Abstract
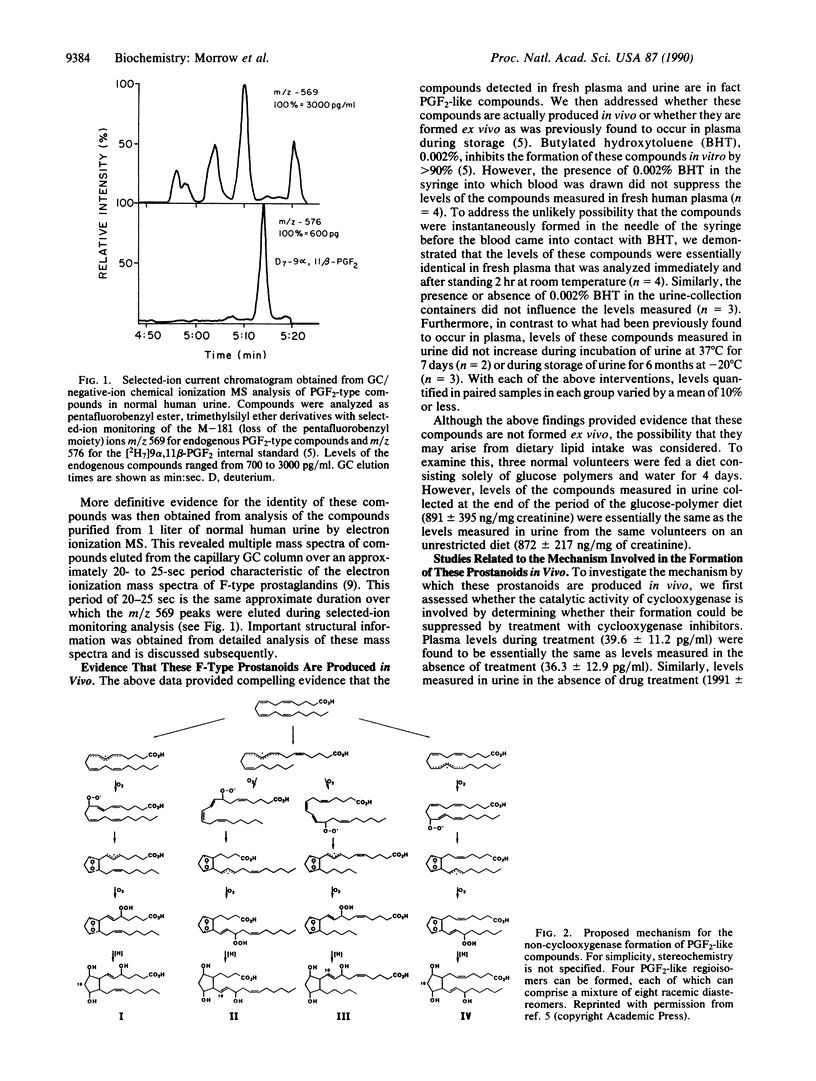
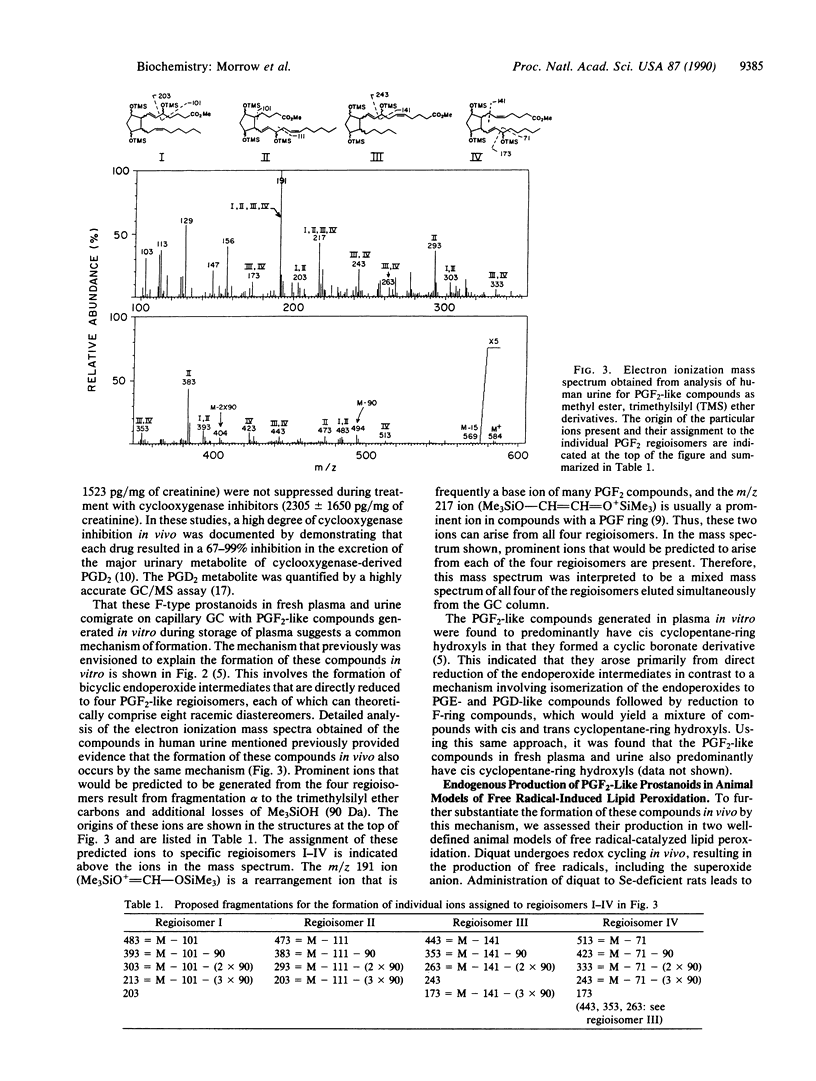
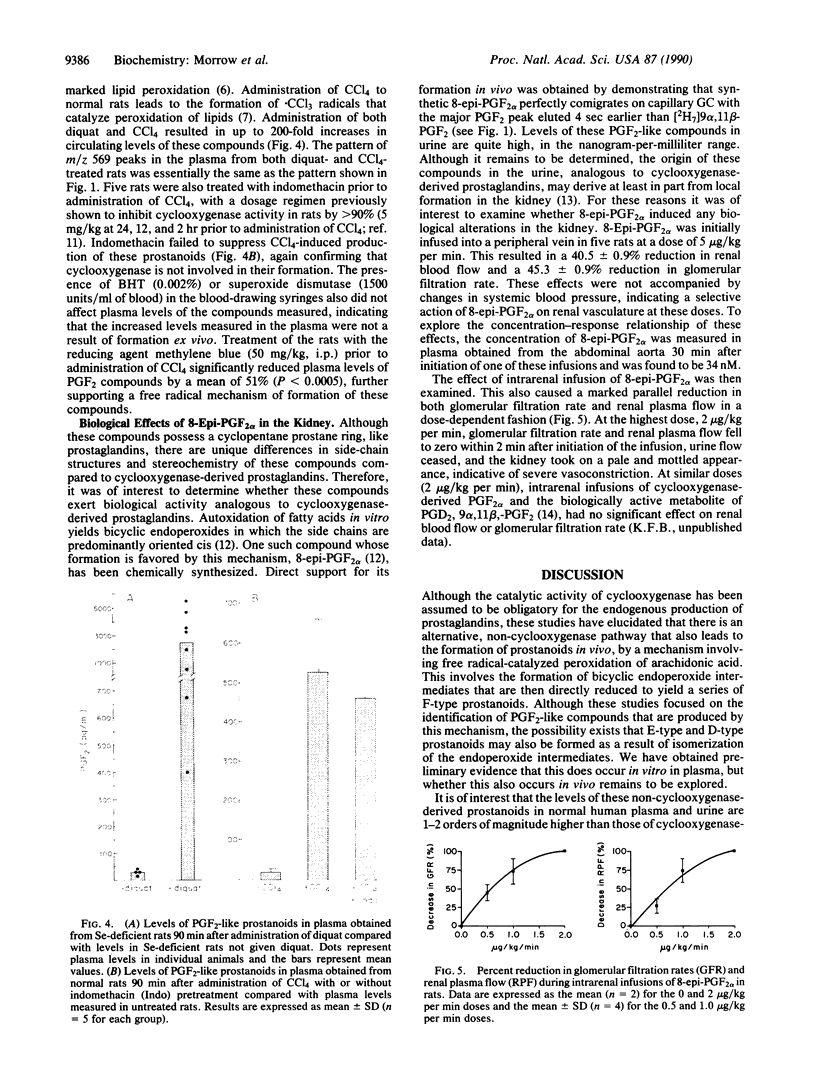
Increasing attention has focused on the role of free radicals derived from oxygen in the pathophysiology of a wide variety of disorders. One of the well-recognized targets of free radical-induced injury is peroxidation of lipids. Using a variety of approaches, we have found that a series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase mechanism involving free radical-catalyzed peroxidation of arachidonic acid. Levels of these compounds in normal human plasma and urine range from 5 to 40 pg/ml and 500 to 4000 pg/mg of creatinine, respectively. In rats, their formation was found to increase as much as 200-fold in association with marked free radical-catalyzed lipid peroxidation induced by administration of CCl4 and diquat. To explore whether these prostanoids can exert biological activity, the effects of one of the compounds formed by this mechanism, 8-epi-prostaglandin F2 alpha, was examined in the kidney in the rat. Infusion of 8-epi-prostaglandin F2 alpha into a peripheral vein (5 micrograms/kg per min) or intrarenally (0.5-2.0 micrograms/kg per min) resulted in marked parallel reductions in renal blood flow and glomerular filtration rate. That the formation of these prostanoids is catalyzed by free radicals and that they can exert potent biological activity suggest that these prostanoids may participate as pathophysiological mediators in oxidant injury. Quantification of these compounds may also provide a noninvasive approach to assess oxidant status in humans. That the formation of these prostanoids occurs independent of the catalytic activity of the cyclooxygenase enzyme suggests that there may be limitations at times regarding the reliability of the use of cyclooxygenase inhibitors to assess the role of prostaglandins in certain pathophysiological processes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badr K. F., DeBoer D. K., Schwartzberg M., Serhan C. N. Lipoxin A4 antagonizes cellular and in vivo actions of leukotriene D4 in rat glomerular mesangial cells: evidence for competition at a common receptor. Proc Natl Acad Sci U S A. 1989 May;86(9):3438–3442. doi: 10.1073/pnas.86.9.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk R. F., Lane J. M. Ethane production and liver necrosis in rats after administration of drugs and other chemicals. Toxicol Appl Pharmacol. 1979 Sep 30;50(3):467–478. doi: 10.1016/0041-008x(79)90400-9. [DOI] [PubMed] [Google Scholar]
- Burk R. F., Lawrence R. A., Lane J. M. Liver necrosis and lipid peroxidation in the rat as the result of paraquat and diquat administration. Effect of selenium deficiency. J Clin Invest. 1980 May;65(5):1024–1031. doi: 10.1172/JCI109754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frölich J. C., Wilson T. W., Sweetman B. J., Smigel M., Nies A. S., Carr K., Watson J. T., Oates J. A. Urinary prostaglandins. Identification and origin. J Clin Invest. 1975 Apr;55(4):763–770. doi: 10.1172/JCI107987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B. The measurement and mechanism of lipid peroxidation in biological systems. Trends Biochem Sci. 1990 Apr;15(4):129–135. doi: 10.1016/0968-0004(90)90206-q. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Grootveld M. The measurement of free radical reactions in humans. Some thoughts for future experimentation. FEBS Lett. 1987 Mar 9;213(1):9–14. doi: 10.1016/0014-5793(87)81455-2. [DOI] [PubMed] [Google Scholar]
- Jackson E. K. Defective modulation of noradrenergic neurotransmission by endogenous prostaglandins in aging spontaneously hypertensive rats. J Pharmacol Exp Ther. 1989 Jul;250(1):9–21. [PubMed] [Google Scholar]
- Liston T. E., Roberts L. J., 2nd Metabolic fate of radiolabeled prostaglandin D2 in a normal human male volunteer. J Biol Chem. 1985 Oct 25;260(24):13172–13180. [PubMed] [Google Scholar]
- Liston T. E., Roberts L. J., 2nd Transformation of prostaglandin D2 to 9 alpha, 11 beta-(15S)-trihydroxyprosta-(5Z,13E)-dien-1-oic acid (9 alpha, 11 beta-prostaglandin F2): a unique biologically active prostaglandin produced enzymatically in vivo in humans. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6030–6034. doi: 10.1073/pnas.82.18.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D., Harris T. M., Roberts L. J., 2nd Noncyclooxygenase oxidative formation of a series of novel prostaglandins: analytical ramifications for measurement of eicosanoids. Anal Biochem. 1990 Jan;184(1):1–10. doi: 10.1016/0003-2697(90)90002-q. [DOI] [PubMed] [Google Scholar]
- Nugteren D. H. The determination of prostaglandin metabolites in human urine. J Biol Chem. 1975 Apr 25;250(8):2808–2812. [PubMed] [Google Scholar]
- Porter N. A., Funk M. O. Letter: Peroxy radical cyclization as a model for prostaglandin biosynthesis. J Org Chem. 1975 Nov 28;40(24):3614–3615. doi: 10.1021/jo00912a037. [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Stanley J. P. Letter: A suggested mechanism for the production of malonaldehyde during the autoxidation of polyunsaturated fatty acids. Nonenzymatic production of prostaglandin endoperoxides during autoxidation. J Org Chem. 1975 Nov 28;40(24):3615–3617. doi: 10.1021/jo00912a038. [DOI] [PubMed] [Google Scholar]



