Abstract
Complementation of a posterior silk gland (psg) extract to a HeLa cell extract specifically enhances the transcription of the Bombyx mori fibroin gene. To map the regions responsible for this enhancement, the fibroin promoter was dissected and the transcriptional function of each region was analyzed. Besides the upstream promoter element 5' to the TATA box, two downstream elements were found to be important for the preferential transcription of the fibroin gene in the complementation system as well as in the psg extract. The minimal fibroin promoter from -37 to +10 (core-promoter) was preferentially transcribed in the psg extract, while the transcription efficiencies of other promoters like one of the Bombyx chorion genes and the adenovirus 2 major late promoter (Ad2MLP) were considerably lower. The transcription from the core-promoter was further enhanced when combined with either the intronic element from +156 to +454 or the upstream element. Both the upstream and intronic elements also stimulated the transcription from the Ad2MLP in an orientation independent manner. These results demonstrate that the transcription of the fibroin gene is mediated through an integration of multiple regulatory elements.
Full text
PDF
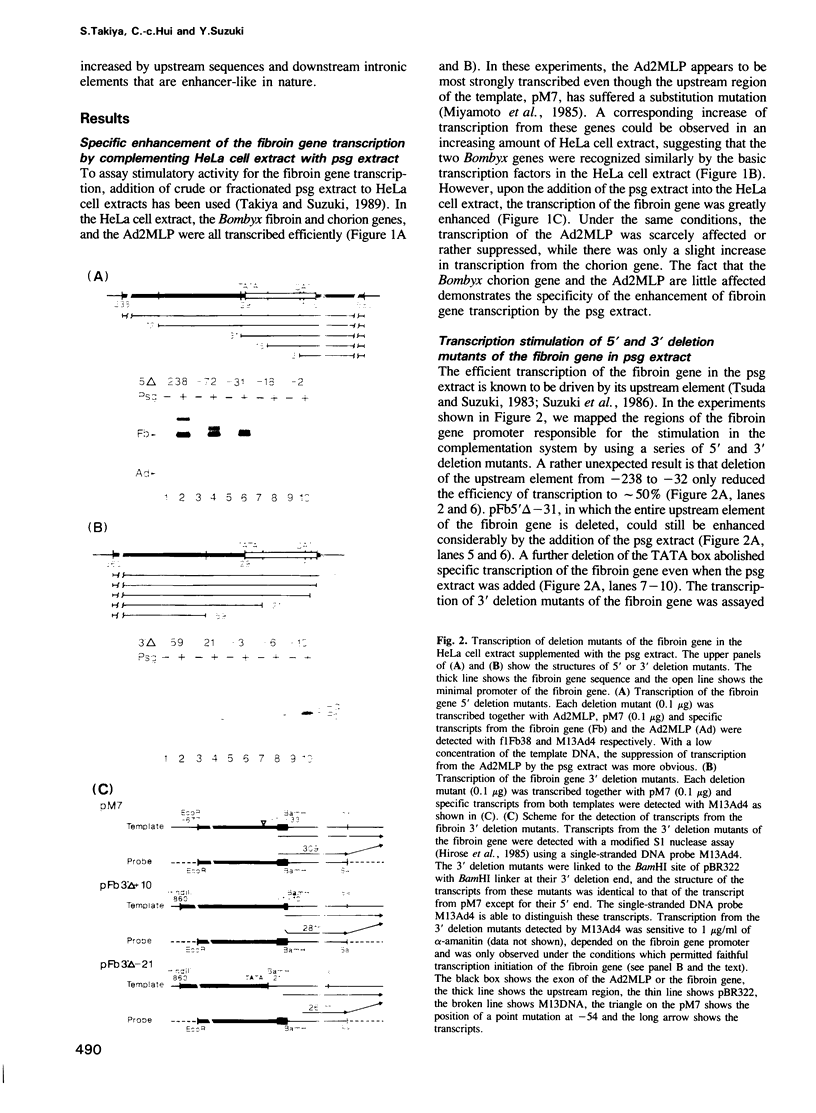





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggin M. D., Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988 Jun 3;53(5):699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- Coen D. M., Weinheimer S. P., McKnight S. L. A genetic approach to promoter recognition during trans induction of viral gene expression. Science. 1986 Oct 3;234(4772):53–59. doi: 10.1126/science.3018926. [DOI] [PubMed] [Google Scholar]
- Couble P., Chevillard M., Moine A., Ravel-Chapuis P., Prudhomme J. C. Structural organization of the P25 gene of Bombyx mori and comparative analysis of its 5' flanking DNA with that of the fibroin gene. Nucleic Acids Res. 1985 Mar 11;13(5):1801–1814. doi: 10.1093/nar/13.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierich A., Gaub M. P., LePennec J. P., Astinotti D., Chambon P. Cell-specificity of the chicken ovalbumin and conalbumin promoters. EMBO J. 1987 Aug;6(8):2305–2312. doi: 10.1002/j.1460-2075.1987.tb02505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
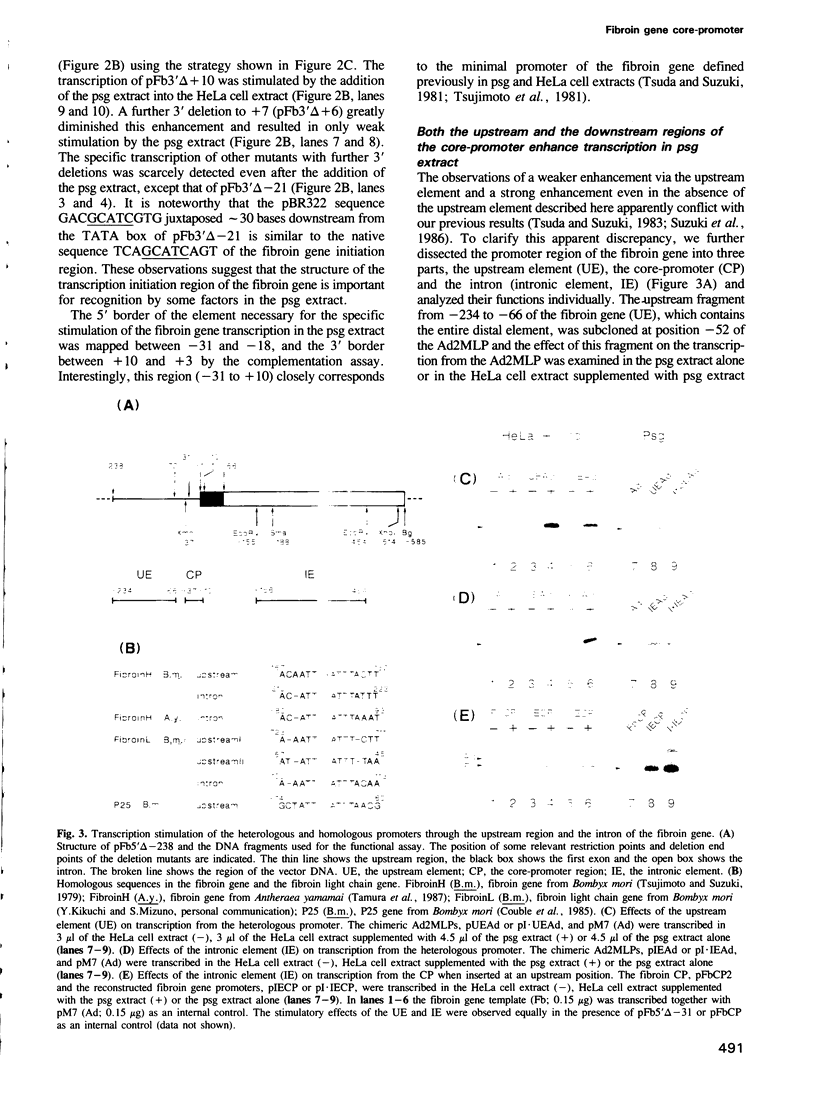
- Fischer J. A., Maniatis T. Drosophila Adh: a promoter element expands the tissue specificity of an enhancer. Cell. 1988 May 6;53(3):451–461. doi: 10.1016/0092-8674(88)90165-1. [DOI] [PubMed] [Google Scholar]
- Garcia J. V., Bich-Thuy L. T., Stafford J., Queen C. Synergism between immunoglobulin enhancers and promoters. Nature. 1986 Jul 24;322(6077):383–385. doi: 10.1038/322383a0. [DOI] [PubMed] [Google Scholar]
- Hirose S., Suzuki Y. In vitro transcription of eukaryotic genes is affected differently by the degree of DNA supercoiling. Proc Natl Acad Sci U S A. 1988 Feb;85(3):718–722. doi: 10.1073/pnas.85.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Takeuchi K., Hori H., Hirose T., Inayama S., Suzuki Y. Contact points between transcription machinery and the fibroin gene promoter deduced by functional tests of single-base substitution mutants. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1394–1397. doi: 10.1073/pnas.81.5.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Takeuchi K., Suzuki Y. In vitro characterization of the fibroin gene promoter by the use of single-base substitution mutants. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7258–7262. doi: 10.1073/pnas.79.23.7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose S., Tsuda M., Suzuki Y. Enhanced transcription of fibroin gene in vitro on covalently closed circular templates. J Biol Chem. 1985 Sep 5;260(19):10557–10562. [PubMed] [Google Scholar]
- Homa F. L., Glorioso J. C., Levine M. A specific 15-bp TATA box promoter element is required for expression of a herpes simplex virus type 1 late gene. Genes Dev. 1988 Jan;2(1):40–53. doi: 10.1101/gad.2.1.40. [DOI] [PubMed] [Google Scholar]
- Homa F. L., Otal T. M., Glorioso J. C., Levine M. Transcriptional control signals of a herpes simplex virus type 1 late (gamma 2) gene lie within bases -34 to +124 relative to the 5' terminus of the mRNA. Mol Cell Biol. 1986 Nov;6(11):3652–3666. doi: 10.1128/mcb.6.11.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horikoshi M., Carey M. F., Kakidani H., Roeder R. G. Mechanism of action of a yeast activator: direct effect of GAL4 derivatives on mammalian TFIID-promoter interactions. Cell. 1988 Aug 26;54(5):665–669. doi: 10.1016/s0092-8674(88)80011-4. [DOI] [PubMed] [Google Scholar]
- Horikoshi M., Hai T., Lin Y. S., Green M. R., Roeder R. G. Transcription factor ATF interacts with the TATA factor to facilitate establishment of a preinitiation complex. Cell. 1988 Sep 23;54(7):1033–1042. doi: 10.1016/0092-8674(88)90118-3. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Klemenz R., Gehring W. J. Translational and transcriptional control elements in the untranslated leader of the heat-shock gene hsp22. Cell. 1986 Feb 14;44(3):429–438. doi: 10.1016/0092-8674(86)90464-2. [DOI] [PubMed] [Google Scholar]
- Manley J. L., Fire A., Cano A., Sharp P. A., Gefter M. L. DNA-dependent transcription of adenovirus genes in a soluble whole-cell extract. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3855–3859. doi: 10.1073/pnas.77.7.3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsialis S. A., Kafatos F. C. Regulatory elements controlling chorion gene expression are conserved between flies and moths. Nature. 1985 Oct 3;317(6036):453–456. doi: 10.1038/317453a0. [DOI] [PubMed] [Google Scholar]
- Miyamoto N. G., Moncollin V., Egly J. M., Chambon P. Specific interaction between a transcription factor and the upstream element of the adenovirus-2 major late promoter. EMBO J. 1985 Dec 16;4(13A):3563–3570. doi: 10.1002/j.1460-2075.1985.tb04118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N. G., Moncollin V., Wintzerith M., Hen R., Egly J. M., Chambon P. Stimulation of in vitro transcription by the upstream element of the adenovirus-2 major late promoter involves a specific factor. Nucleic Acids Res. 1984 Dec 11;12(23):8779–8799. doi: 10.1093/nar/12.23.8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins K. K., Dailey G. M., Tjian R. In vitro analysis of the Antennapedia P2 promoter: identification of a new Drosophila transcription factor. Genes Dev. 1988 Dec;2(12A):1615–1626. doi: 10.1101/gad.2.12a.1615. [DOI] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Paterson B. M. Cell cycle regulation of a mouse histone H4 gene requires the H4 promoter. Mol Cell Biol. 1987 Mar;7(3):1048–1054. doi: 10.1128/mcb.7.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapira M., Homa F. L., Glorioso J. C., Levine M. Regulation of the herpes simplex virus type 1 late (gamma 2) glycoprotein C gene: sequences between base pairs -34 to +29 control transient expression and responsiveness to transactivation by the products of the immediate early (alpha) 4 and 0 genes. Nucleic Acids Res. 1987 Apr 10;15(7):3097–3111. doi: 10.1093/nar/15.7.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. C., Fisch T. M., Benecke B. J., Nevins J. R., Heintz N. Definition of multiple, functionally distinct TATA elements, one of which is a target in the hsp70 promoter for E1A regulation. Cell. 1988 Mar 11;52(5):723–729. doi: 10.1016/0092-8674(88)90410-2. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Soeller W. C., Poole S. J., Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988 Jan;2(1):68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Suzuki Y. Interaction of composite protein complex with the fibroin enhancer sequence. J Biol Chem. 1988 Apr 25;263(12):5979–5986. [PubMed] [Google Scholar]
- Suzuki Y., Tsuda M., Takiya S., Hirose S., Suzuki E., Kameda M., Ninaki O. Tissue-specific transcription enhancement of the fibroin gene characterized by cell-free systems. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9522–9526. doi: 10.1073/pnas.83.24.9522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiya S., Suzuki Y. Factors involved in preferential transcription of the fibroin gene. Eur J Biochem. 1989 Jan 15;179(1):1–9. doi: 10.1111/j.1432-1033.1989.tb14513.x. [DOI] [PubMed] [Google Scholar]
- Takiya S., Takahashi K., Iwabuchi M., Suzuki Y. Efficiency of in vitro transcription of Dictyostelium discoideum actin gene is affected by the nucleotide sequence of the transcription initiation region. Biochemistry. 1985 Feb 12;24(4):1040–1047. doi: 10.1021/bi00325a036. [DOI] [PubMed] [Google Scholar]
- Talkington C. A., Leder P. Rescuing the in vitro function of a globin pseudogene promoter. Nature. 1982 Jul 8;298(5870):192–195. doi: 10.1038/298192a0. [DOI] [PubMed] [Google Scholar]
- Theill L. E., Wiborg O., Vuust J. Cell-specific expression of the human gastrin gene: evidence for a control element located downstream of the TATA box. Mol Cell Biol. 1987 Dec;7(12):4329–4336. doi: 10.1128/mcb.7.12.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga K., Hirose S., Suzuki Y. In monkey COS cells only the TATA box and the cap site region are required for faithful and efficient initiation of the fibroin gene transcription. Nucleic Acids Res. 1984 Feb 10;12(3):1543–1558. doi: 10.1093/nar/12.3.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Ohshima Y., Suzuki Y. Assumed initiation site of fibroin gene transcription. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4872–4876. doi: 10.1073/pnas.76.10.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M., Suzuki Y. Faithful transcription initiation of fibroin gene in a homologous cell-free system reveals an enhancing effect of 5' flanking sequence far upstream. Cell. 1981 Nov;27(1 Pt 2):175–182. doi: 10.1016/0092-8674(81)90371-8. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Suzuki Y. Transcription modulation in vitro of the fibroin gene exerted by a 200-base-pair region upstream from the "TATA" box. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7442–7446. doi: 10.1073/pnas.80.24.7442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Hirose S., Tsuda M., Suzuki Y. Promoter sequence of fibroin gene assigned by in vitro transcription system. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4838–4842. doi: 10.1073/pnas.78.8.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Suzuki Y. The DNA sequence of Bombyx mori fibroin gene including the 5' flanking, mRNA coding, entire intervening and fibroin protein coding regions. Cell. 1979 Oct;18(2):591–600. doi: 10.1016/0092-8674(79)90075-8. [DOI] [PubMed] [Google Scholar]
- Zajchowski D. A., Boeuf H., Kédinger C. E1a inducibility of the adenoviral early E2a promoter is determined by specific combinations of sequence elements. Gene. 1987;58(2-3):243–256. doi: 10.1016/0378-1119(87)90379-9. [DOI] [PubMed] [Google Scholar]