Abstract
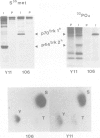
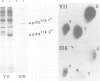
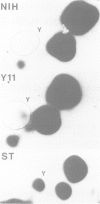
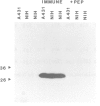
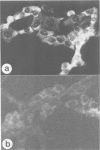
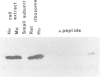
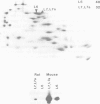
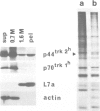
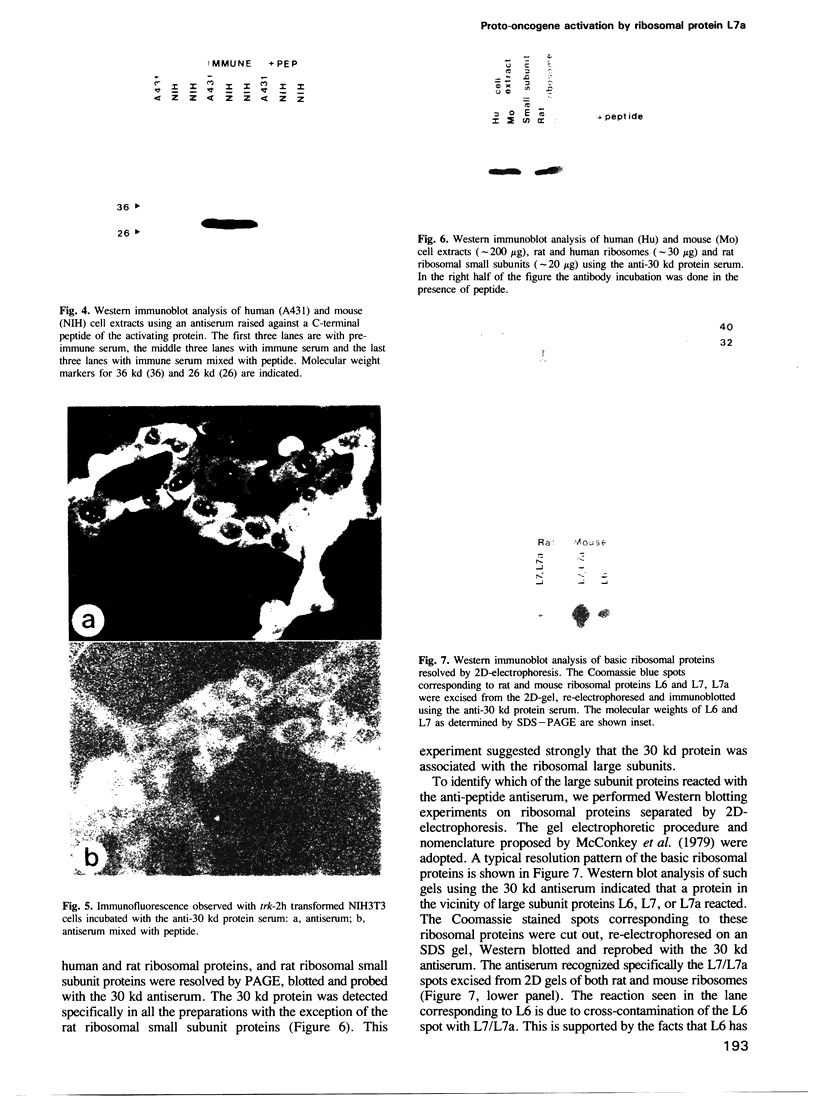
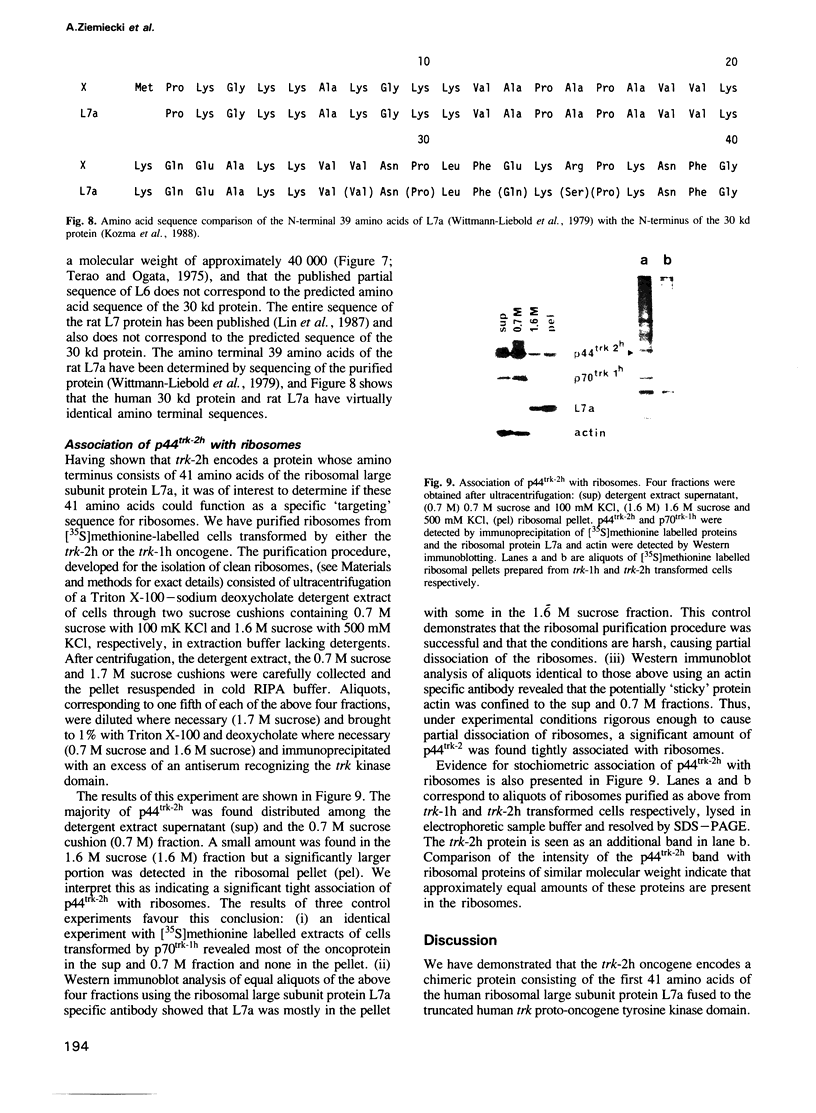
The trk-2h oncogene, isolated from the human breast carcinoma cell line MDA-MB 231 by genomic DNA-transfection into NIH3T3 cells, consists of the trk proto-oncogene receptor kinase domain fused to a N-terminal 41 amino acid activating sequence (Kozma, S.C., Redmond, S.M.S., Xiao-Chang, F., Saurer, S.M., Groner, B. and Hynes, N.E. (1988) EMBO J., 7, 147-154). Antibodies raised against a bacterially produced beta gal-trk receptor kinase fusion protein recognized a 44 kd phosphoprotein phosphorylated on serine, threonine and tyrosine in extracts of trk-2h transformed NIH3T3 cells. In vitro, in the presence of Mn2+/gamma ATP, this protein became phosphorylated extensively on tyrosine. Cells transformed by trk-2h did not, however, show an elevation in total phosphotyrosine. We have cloned and sequenced the cDNA encoding the amino terminal activating sequences of trk-2h (Kozma et al., 1988). The encoded protein has a high basic amino acid content and the gene is expressed as an abundant 1.2 kb mRNA in human, rat and mouse cells. Antipeptide antibodies raised against a C-terminal peptide recognized specifically a 30 kd protein on Western blots of human, rat and mouse cell extracts. Immunofluorescence revealed, in addition to granular cytoplasmic fluorescence, intense nucleolar staining. The high basic amino acid content and nucleolar staining prompted us to investigate whether the 30 kd protein could be a ribosomal protein. Western immunoblotting analysis of 2D-electrophoretically resolved ribosomal proteins indicated that the 30 kd protein is the ribosomal large subunit protein L7a.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbacid M., Lauver A. V. Gene products of McDonough feline sarcoma virus have an in vitro-associated protein kinase that phosphorylates tyrosine residues: lack of detection of this enzymatic activity in vivo. J Virol. 1981 Dec;40(3):812–821. doi: 10.1128/jvi.40.3.812-821.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Coulier F., Martin-Zanca D., Ernst M., Barbacid M. Mechanism of activation of the human trk oncogene. Mol Cell Biol. 1989 Jan;9(1):15–23. doi: 10.1128/mcb.9.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Giallongo A., Yon J., Fried M. Ribosomal protein L7a is encoded by a gene (Surf-3) within the tightly clustered mouse surfeit locus. Mol Cell Biol. 1989 Jan;9(1):224–231. doi: 10.1128/mcb.9.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley C., Williams T., Fried M. One of the tightly clustered genes of the mouse surfeit locus is a highly expressed member of a multigene family whose other members are predominantly processed pseudogenes. Mol Cell Biol. 1988 Sep;8(9):3898–3905. doi: 10.1128/mcb.8.9.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Identification of multiple novel polypeptide substrates of the v-src, v-yes, v-fps, v-ros, and v-erb-B oncogenic tyrosine protein kinases utilizing antisera against phosphotyrosine. Oncogene. 1988 Apr;2(4):305–315. [PubMed] [Google Scholar]
- Kappes B., Ziemiecki A., Müller R. G., Theilen G. H., Bauer H., Barnekow A. The TP1 isolate of feline sarcoma virus encodes a fgr-related oncogene lacking gamma actin sequences. Oncogene. 1989 Mar;4(3):363–372. [PubMed] [Google Scholar]
- Kozma S. C., Redmond S. M., Fu X. C., Saurer S. M., Groner B., Hynes N. E. Activation of the receptor kinase domain of the trk oncogene by recombination with two different cellular sequences. EMBO J. 1988 Jan;7(1):147–154. doi: 10.1002/j.1460-2075.1988.tb02794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin A., Chan Y. L., McNally J., Peleg D., Meyuhas O., Wool I. G. The primary structure of rat ribosomal protein L7. The presence near the amino terminus of L7 of five tandem repeats of a sequence of 12 amino acids. J Biol Chem. 1987 Sep 15;262(26):12665–12671. [PubMed] [Google Scholar]
- Martin-Zanca D., Hughes S. H., Barbacid M. A human oncogene formed by the fusion of truncated tropomyosin and protein tyrosine kinase sequences. 1986 Feb 27-Mar 5Nature. 319(6056):743–748. doi: 10.1038/319743a0. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Mitra G., Long L. K., Barbacid M. Molecular characterization of the human trk oncogene. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):983–992. doi: 10.1101/sqb.1986.051.01.112. [DOI] [PubMed] [Google Scholar]
- Martin-Zanca D., Oskam R., Mitra G., Copeland T., Barbacid M. Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol. 1989 Jan;9(1):24–33. doi: 10.1128/mcb.9.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey E. H., Bielka H., Gordon J., Lastick S. M., Lin A., Ogata K., Reboud J. P., Traugh J. A., Traut R. R., Warner J. R. Proposed uniform nomenclature for mammalian ribosomal proteins. Mol Gen Genet. 1979 Jan 16;169(1):1–6. doi: 10.1007/BF00267538. [DOI] [PubMed] [Google Scholar]
- Mitra G., Martin-Zanca D., Barbacid M. Identification and biochemical characterization of p70TRK, product of the human TRK oncogene. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6707–6711. doi: 10.1073/pnas.84.19.6707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neckameyer W. S., Shibuya M., Hsu M. T., Wang L. H. Proto-oncogene c-ros codes for a molecule with structural features common to those of growth factor receptors and displays tissue specific and developmentally regulated expression. Mol Cell Biol. 1986 May;6(5):1478–1486. doi: 10.1128/mcb.6.5.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskam R., Coulier F., Ernst M., Martin-Zanca D., Barbacid M. Frequent generation of oncogenes by in vitro recombination of TRK protooncogene sequences. Proc Natl Acad Sci U S A. 1988 May;85(9):2964–2968. doi: 10.1073/pnas.85.9.2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Van de Ven W. J., Blomberg J., Stephenson J. R. Differences in mechanisms of transformation by independent feline sarcoma virus isolates. J Virol. 1981 Jun;38(3):1084–1089. doi: 10.1128/jvi.38.3.1084-1089.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Siegmann M., Thomas G. Separation of multiple phosphorylated forms of 40 S ribosomal protein S6 by two-dimensional polyacrylamide gel electrophoresis. Methods Enzymol. 1987;146:362–369. doi: 10.1016/s0076-6879(87)46037-0. [DOI] [PubMed] [Google Scholar]
- Terao K., Ogata K. Studies on structural proteins of the rat liver ribosomes. I. Molecular wights of the proteins of large and small subunits. Biochim Biophys Acta. 1975 Aug 21;402(2):214–229. doi: 10.1016/0005-2787(75)90041-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Geissler A. W., Lin A., Wool I. G. Sequence of the amino-terminal region of rat liver ribosomal proteins S4, S6, S8, L6, L7a, L18, L27, L30, L37, L37a, and L39. J Supramol Struct. 1979;12(4):425–433. doi: 10.1002/jss.400120403. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Ullrich A. Growth factor receptor tyrosine kinases. Annu Rev Biochem. 1988;57:443–478. doi: 10.1146/annurev.bi.57.070188.002303. [DOI] [PubMed] [Google Scholar]
- Ziemiecki A., Hennig D., Gardner L., Ferdinand F. J., Friis R. R., Bauer H., Pedersen N. C., Johnson L., Theilen G. H. Biological and biochemical characterization of a new isolate of feline sarcoma virus: Theilen-Pedersen (TP1-FeSV). Virology. 1984 Oct 30;138(2):324–331. doi: 10.1016/0042-6822(84)90355-6. [DOI] [PubMed] [Google Scholar]