Abstract
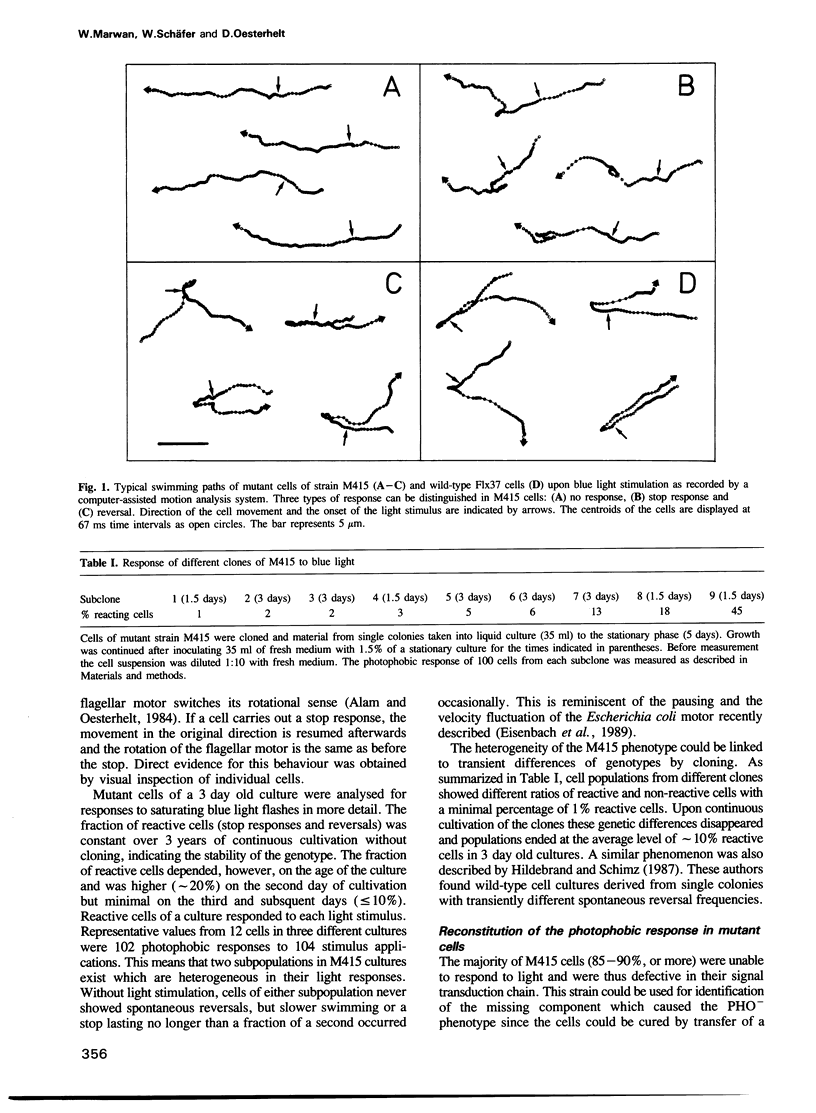
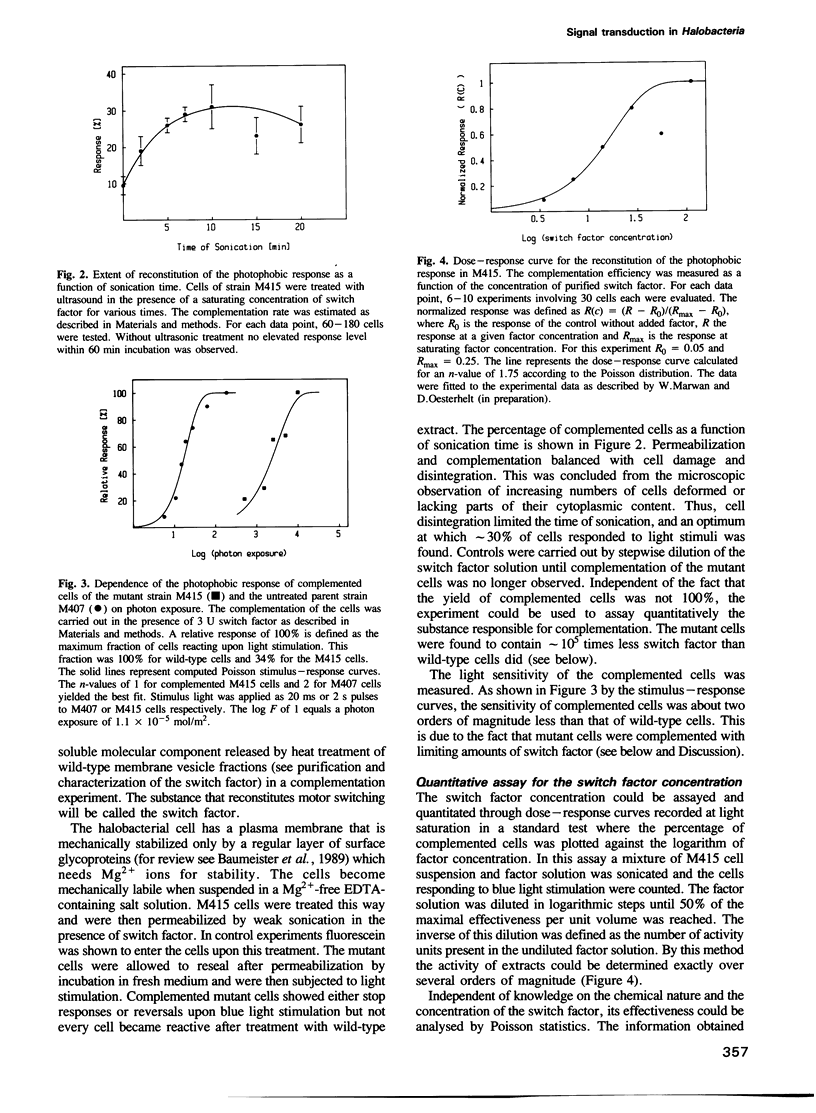
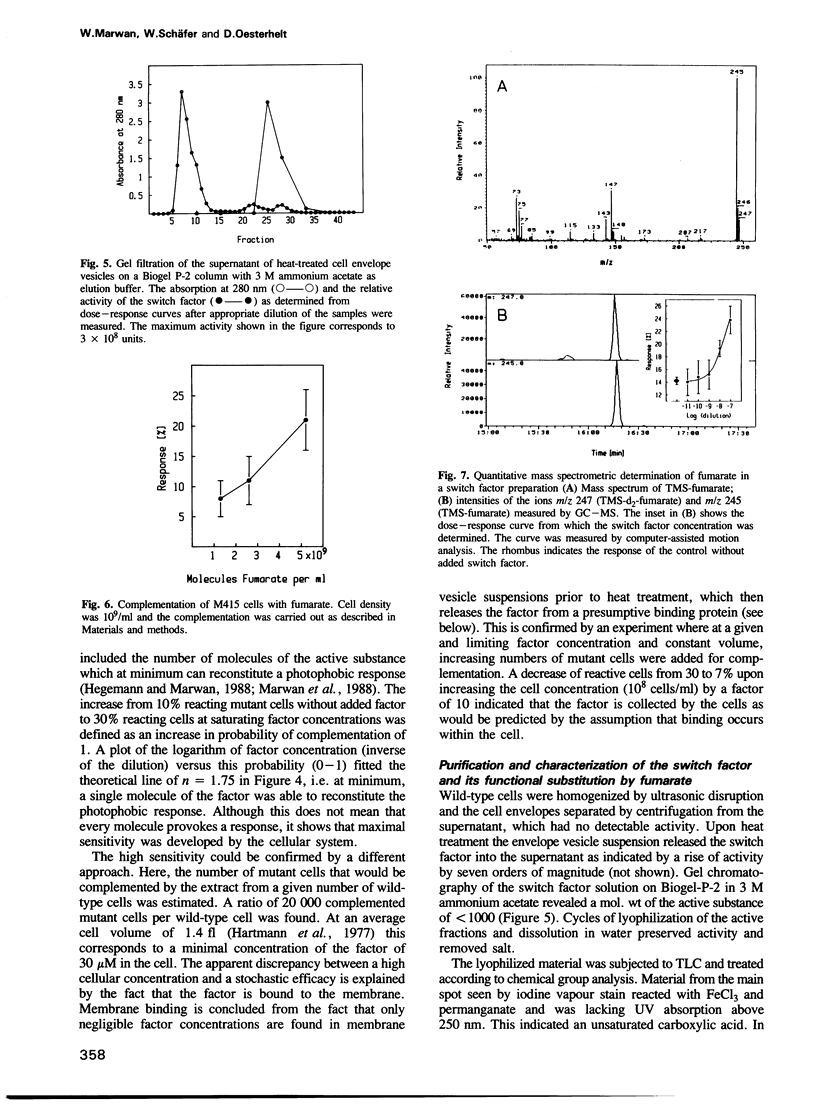
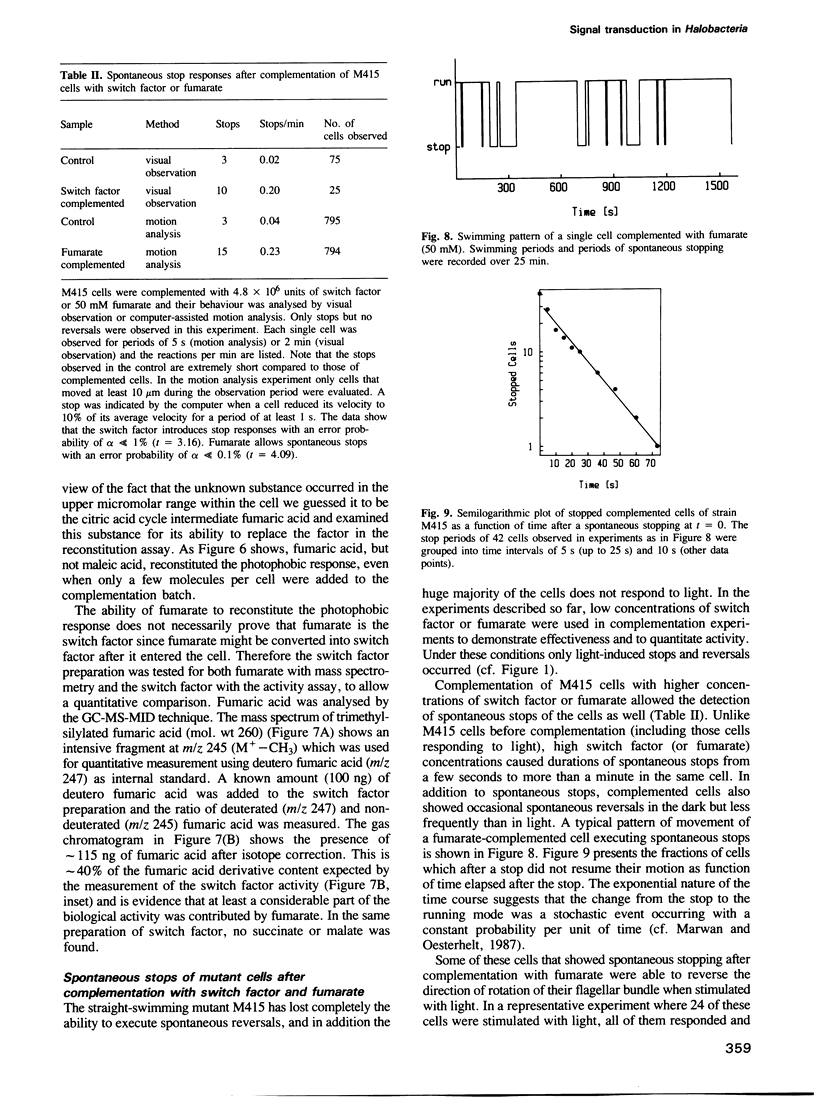
The isolation of a straight-swimming mutant of Halobacterium halobium is reported which has a defect in switching the rotational sense of its flagellar motor. Cells of this mutant strain could be complemented with an extract from wild-type cells by mild sonication and resealing of the cells in fresh medium. The switch factor responsible for restoration of wild-type behaviour was isolated from membrane vesicle preparations. Its chemical nature is proposed to be that of fumarate on the basis of chemical, chromatographic and mass spectrometric analysis. Since the switch factor (fumarate) was released from a membrane-bound state by heat and was accumulated into mutant cells that lack this compound, it is proposed that a membrane-bound protein exists which specifically binds the switch factor. Both the switch factor and fumarate cause stimulus-induced responses in cells at the level of one or few molecules.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam M., Lebert M., Oesterhelt D., Hazelbauer G. L. Methyl-accepting taxis proteins in Halobacterium halobium. EMBO J. 1989 Feb;8(2):631–639. doi: 10.1002/j.1460-2075.1989.tb03418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam M., Oesterhelt D. Morphology, function and isolation of halobacterial flagella. J Mol Biol. 1984 Jul 15;176(4):459–475. doi: 10.1016/0022-2836(84)90172-4. [DOI] [PubMed] [Google Scholar]
- Baumeister W., Wildhaber I., Phipps B. M. Principles of organization in eubacterial and archaebacterial surface proteins. Can J Microbiol. 1989 Jan;35(1):215–227. doi: 10.1139/m89-034. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Sickinger H. D., Oesterhelt D. Quantitative aspects of energy conversion in halobacteria. FEBS Lett. 1977 Oct 1;82(1):1–6. doi: 10.1016/0014-5793(77)80873-9. [DOI] [PubMed] [Google Scholar]
- Hildebrand E., Dencher N. Two photosystems controlling behavioural responses of Halobacterium halobium. Nature. 1975 Sep 4;257(5521):46–48. doi: 10.1038/257046a0. [DOI] [PubMed] [Google Scholar]
- Hildebrand E., Schimz A. Role of the response oscillator in inverse responses of Halobacterium halobium to weak light stimuli. J Bacteriol. 1987 Jan;169(1):254–259. doi: 10.1128/jb.169.1.254-259.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus I. R., Welch M., Eisenbach M. Pausing of flagellar rotation is a component of bacterial motility and chemotaxis. J Bacteriol. 1988 Aug;170(8):3627–3632. doi: 10.1128/jb.170.8.3627-3632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson M. D., Tedesco P. M., Berg H. C. Energetics of flagellar rotation in bacteria. J Mol Biol. 1980 Apr 15;138(3):541–561. doi: 10.1016/s0022-2836(80)80017-9. [DOI] [PubMed] [Google Scholar]
- Marwan W., Hegemann P., Oesterhelt D. Single photon detection by an archaebacterium. J Mol Biol. 1988 Feb 20;199(4):663–664. doi: 10.1016/0022-2836(88)90309-9. [DOI] [PubMed] [Google Scholar]
- Marwan W., Oesterhelt D. Signal formation in the halobacterial photophobic response mediated by a fourth retinal protein (P480). J Mol Biol. 1987 May 20;195(2):333–342. doi: 10.1016/0022-2836(87)90654-1. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Krippahl G. Phototrophic growth of halobacteria and its use for isolation of photosynthetically-deficient mutants. Ann Microbiol (Paris) 1983 Jul-Aug;134B(1):137–150. doi: 10.1016/s0769-2609(83)80101-x. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Marwan W. Change of membrane potential is not a component of the photophobic transduction chain in Halobacterium halobium. J Bacteriol. 1987 Aug;169(8):3515–3520. doi: 10.1128/jb.169.8.3515-3520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J. Two pumps, one principle: light-driven ion transport in halobacteria. Trends Biochem Sci. 1989 Feb;14(2):57–61. doi: 10.1016/0968-0004(89)90044-3. [DOI] [PubMed] [Google Scholar]
- Schegk E. S., Oesterhelt D. Isolation of a prokaryotic photoreceptor: sensory rhodopsin from halobacteria. EMBO J. 1988 Sep;7(9):2925–2933. doi: 10.1002/j.1460-2075.1988.tb03151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimz A., Hildebrand E. Chemosensory responses of Halobacterium halobium. J Bacteriol. 1979 Dec;140(3):749–753. doi: 10.1128/jb.140.3.749-753.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimz A. Methylation of membrane proteins is involved in chemosensory and photosensory behavior of Halobacterium halobium. FEBS Lett. 1981 Mar 23;125(2):205–207. doi: 10.1016/0014-5793(81)80719-3. [DOI] [PubMed] [Google Scholar]
- Spudich E. N., Spudich J. L. Control of transmembrane ion fluxes to select halorhodopsin-deficient and other energy-transduction mutants of Halobacterium halobium. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4308–4312. doi: 10.1073/pnas.79.14.4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Bogomolni R. A. Sensory rhodopsins of halobacteria. Annu Rev Biophys Biophys Chem. 1988;17:193–215. doi: 10.1146/annurev.bb.17.060188.001205. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Wolff E. K., Hess B. A rapid population method for action spectra applied to Halobacterium halobium. J Bacteriol. 1988 Jun;170(6):2790–2795. doi: 10.1128/jb.170.6.2790-2795.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundberg S. A., Bogomolni R. A., Spudich J. L. Selection and properties of phototaxis-deficient mutants of Halobacterium halobium. J Bacteriol. 1985 Oct;164(1):282–287. doi: 10.1128/jb.164.1.282-287.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]


