Abstract
We have analyzed t(1;14)(p32;q11) chromosome translocations from two patients with T cell acute lymphocytic leukemia. The chromosome 1 breakpoints of these patients lie within a kilobasepair of each other, and thus define a genetic locus (designated tal) involved in T cell oncogenesis. Moreover, we have identified sequences within tal that potentially encode an amphipathic helix-loop-helix motif, a DNA-binding domain found in a variety of proteins that control cell growth and differentiation. The homology domain of tal is especially related to that of lyl-1, a gene on chromosome 19 that has also been implicated in T cell oncogenesis. Hence, tal and lyl-1 encode a distinct family of helix-loop-helix proteins involved in the malignant development of lymphocytes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso M. C., Cabrera C. V. The achaete-scute gene complex of Drosophila melanogaster comprises four homologous genes. EMBO J. 1988 Aug;7(8):2585–2591. doi: 10.1002/j.1460-2075.1988.tb03108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., DePinho R., Zimmerman K., Legouy E., Hatton K., Ferrier P., Tesfaye A., Yancopoulos G., Nisen P. The human myc gene family. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):931–941. doi: 10.1101/sqb.1986.051.01.106. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Boehm T., Yssel H., Spits H., Rabbitts T. H. Complex rearrangements within the human J delta-C delta/J alpha-C alpha locus and aberrant recombination between J alpha segments. EMBO J. 1988 Jun;7(6):1661–1668. doi: 10.1002/j.1460-2075.1988.tb02993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Chen K. C., Smith S. D., Rabbitts T. H. Fusion of an immunoglobulin variable gene and a T cell receptor constant gene in the chromosome 14 inversion associated with T cell tumors. Cell. 1985 Dec;43(3 Pt 2):705–713. doi: 10.1016/0092-8674(85)90243-0. [DOI] [PubMed] [Google Scholar]
- Begley C. G., Aplan P. D., Davey M. P., Nakahara K., Tchorz K., Kurtzberg J., Hershfield M. S., Haynes B. F., Cohen D. I., Waldmann T. A. Chromosomal translocation in a human leukemic stem-cell line disrupts the T-cell antigen receptor delta-chain diversity region and results in a previously unreported fusion transcript. Proc Natl Acad Sci U S A. 1989 Mar;86(6):2031–2035. doi: 10.1073/pnas.86.6.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Boehm T., Baer R., Lavenir I., Forster A., Waters J. J., Nacheva E., Rabbitts T. H. The mechanism of chromosomal translocation t(11;14) involving the T-cell receptor C delta locus on human chromosome 14q11 and a transcribed region of chromosome 11p15. EMBO J. 1988 Feb;7(2):385–394. doi: 10.1002/j.1460-2075.1988.tb02825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm T., Buluwela L., Williams D., White L., Rabbitts T. H. A cluster of chromosome 11p13 translocations found via distinct D-D and D-D-J rearrangements of the human T cell receptor delta chain gene. EMBO J. 1988 Jul;7(7):2011–2017. doi: 10.1002/j.1460-2075.1988.tb03040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera C. V., Martinez-Arias A., Bate M. The expression of three members of the achaete-scute gene complex correlates with neuroblast segregation in Drosophila. Cell. 1987 Jul 31;50(3):425–433. doi: 10.1016/0092-8674(87)90496-x. [DOI] [PubMed] [Google Scholar]
- Caudy M., Vässin H., Brand M., Tuma R., Jan L. Y., Jan Y. N. daughterless, a Drosophila gene essential for both neurogenesis and sex determination, has sequence similarities to myc and the achaete-scute complex. Cell. 1988 Dec 23;55(6):1061–1067. doi: 10.1016/0092-8674(88)90250-4. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Dubé I. D., Raimondi S. C., Pi D., Kalousek D. K. A new translocation, t(10;14)(q24;q11), in T cell neoplasia. Blood. 1986 Apr;67(4):1181–1184. [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finger L. R., Harvey R. C., Moore R. C., Showe L. C., Croce C. M. A common mechanism of chromosomal translocation in T- and B-cell neoplasia. Science. 1986 Nov 21;234(4779):982–985. doi: 10.1126/science.3490692. [DOI] [PubMed] [Google Scholar]
- Finger L. R., Kagan J., Christopher G., Kurtzberg J., Hershfield M. S., Nowell P. C., Croce C. M. Involvement of the TCL5 gene on human chromosome 1 in T-cell leukemia and melanoma. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5039–5043. doi: 10.1073/pnas.86.13.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht F., Morgan R., Hecht B. K., Smith S. D. Common region on chromosome 14 in T-cell leukemia and lymphoma. Science. 1984 Dec 21;226(4681):1445–1447. doi: 10.1126/science.6438800. [DOI] [PubMed] [Google Scholar]
- Hershfield M. S., Kurtzberg J., Harden E., Moore J. O., Whang-Peng J., Haynes B. F. Conversion of a stem cell leukemia from a T-lymphoid to a myeloid phenotype induced by the adenosine deaminase inhibitor 2'-deoxycoformycin. Proc Natl Acad Sci U S A. 1984 Jan;81(1):253–257. doi: 10.1073/pnas.81.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J., Finan J., Letofsky J., Besa E. C., Nowell P. C., Croce C. M. Alpha-chain locus of the T-cell antigen receptor is involved in the t(10;14) chromosome translocation of T-cell acute lymphocytic leukemia. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4543–4546. doi: 10.1073/pnas.84.13.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Matthes H. W., Gait M. J., Brenner S. A new selective phage cloning vector, lambda 2001, with sites for XbaI, BamHI, HindIII, EcoRI, SstI and XhoI. Gene. 1984 Dec;32(1-2):217–224. doi: 10.1016/0378-1119(84)90049-0. [DOI] [PubMed] [Google Scholar]
- Klämbt C., Knust E., Tietze K., Campos-Ortega J. A. Closely related transcripts encoded by the neurogenic gene complex enhancer of split of Drosophila melanogaster. EMBO J. 1989 Jan;8(1):203–210. doi: 10.1002/j.1460-2075.1989.tb03365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Kurtzberg J., Bigner S. H., Hershfield M. S. Establishment of the DU.528 human lymphohemopoietic stem cell line. J Exp Med. 1985 Nov 1;162(5):1561–1578. doi: 10.1084/jem.162.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampert F., Harbott J., Ritterbach J., Ludwig W. D., Fonatsch C., Schwamborn D., Stier B., Gnekow A., Gerein V., Stollmann B. T-cell acute childhood lymphoblastic leukemia with chromosome 14 q 11 anomaly: a morphologic, immunologic, and cytogenetic analysis of 10 patients. Blut. 1988 Mar;56(3):117–123. doi: 10.1007/BF00320016. [DOI] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Mathieu-Mahul D., Bernheim A., Sigaux F., Daniel M. T., Larsen C. J., Berger R. Translocation t(1; 14) et remaniement du gène de la chaîne alpha du récepteur T dans une leucémie aiguë lymphoblastique T. C R Acad Sci III. 1986;302(14):525–528. [PubMed] [Google Scholar]
- McKeithan T. W., Shima E. A., Le Beau M. M., Minowada J., Rowley J. D., Diaz M. O. Molecular cloning of the breakpoint junction of a human chromosomal 8;14 translocation involving the T-cell receptor alpha-chain gene and sequences on the 3' side of MYC. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6636–6640. doi: 10.1073/pnas.83.17.6636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
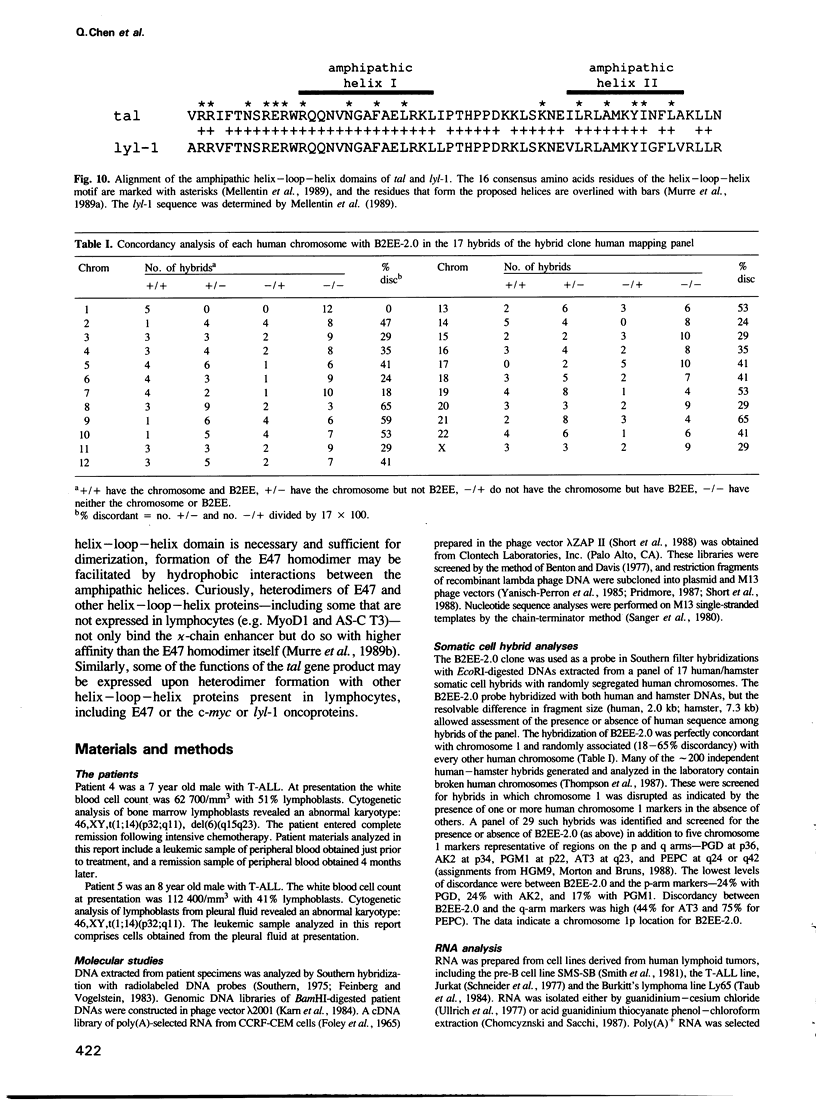
- Mellentin J. D., Smith S. D., Cleary M. L. lyl-1, a novel gene altered by chromosomal translocation in T cell leukemia, codes for a protein with a helix-loop-helix DNA binding motif. Cell. 1989 Jul 14;58(1):77–83. doi: 10.1016/0092-8674(89)90404-2. [DOI] [PubMed] [Google Scholar]
- Morton N. E., Bruns G. A. Report of the committee on the genetic-constitution of chromosomes 1 and 2. Cytogenet Cell Genet. 1987;46(1-4):102–130. doi: 10.1159/000132473. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Pearson-White S. H., Konieczny S. F., Latham K. E., Emerson C. P., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell. 1988 Jun 3;53(5):781–793. doi: 10.1016/0092-8674(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Pridmore R. D. New and versatile cloning vectors with kanamycin-resistance marker. Gene. 1987;56(2-3):309–312. doi: 10.1016/0378-1119(87)90149-1. [DOI] [PubMed] [Google Scholar]
- Raimondi S. C., Behm F. G., Roberson P. K., Pui C. H., Rivera G. K., Murphy S. B., Williams D. L. Cytogenetics of childhood T-cell leukemia. Blood. 1988 Nov;72(5):1560–1566. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Schneider U., Schwenk H. U., Bornkamm G. Characterization of EBV-genome negative "null" and "T" cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977 May 15;19(5):621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- Shima E. A., Le Beau M. M., McKeithan T. W., Minowada J., Showe L. C., Mak T. W., Minden M. D., Rowley J. D., Diaz M. O. Gene encoding the alpha chain of the T-cell receptor is moved immediately downstream of c-myc in a chromosomal 8;14 translocation in a cell line from a human T-cell leukemia. Proc Natl Acad Sci U S A. 1986 May;83(10):3439–3443. doi: 10.1073/pnas.83.10.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. G., Dev V. G., Shannon W. A., Jr Characterization of a novel human pre-B leukemia cell line. J Immunol. 1981 Feb;126(2):596–602. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stallings R. L., Olson E., Strauss A. W., Thompson L. H., Bachinski L. L., Siciliano M. J. Human creatine kinase genes on chromosomes 15 and 19, and proximity of the gene for the muscle form to the genes for apolipoprotein C2 and excision repair. Am J Hum Genet. 1988 Aug;43(2):144–151. [PMC free article] [PubMed] [Google Scholar]
- Taub R., Moulding C., Battey J., Murphy W., Vasicek T., Lenoir G. M., Leder P. Activation and somatic mutation of the translocated c-myc gene in burkitt lymphoma cells. Cell. 1984 Feb;36(2):339–348. doi: 10.1016/0092-8674(84)90227-7. [DOI] [PubMed] [Google Scholar]
- Thayer M. J., Tapscott S. J., Davis R. L., Wright W. E., Lassar A. B., Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989 Jul 28;58(2):241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- Thisse B., Stoetzel C., Gorostiza-Thisse C., Perrin-Schmitt F. Sequence of the twist gene and nuclear localization of its protein in endomesodermal cells of early Drosophila embryos. EMBO J. 1988 Jul;7(7):2175–2183. doi: 10.1002/j.1460-2075.1988.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Carrano A. V., Sato K., Salazar E. P., White B. F., Stewart S. A., Minkler J. L., Siciliano M. J. Identification of nucleotide-excision-repair genes on human chromosomes 2 and 13 by functional complementation in hamster-human hybrids. Somat Cell Mol Genet. 1987 Sep;13(5):539–551. doi: 10.1007/BF01534495. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Villares R., Cabrera C. V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987 Jul 31;50(3):415–424. doi: 10.1016/0092-8674(87)90495-8. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Look A. T., Melvin S. L., Roberson P. K., Dahl G., Flake T., Stass S. New chromosomal translocations correlate with specific immunophenotypes of childhood acute lymphoblastic leukemia. Cell. 1984 Jan;36(1):101–109. doi: 10.1016/0092-8674(84)90078-3. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]





