Abstract
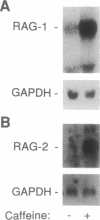
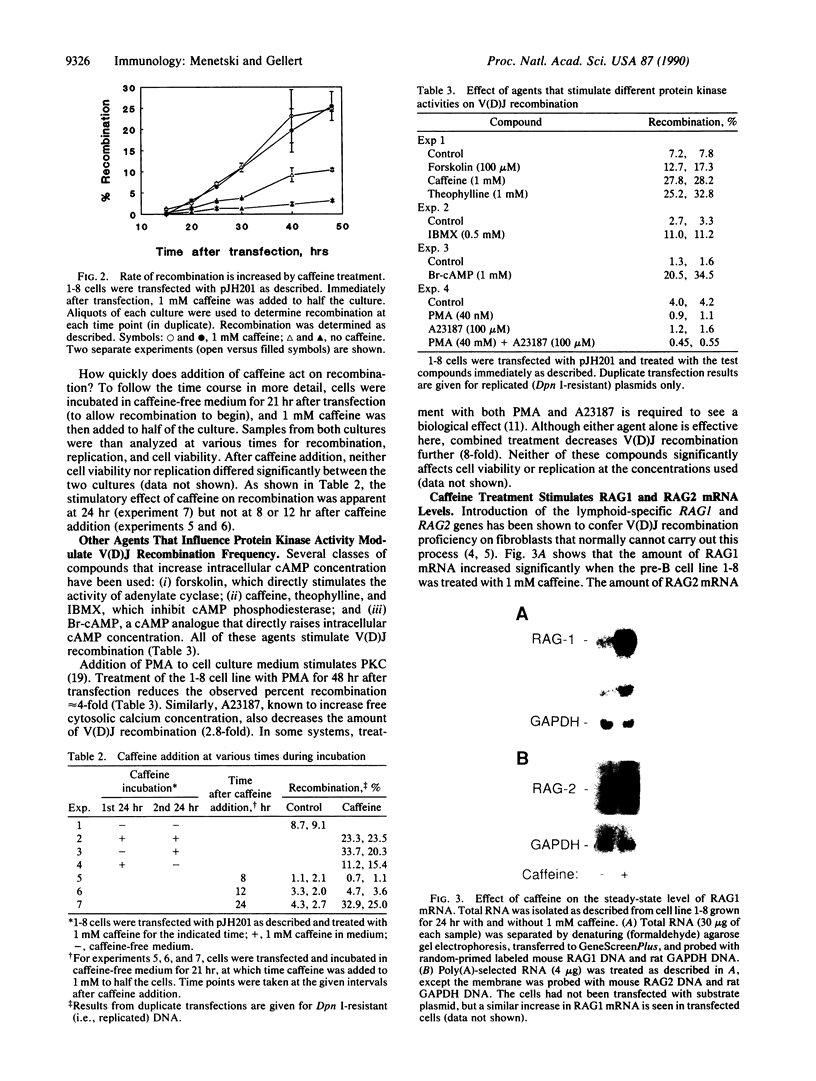
V(D)J [variable--(diversity)--joining] recombination is regulated developmentally, being restricted to cells of the early B- and T-lymphocyte lineages. In this report we show that recombination activity can also be regulated in response to chemical effectors. Compounds that increase intracellular cAMP increase V(D)J recombination of extrachromosomal substrates in pre-B-cell lines as much as 10-fold. In contrast, V(D)J recombination is reduced 5- to 8-fold in response to phorbol 12-myristate 13-acetate or to the calcium ionophore A23187. The effect of cAMP agonists on recombination appears to reflect an increase in cellular recombination activity, as indicated by the caffeine-induced rise in the level of mRNA from the recombination-activating genes RAG1 and RAG2. Our data demonstrate that intracellular second messengers modulate recombination activity in lymphoid cell lines, implying that recombination activity can be regulated by these signals in developing B and T lymphocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham R. T., Ho S. N., Barna T. J., McKean D. J. Transmembrane signaling during interleukin 1-dependent T cell activation. Interactions of signal 1- and signal 2-type mediators with the phosphoinositide-dependent signal transduction mechanism. J Biol Chem. 1987 Feb 25;262(6):2719–2728. [PubMed] [Google Scholar]
- Blackwell T. K., Moore M. W., Yancopoulos G. D., Suh H., Lutzker S., Selsing E., Alt F. W. Recombination between immunoglobulin variable region gene segments is enhanced by transcription. Nature. 1986 Dec 11;324(6097):585–589. doi: 10.1038/324585a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Burchiel S. W., Warner N. L. Cyclic AMP modulation of Fc receptor expression on a pre-B cell lymphoma. J Immunol. 1980 Mar;124(3):1016–1021. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Desiderio S. V., Yancopoulos G. D., Paskind M., Thomas E., Boss M. A., Landau N., Alt F. W., Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984 Oct 25;311(5988):752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Fevrier M. Antigen-nonspecific macrophage factors modulating the antibody response in vitro. Comp Immunol Microbiol Infect Dis. 1985;8(2):159–170. doi: 10.1016/0147-9571(85)90042-6. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Gellert M., Mizuuchi K. Extrachromosomal DNA substrates in pre-B cells undergo inversion or deletion at immunoglobulin V-(D)-J joining signals. Cell. 1987 Jun 19;49(6):775–783. doi: 10.1016/0092-8674(87)90615-5. [DOI] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Irving S. G., June C. H., Zipfel P. F., Siebenlist U., Kelly K. Mitogen-induced genes are subject to multiple pathways of regulation in the initial stages of T-cell activation. Mol Cell Biol. 1989 Mar;9(3):1034–1040. doi: 10.1128/mcb.9.3.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- Malynn B. A., Blackwell T. K., Fulop G. M., Rathbun G. A., Furley A. J., Ferrier P., Heinke L. B., Phillips R. A., Yancopoulos G. D., Alt F. W. The scid defect affects the final step of the immunoglobulin VDJ recombinase mechanism. Cell. 1988 Aug 12;54(4):453–460. doi: 10.1016/0092-8674(88)90066-9. [DOI] [PubMed] [Google Scholar]
- McKearn J. P., Rosenberg N. Mapping cell surface antigens on mouse pre-B cell lines. Eur J Immunol. 1985 Mar;15(3):295–298. doi: 10.1002/eji.1830150316. [DOI] [PubMed] [Google Scholar]
- Mellon P. L., Clegg C. H., Correll L. A., McKnight G. S. Regulation of transcription by cyclic AMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4887–4891. doi: 10.1073/pnas.86.13.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Plaut M. Lymphocyte hormone receptors. Annu Rev Immunol. 1987;5:621–669. doi: 10.1146/annurev.iy.05.040187.003201. [DOI] [PubMed] [Google Scholar]
- Schatz D. G., Oettinger M. A., Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989 Dec 22;59(6):1035–1048. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- Schlissel M. S., Baltimore D. Activation of immunoglobulin kappa gene rearrangement correlates with induction of germline kappa gene transcription. Cell. 1989 Sep 8;58(5):1001–1007. doi: 10.1016/0092-8674(89)90951-3. [DOI] [PubMed] [Google Scholar]
- Schuler W., Weiler I. J., Schuler A., Phillips R. A., Rosenberg N., Mak T. W., Kearney J. F., Perry R. P., Bosma M. J. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell. 1986 Sep 26;46(7):963–972. doi: 10.1016/0092-8674(86)90695-1. [DOI] [PubMed] [Google Scholar]
- Shirakawa F., Chedid M., Suttles J., Pollok B. A., Mizel S. B. Interleukin 1 and cyclic AMP induce kappa immunoglobulin light-chain expression via activation of an NF-kappa B-like DNA-binding protein. Mol Cell Biol. 1989 Mar;9(3):959–964. doi: 10.1128/mcb.9.3.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siden E. J., Gifford A., Baltimore D. Cyclic AMP induces terminal deoxynucleotidyl transferase in immature B cell leukemia lines. J Immunol. 1985 Aug;135(2):1518–1522. [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Nolan G. P., Pollock R., Prockop S., Li S. C., Herzenberg L. A., Alt F. W. A novel fluorescence-based system for assaying and separating live cells according to VDJ recombinase activity. Mol Cell Biol. 1990 Apr;10(4):1697–1704. doi: 10.1128/mcb.10.4.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]