DC, de Koning EJ. Peritubular endothelium: the Achilles heel of the kidney? Kidney Int. 2007;72:926–930. doi: 10.1038/sj.ki.5002414. [DOI] [PubMed] [Google Scholar]
- 2.Steegh FM, Gelens MA, Nieman FH, et al. Early loss of peritubular capillaries after kidney transplantation. J Am Soc Nephrol. 2011;22:1024–1029. doi: 10.1681/ASN.2010050531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008;86:1340–1348. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruneau S, Woda CB, Daly KP, et al. Key Features of the Intragraft Microenvironment that Determine Long-Term Survival Following Transplantation. Front Immunol. 2012;3:54. doi: 10.3389/fimmu.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruneau S, Wedel J, Fakhouri F, et al. Translational implications of endothelial cell dysfunction in association with chronic allograft rejection. Pediatr Nephrol. 2016;31:41–51. doi: 10.1007/s00467-015-3094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Contreras AG, Briscoe DM. Every allograft needs a silver lining. J Clin Invest. 2007;117:3645–3648. doi: 10.1172/JCI34238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babu AN, Murakawa T, Thurman JM, et al. Microvascular destruction identifies murine allografts that cannot be rescued from airway fibrosis. J Clin Invest. 2007;117:3774–3785. doi: 10.1172/JCI32311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pober JS, Jane-wit D, Qin L, Tellides G. Interacting mechanisms in the pathogenesis of cardiac allograft vasculopathy. Arterioscler Thromb Vasc Biol. 2014;34:1609–1614. doi: 10.1161/ATVBAHA.114.302818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang X, Sung YK, Tian W, et al. Graft microvascular disease in solid organ transplantation. J Mol Med (Berl) 2014;92:797–810. doi: 10.1007/s00109-014-1173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wedel J, Bruneau S, Kochupurakkal N, et al. Chronic allograft rejection: a fresh look. Curr Opin Organ Transplant. 2015;20:13–20. doi: 10.1097/MOT.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dang EV, Barbi J, Yang HY, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ostergaard L, Tietze A, Nielsen T, et al. The relationship between tumor blood flow, angiogenesis, tumor hypoxia, and aerobic glycolysis. Cancer Res. 2013;73:5618–5624. doi: 10.1158/0008-5472.CAN-13-0964. [DOI] [PubMed] [Google Scholar]
- 13.Jiang X, Khan MA, Tian W, et al. Adenovirus-mediated HIF-1alpha gene transfer promotes repair of mouse airway allograft microvasculature and attenuates chronic rejection. J Clin Invest. 2011;121:2336–2349. doi: 10.1172/JCI46192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sidky YA, Auerbach R. Lymphocyte-induced angiogenesis: a quantitative and sensitive assay of the graft-vs.-host reaction. J Exp Med. 1975 May 1;141:1084–1100. doi: 10.1084/jem.141.5.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auerbach R, Sidky YA. Nature of the stimulus leading to lymphocyte-induced angiogenesis. J Immunol. 1979 Aug;123:751–754. [PubMed] [Google Scholar]
- 16.Kaminski M, Auerbach R. Angiogenesis induction by CD-4 positive lymphocytes. Proceedings of the Society for Experimental Biology and Medicine. 1988;188:440–443. doi: 10.3181/00379727-188-42757. [DOI] [PubMed] [Google Scholar]
- 17.Leibovich SJ, Polverini PJ, Shepard HM, et al. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 18.Ferrara N. The role of VEGF in the regulation of physiological and pathological angiogenesis. EXS. 2005:209–231. doi: 10.1007/3-7643-7311-3_15. [DOI] [PubMed] [Google Scholar]
- 19.Freeman MR, Schneck FX, Gagnon ML, et al. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis. Cancer Res. 1995;55:4140–4145. [PubMed] [Google Scholar]
- 20.Melter M, Reinders ME, Sho M, et al. Ligation of CD40 induces the expression of vascular endothelial growth factor by endothelial cells and monocytes and promotes angiogenesis in vivo. Blood. 2000;96:3801–3808. [PubMed] [Google Scholar]
- 21.Reinders ME, Sho M, Izawa A, et al. Proinflammatory functions of vascular endothelial growth factor in alloimmunity. J Clin Invest. 2003;112:1655–1665. doi: 10.1172/JCI17712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection. J Am Soc Nephrol. 2006;17:932–942. doi: 10.1681/ASN.2005121250. [DOI] [PubMed] [Google Scholar]
- 23.Reinders ME, Fang JC, Wong W, et al. Expression patterns of vascular endothelial growth factor in human cardiac allografts: association with rejection. Transplantation. 2003;76:224–230. doi: 10.1097/01.TP.0000071363.55007.D0. [DOI] [PubMed] [Google Scholar]
- 24.Daly KP, Seifert ME, Chandraker A, et al. VEGF-C, VEGF-A and related angiogenesis factors as biomarkers of allograft vasculopathy in cardiac transplant recipients. J Heart Lung Transplant. 2013;32:120–128. doi: 10.1016/j.healun.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *25.Daly KP, Stack M, Eisenga MF, et al. VEGF-A is associated with the subsequent development of moderate or severe cardiac allograft vasculopathy in pediatric heart transplant recipients☆. The Journal of Heart and Lung Transplantation. 2016 doi: 10.1016/j.healun.2016.09.013. This cohort study identified a statistically significant correlation between high serum levels of VEGF-A and the subsequent development of allograft vasculopathy in pediatric heart transplant recipients. It suggests that endothelial injury and their physiological repair mechanisms could serve as a predictive biomarker of chronic rejection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain RK. Taming vessels to treat cancer. Sci Am. 2008;298:56–63. doi: 10.1038/scientificamerican0108-56. [DOI] [PubMed] [Google Scholar]
- 27.Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91:1071–1121. doi: 10.1152/physrev.00038.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Basu A, Hoerning A, Datta D, et al. Cutting edge: Vascular endothelial growth factor-mediated signaling in human CD45RO+ CD4+ T cells promotes Akt and ERK activation and costimulates IFN-gamma production. J Immunol. 2010;184:545–549. doi: 10.4049/jimmunol.0900397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edelbauer M, Datta D, Vos IH, et al. Effect of vascular endothelial growth factor and its receptor KDR on the transendothelial migration and local trafficking of human T cells in vitro and in vivo. Blood. 2010;116:1980–1989. doi: 10.1182/blood-2009-11-252460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **30.Chalasani G, Li Q, Konieczny BT, et al. The allograft defines the type of rejection (acute versus chronic) in the face of an established effector immune response. J Immunol. 2004;172:7813–7820. doi: 10.4049/jimmunol.172.12.7813. This paper sets the foundation for the hypothesis that the intragraft microenvironment can influence the phenotype of rejection (acute/chronic) independent of the recipient’s immune response. Adoptive transfer of the same number and composition of T cells at day 2 post-transplantation resulted in an acute rejection response, while transfer at day 50 resulted in chronic allograft rejection. Ischemia/reperfusion injury/retransplantation of healed-in allografts recapitulated the acute rejection response, further suggesting that events within microenvironment within the allograft dictates the type of local immune response. [DOI] [PubMed] [Google Scholar]
- 31.Hughes CC, Savage CO, Pober JS. The endothelial cell as a regulator of T-cell function. Immunol Rev. 1990;117:85–102. doi: 10.1111/j.1600-065x.1990.tb00568.x. [DOI] [PubMed] [Google Scholar]
- 32.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 33.Denton MD, Davis SF, Baum MA, et al. The role of the graft endothelium in transplant rejection: evidence that endothelial activation may serve as a clinical marker for the development of chronic rejection. Pediatr Transplant. 2000;4:252–260. doi: 10.1034/j.1399-3046.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- 34.Denton MD, Geehan CS, Alexander SI, et al. Endothelial cells modify the costimulatory capacity of transmigrating leukocytes and promote CD28-mediated CD4(+) T cell alloactivation. J Exp Med. 1999;190:555–566. doi: 10.1084/jem.190.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodig N, Ryan T, Allen JA, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33:3117–3126. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 36.Murakami K, Ma W, Fuleihan R, Pober JS. Human endothelial cells augment early CD40 ligand expression in activated CD4+ T cells through LFA-3-mediated stabilization of mRNA. J Immunol. 1999;163:2667–2673. [PubMed] [Google Scholar]
- 37.Wang C, Yi T, Qin L, et al. Rapamycin-treated human endothelial cells preferentially activate allogeneic regulatory T cells. J Clin Invest. 2013;123:1677–1693. doi: 10.1172/JCI66204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briscoe DM, Sayegh MH. A rendezvous before rejection: where do T cells meet transplant antigens? Nat Med. 2002;8:220–222. doi: 10.1038/nm0302-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes CC, Savage CO, Pober JS. Endothelial cells augment T cell interleukin 2 production by a contact-dependent mechanism involving CD2/LFA-3 interaction. J Exp Med. 1990;171:1453–1467. doi: 10.1084/jem.171.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valujskikh A, Lantz O, Celli S, et al. Cross-primed CD8(+) T cells mediate graft rejection via a distinct effector pathway. Nat Immunol. 2002;3:844–851. doi: 10.1038/ni831. [DOI] [PubMed] [Google Scholar]
- 41.Kreisel D, Krupnick AS, Gelman AE, et al. Non-hematopoietic allograft cells directly activate CD8+ T cells and trigger acute rejection: an alternative mechanism of allorecognition. Nat Med. 2002;8:233–239. doi: 10.1038/nm0302-233. [DOI] [PubMed] [Google Scholar]
- 42.Shiao SL, Kirkiles-Smith NC, Shepherd BR, et al. Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo. J Immunol. 2007;179:4397–4404. doi: 10.4049/jimmunol.179.7.4397. [DOI] [PubMed] [Google Scholar]
- *43.Abrahimi P, Qin L, Chang WG, et al. Blocking MHC class II on human endothelium mitigates acute rejection. JCI Insight. 2016;1 doi: 10.1172/jci.insight.85293. This group has published several studies defining the function of MHC II-expressing EC in the reactivation of CD4+ and CD8+ T cells. The current study demonstrates that the MHC class II expression on allograft EC in vivo promotes the differentiation of effector memory CD8+ into cytotoxic T lymphocytes with the help of paracrine IL-2 derived from alloreactive CD4+ cells. Since EC may activate effector memory CD8+ subsets (that are exclusively present in humans and not mice) they concluded that rejection can be initiated locally in the absence of secondary lymphoid tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma W, Pober JS. Human endothelial cells effectively costimulate cytokine production by, but not differentiation of, naive CD4+ T cells. J Immunol. 1998;161:2158–2167. [PubMed] [Google Scholar]
- 45.Khayyamian S, Hutloff A, Buchner K, et al. ICOS-ligand, expressed on human endothelial cells, costimulates Th1 and Th2 cytokine secretion by memory CD4+ T cells. Proc Natl Acad Sci U S A. 2002;99:6198–6203. doi: 10.1073/pnas.092576699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Briscoe DM, Yeung AC, Schoen FJ, et al. Predictive value of inducible endothelial cell adhesion molecule expression for acute rejection of human cardiac allografts. Transplantation. 1995;59:204–211. [PubMed] [Google Scholar]
- 47.Pober JS, Gimbrone MA, Jr, Collins T, et al. Interactions of T lymphocytes with human vascular endothelial cells: role of endothelial cells surface antigens. Immunobiology. 1984;168:483–494. doi: 10.1016/s0171-2985(84)80132-1. [DOI] [PubMed] [Google Scholar]
- 48.Tellides G, Tereb DA, Kirkiles-Smith NC, et al. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature. 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 49.Pober JS, Bothwell AL, Lorber MI, et al. Immunopathology of human T cell responses to skin, artery and endothelial cell grafts in the human peripheral blood lymphocyte/severe combined immunodeficient mouse. Springer Semin Immunopathol. 2003;25:167–180. doi: 10.1007/s00281-003-0135-1. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Bai Y, Qin L, et al. Interferon-gamma induces human vascular smooth muscle cell proliferation and intimal expansion by phosphatidylinositol 3-kinase dependent mammalian target of rapamycin raptor complex 1 activation. Circ Res. 2007;101:560–569. doi: 10.1161/CIRCRESAHA.107.151068. [DOI] [PubMed] [Google Scholar]
- 51.Rao DA, Eid RE, Qin L, et al. Interleukin (IL)-1 promotes allogeneic T cell intimal infiltration and IL-17 production in a model of human artery rejection. J Exp Med. 2008;205:3145–3158. doi: 10.1084/jem.20081661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fogal B, Yi T, Wang C, et al. Neutralizing IL-6 reduces human arterial allograft rejection by allowing emergence of CD161+ CD4+ regulatory T cells. J Immunol. 2011;187:6268–6280. doi: 10.4049/jimmunol.1003774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *53.Lion J, Taflin C, Cross AR, et al. HLA Class II Antibody Activation of Endothelial Cells Promotes Th17 and Disrupts Regulatory T Lymphocyte Expansion. Am J Transplant. 2016;16:1408–1420. doi: 10.1111/ajt.13644. This study shows that EC can produce cytokines that regulate CD4+ T helper cell differentiation. HLA class II stimulation led to activation of MAPK and mTOR pathways and resulted in the secretion of IL-6. Co-culture with PBMC showed that paracrine IL-6 was sufficient to promote Th17 responses and to decrease the frequency of CD4+FoxP3+ T cells. [DOI] [PubMed] [Google Scholar]
- 54.Krupnick AS, Gelman AE, Barchet W, et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol. 2005;175:6265–6270. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 55.Grabie N, Gotsman I, DaCosta R, et al. Endothelial programmed death-1 ligand 1 (PD-L1) regulates CD8+ T-cell mediated injury in the heart. Circulation. 2007;116:2062–2071. doi: 10.1161/CIRCULATIONAHA.107.709360. [DOI] [PubMed] [Google Scholar]
- 56.Wang C, Qin L, Manes TD, et al. Rapamycin antagonizes TNF induction of VCAM-1 on endothelial cells by inhibiting mTORC2. J Exp Med. 2014;211:395–404. doi: 10.1084/jem.20131125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mazanet MM, Hughes CC. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J Immunol. 2002;169:3581–3588. doi: 10.4049/jimmunol.169.7.3581. [DOI] [PubMed] [Google Scholar]
- 58.Koga N, Suzuki J, Kosuge H, et al. Blockade of the interaction between PD-1 and PD-L1 accelerates graft arterial disease in cardiac allografts. Arterioscler Thromb Vasc Biol. 2004;24:2057–2062. doi: 10.1161/01.ATV.0000145015.23656.e4. [DOI] [PubMed] [Google Scholar]
- 59.Ozkaynak E, Wang L, Goodearl A, et al. Programmed death-1 targeting can promote allograft survival. J Immunol. 2002;169:6546–6553. doi: 10.4049/jimmunol.169.11.6546. [DOI] [PubMed] [Google Scholar]
- 60.Sandner SE, Clarkson MR, Salama AD, et al. Role of the programmed death-1 pathway in regulation of alloimmune responses in vivo. J Immunol. 2005;174:3408–3415. doi: 10.4049/jimmunol.174.6.3408. [DOI] [PubMed] [Google Scholar]
- 61.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 62.Yang J, Popoola J, Khandwala S, et al. Critical role of donor tissue expression of programmed death ligand-1 in regulating cardiac allograft rejection and vasculopathy. Circulation. 2008;117:660–669. doi: 10.1161/CIRCULATIONAHA.107.741025. [DOI] [PubMed] [Google Scholar]
- **63.Motz GT, Santoro SP, Wang LP, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. 2014;20:607–615. doi: 10.1038/nm.3541. This study focuses on the role of the EC in tumor immunity and specifically how cell surface molecules on the may promote local tissue immunoregulation. Endothelial FasL upregulation was identified to selectively kill infiltrating CD8+ effector T cells but promote Treg influx within the tumor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sata M, Luo Z, Walsh K. Fas ligand overexpression on allograft endothelium inhibits inflammatory cell infiltration and transplant-associated intimal hyperplasia. J Immunol. 2001;166:6964–6971. doi: 10.4049/jimmunol.166.11.6964. [DOI] [PubMed] [Google Scholar]
- 65.Riella LV, Watanabe T, Sage PT, et al. Essential role of PDL1 expression on nonhematopoietic donor cells in acquired tolerance to vascularized cardiac allografts. Am J Transplant. 2011;11:832–840. doi: 10.1111/j.1600-6143.2011.03451.x. [DOI] [PubMed] [Google Scholar]
- 66.Chaturvedi S, Yuen DA, Bajwa A, et al. Slit2 prevents neutrophil recruitment and renal ischemia-reperfusion injury. J Am Soc Nephrol. 2013;24:1274–1287. doi: 10.1681/ASN.2012090890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *67.Boneschansker L, Nakayama H, Eisenga M, et al. Netrin-1 Augments Chemokinesis in CD4+ T Cells In Vitro and Elicits a Proinflammatory Response In Vivo. J Immunol. 2016;197:1389–1398. doi: 10.4049/jimmunol.1502432. This study investigates the influence of the axonal migration cue Netrin-1 on the migratory behavior of CD4+ T cells. While Netrin-1 has been previously identified as a chemorepellent, these authors used a novel microfluidics device and found that it is potent to induce chemokinesis of CD4+ effector T cells and bidirectional migration in vitro. This suggests that Netrin-1 is a component of leukocyte recruitment, but that other cues (chemoattactants/chemorepellants) are required to define directionality of migration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coulthard MG, Morgan M, Woodruff TM, et al. Eph/Ephrin signaling in injury and inflammation. Am J Pathol. 2012;181:1493–1503. doi: 10.1016/j.ajpath.2012.06.043. [DOI] [PubMed] [Google Scholar]
- 69.Mizui M, Kumanogoh A, Kikutani H. Immune Semaphorins: Novel Features of Neural Guidance Molecules. J Clin Immunol. 2008 doi: 10.1007/s10875-008-9263-7. [DOI] [PubMed] [Google Scholar]
- 70.Battaglia A, Buzzonetti A, Monego G, et al. Neuropilin-1 expression identifies a subset of regulatory T cells in human lymph nodes that is modulated by preoperative chemoradiation therapy in cervical cancer. Immunology. 2008;123:129–138. doi: 10.1111/j.1365-2567.2007.02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kumanogoh A, Kikutani H. Immunological functions of the neuropilins and plexins as receptors for semaphorins. Nat Rev Immunol. 2013;13:802–814. doi: 10.1038/nri3545. [DOI] [PubMed] [Google Scholar]
- 72.Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9:17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- 73.Takamatsu H, Okuno T, Kumanogoh A. Regulation of immune cell responses by semaphorins and their receptors. Cell Mol Immunol. 2010;7:83–88. doi: 10.1038/cmi.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lampropoulou A, Ruhrberg C. Neuropilin regulation of angiogenesis. Biochem Soc Trans. 2014;42:1623–1628. doi: 10.1042/BST20140244. [DOI] [PubMed] [Google Scholar]
- 75.Soker S, Takashima S, Miao HQ, et al. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 76.Klagsbrun M, Takashima S, Mamluk R. The role of neuropilin in vascular and tumor biology. Adv Exp Med Biol. 2002;515:33–48. doi: 10.1007/978-1-4615-0119-0_3. [DOI] [PubMed] [Google Scholar]
- 77.Bielenberg DR, Klagsbrun M. Targeting endothelial and tumor cells with semaphorins. Cancer Metastasis Rev. 2007;26:421–431. doi: 10.1007/s10555-007-9097-4. [DOI] [PubMed] [Google Scholar]
- *78.Mucka P, Levonyak N, Geretti E, et al. Inflammation and Lymphedema Are Exacerbated and Prolonged by Neuropilin 2 Deficiency. Am J Pathol. 2016 doi: 10.1016/j.ajpath.2016.07.022. In Press. This study shows that NRP-2 deficiency is associated with profound blood vessel permeability and following delayed-type hypersensitivity. The injection of SEMA3F into skin was potent to inhibit vascular leak induced by VEGF, suggesting that it competes with VEGF for binding to the receptor and has potential to stabilize the microvasculature in association with cell mediated immune responses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kikutani H, Kumanogoh A. Semaphorins in interactions between T cells and antigen-presenting cells. Nat Rev Immunol. 2003;3:159–167. doi: 10.1038/nri1003. [DOI] [PubMed] [Google Scholar]
- 80.Shimizu A, Mammoto A, Italiano JE, Jr, et al. ABL2/ARG tyrosine kinase mediates SEMA3F-induced RhoA inactivation and cytoskeleton collapse in human glioma cells. J Biol Chem. 2008;283:27230–27238. doi: 10.1074/jbc.M804520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *81.Nakayama H, Bruneau S, Kochupurakkal N, et al. Regulation of mTOR Signaling by Semaphorin 3F-Neuropilin 2 Interactions In Vitro and In Vivo. Sci Rep. 2015;5:11789. doi: 10.1038/srep11789. This study shows that SEMA3F-neuropilin-2 interactions inhibit mTOC2-Akt signaling and associated cellular activation responses in diverse human cell types including T cells and EC. It also functions to inhibit VEGF transcriptional activation (perhaps via the regulation of mTOR/Akt signals) and functions in vivo to inhibit neoangiogenesis and tumor growth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Delgoffe GM, Woo SR, Turnis ME, et al. Stability and function of regulatory T cells is maintained by a neuropilin-1-semaphorin-4a axis. Nature. 2013;501:252–256. doi: 10.1038/nature12428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takamatsu H, Kumanogoh A. Diverse roles for semaphorin-plexin signaling in the immune system. Trends Immunol. 2012;33:127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 84.Gaur P, Bielenberg DR, Samuel S, et al. Role of class 3 semaphorins and their receptors in tumor growth and angiogenesis. Clin Cancer Res. 2009;15:6763–6770. doi: 10.1158/1078-0432.CCR-09-1810. [DOI] [PubMed] [Google Scholar]
- 85.Capparuccia L, Tamagnone L. Semaphorin signaling in cancer cells and in cells of the tumor microenvironment--two sides of a coin. J Cell Sci. 2009;122:1723–1736. doi: 10.1242/jcs.030197. [DOI] [PubMed] [Google Scholar]
- 86.Sarris M, Andersen KG, Randow F, et al. Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008;28:402–413. doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Curreli S, Arany Z, Gerardy-Schahn R, et al. Polysialylated neuropilin-2 is expressed on the surface of human dendritic cells and modulates dendritic cell-T lymphocyte interactions. J Biol Chem. 2007;282:30346–30356. doi: 10.1074/jbc.M702965200. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto M, Suzuki K, Okuno T, et al. Plexin-A4 negatively regulates T lymphocyte responses. Int Immunol. 2008;20:413–420. doi: 10.1093/intimm/dxn006. [DOI] [PubMed] [Google Scholar]
- 89.Takamatsu H, Takegahara N, Nakagawa Y, et al. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol. 2010;11:594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Geretti E, Shimizu A, Klagsbrun M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis. 2008;11:31–39. doi: 10.1007/s10456-008-9097-1. [DOI] [PubMed] [Google Scholar]
- 91.Hansen W, Hutzler M, Abel S, et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J Exp Med. 2012;209:2001–2016. doi: 10.1084/jem.20111497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yadav M, Louvet C, Davini D, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209:1713–1722. S1711–1719. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sarris: Neuropilin-1 expression on regulatory T cells enhances their interactions with dendritic cells during antigen recognition. Immunity. 2008 doi: 10.1016/j.immuni.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nukazuka A, Tamaki S, Matsumoto K, et al. A shift of the TOR adaptor from Rictor towards Raptor by semaphorin in C. elegans. Nat Commun. 2011;2:484. doi: 10.1038/ncomms1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **95.Eil R, Vodnala SK, Clever D, et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature. 2016 doi: 10.1038/nature19364. This study demonstrates that increased potassium levels within the tumor microenvironment impairs effector T cell function. Attenuated anti-tumor immune responses were due to increased intra-T cell potassium levels and not due to changes in plasma membrane potential. Augmenting potassium efflux from T cells enhances anti-tumor activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kleinewietfeld M, Manzel A, Titze J, et al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518–522. doi: 10.1038/nature11868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *97.Hernandez AL, Kitz A, Wu C, et al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest. 2015;125:4212–4222. doi: 10.1172/JCI81151. This study demonstrates that high sodium levels stimulates IFN-γ production by Tregs, and that IFN-γ secretion is associated with a loss of regulatory function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **98.Safa K, Ohori S, Borges TJ, et al. Salt Accelerates Allograft Rejection through Serum- and Glucocorticoid-Regulated Kinase-1-Dependent Inhibition of Regulatory T Cells. J Am Soc Nephrol. 2015;26:2341–2347. doi: 10.1681/ASN.2014090914. This study uses a transplant model to determine the effect high sodium levels on effector and regulatory alloimmune responses. It is based on a previous report demonstrating that sodium favors Th17 effector responses [96]. Here, they demonstrate that high sodium levels results in accelerated allograft rejection response, a loss of immunoregulation and a shift the circulating ration of Treg:Th17 cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chang CH, Curtis JD, Maggi LB, Jr, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **101.Chang CH, Qiu J, O’Sullivan D, et al. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. This study demonstrates that low glucose levels restrict the metabolic activity of T cells and their effector function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **102.Beier UH, Angelin A, Akimova T, et al. Essential role of mitochondrial energy metabolism in Foxp3(+) T-regulatory cell function and allograft survival. FASEB J. 2015;29:2315–2326. doi: 10.1096/fj.14-268409. This study demonstates that oxidative phosphorylation is required for both T effector and Treg function in vivo. However, Tregs use the oxidative phosphorylation pathway to a greater extent that T effectors in vivo following cardiac transplantation in a well established model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **103.Lee CF, Lo YC, Cheng CH, et al. Preventing Allograft Rejection by Targeting Immune Metabolism. Cell Rep. 2015;13:760–770. doi: 10.1016/j.celrep.2015.09.036. This study demonstrates that blocking both glycolysis and glutamine metabolic pathways is sufficient to inhibit alloimmune effector responses and to promote allograft survival following skin and cardiac transplantation. Importantly, this regime increased the frequency of CD4+Foxp3+ Tregs suggesting that it does not inhibit immunoregulation. [DOI] [PMC free article] [PubMed] [Google Scholar]