Abstract
Background and Purpose
Dysregulation of the miR-15a/16-1 cluster in plasma has been reported in stroke patients as a potential biomarker for diagnostic and prognostic use. However, the essential role and therapeutic potential of the miR-15a/16-1 cluster in ischemic stroke are poorly understood. This study is aimed at investigating the regulatory role of the miR-15a/16-1 cluster in ischemic brain injury and insight mechanisms.
Methods
Adult male miR-15a/16-1 knockout and wild-type mice, or adult male C57 BL/6J mice injected via tail vein with the miR-15a/16-1 specific inhibitor (antagomir, 30 pmol/g), were subjected to 1h of middle cerebral artery occlusion (MCAO) and 72h of reperfusion. The neurological scores, brain infarct volume, brain water content, and neurobehavioral tests were then evaluated and analyzed. To explore underlying signaling pathways associated with alteration of miR-15a/16-1 activity, major pro-inflammatory cytokines were measured by qPCR or ELISA and anti-apoptotic proteins were examined by western blotting.
Results
Genetic deletion of the miR-15a/16-1 cluster or intravenous delivery of miR-15a/16-1 antagomir significantly reduced cerebral infarct size, decreased brain water content and improved neurological outcomes in stroke mice. Inhibition of miR-15a/16-1 significantly decreased the expression of the pro-inflammatory cytokines IL-6, MCP-1, VCAM-1 and TNF-α, and increased Bcl-2 and Bcl-w levels in the ischemic brain regions.
Conclusions
Our data indicate that pharmacological inhibition of the miR-15a/16-1 cluster reduces ischemic brain injury via both upregulation of anti-apoptotic proteins and suppression of pro-inflammatory molecules. These results suggest that the miR-15a/16-1 cluster is a novel therapeutic target for ischemic stroke.
Keywords: microRNAs, miR-15a/16-1, microRNA antagomir, apoptosis, inflammation, cerebral ischemia
Introduction
MicroRNAs (miRs) are small endogenous RNA molecules (∼21-25 nucleotides) that repress gene translation by hybridizing to 3′-UTRs of one or more mRNAs in a sequence-specific manner.1 By regulating expression of at least one-third of the human genome, miRs play a critical role in cell proliferation and differentiation, apoptosis, metabolism, and other biological processes.1 MiRs have also been implicated in neurological diseases.2, 3 We and others have demonstrated the essential role of miRs in the pathogenesis of ischemic injury in rodent stroke models, suggesting that miRs are potential therapeutic targets.4-8 Indeed, recent in vivo manipulation of cerebral miR activity via intracerebroventricular or intravenous delivery of synthetic miR inhibitors and mimics has strengthened the rationale for the development of miR-based therapeutic drugs to reduce ischemic brain injury and promote post-stroke neurological recovery.9
MiR-15a and miR-16-1 are two highly conserved miRs that are located in a cluster 250bp apart on chromosome 13q14 in humans and only 54bp apart on chromosome 14 in mice. Generally, they act similarly by binding to their common mRNA targets, thus forming both a structural and functional cluster (the miR-15a/16-1 cluster).10, 11 The miR-15a/16-1 cluster was the first identified miR group associated with human carcinogenesis.10 Recently, dysregulation of plasma miR-15a/16-1 levels have been described in stroke patients,12 suggesting the potential for following this miR cluster as diagnostic and prognostic biomarkers. Beyond its use as biomarkers, inhibition of miR-15 has been shown to protect against myocardial infarction (MI),13 with strong findings that several pharmaceutical companies14 consider miR-15 one of the most important targets for miR-based drug development for improving post-MI recovery. However, the functional significance, molecular mechanisms, and future therapeutic potential of the miR-15a/16-1 cluster in stroke are poorly understood, and need further investigation.
By using genetically-manipulated miR-15a/16-1 knockout mice and pharmacological intervention of cerebral miR-15a/16-1 levels, we have identified that cerebral miR-15a/16-1 functions as a critical negative regulator in ischemic stroke. Furthermore, we demonstrated that the miR-15a/16-1 cluster inhibits the anti-apoptotic genes bcl-2 and bcl-w, as well as promotes inflammatory responses to trigger ischemic brain damage.
Materials and Methods
All procedures using laboratory animals were approved by the University of Pittsburgh Institutional Animal Care and Use Committee, and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were randomly assigned to various experimental groups using a lottery box. All stroke outcome assessments were performed in blinded manner.
Animal models
Male homozygous miR-15a/16-1 knockout mice (kindly provided by Dr. Riccardo Dalla-Favera)11 and littermate wild-type controls, or male C57BL/6J mice (8-10w, 23-25g; Jackson Laboratory) were subjected to middle cerebral artery occlusion (MCAO) for 1h and followed by 1-7d reperfusion.8, 15 Regional cerebral blood flow was measured before, during and after MCAO. Animals that did not show a more than 75% CBF reduction or a less than 60% CBF reperfusion over baseline levels or died after ischemia induction (∼10% of stroke animals) were excluded from further experimentation.
Intravenous administration of miR-15a/16-1 antagomir
Immediately after onset of MCA occlusion or at 2h reperfusion after 1h MCAO, mice were injected with miR-15a/16-1 specific inhibitor/antagomir (a mixture of miR-15a and miR-16-1 antagomirs each at final concentration of 30 pmol/g) or scramble control (30 pmol/g) by tail vein.16 Sham-operated mice were also treated with either miR-15a/16-1 antagomir or scramble control. All mice were sacrificed 1-7 d after MCAO.
Measurement of infarct volume, edema, neurological deficit and sensorimotor function
After MCAO, brain slices were stained with 2% TTC to calculate the infarct volume. 8, 15 Brain water content was measured by using a dry-wet method.17 Neurological deficits were examined and scored on a 5-point scale.8, 15 Neurobehavioral outcomes were also determined by the rotarod test, foot fault test and adhesive tape removal test at 1, 3, 5 and 7 d after MCAO.15, 18
Real time PCR and TaqMan® miRNA assay
IL-6, MCP-1, VCAM-1 and TNF-α mRNAs were detected by a quantitative PCR.15 MiR-15a, miR-16-1 and miR-21 expressions were determined by TaqMan® MicroRNA Assay.8, 15
Western blot analysis and ELISA
Total protein was isolated from mouse brains. The protein levels of Bcl-2, Bcl-w, Bcl-xl and β-actin were determined by Western blot analysis. The concentrations of IL-6, MCP-1, VCAM-1 and TNF-α were measured by ELISA kits.
Statistical analysis
Quantitative data were expressed as mean ± SEM. Differences among multiple groups were statistically analyzed by one- or two-way analysis of variance followed by the Bonferroni/Dunn post hoc correction. Comparison between two experimental groups was based on a two-tailed t-test. A p-value less than 0.05 was considered significant.
Methodological details are available in the online-only Data Supplement.
Results
The miR-15a/16-1 expression was increased in mouse brains after cerebral ischemia
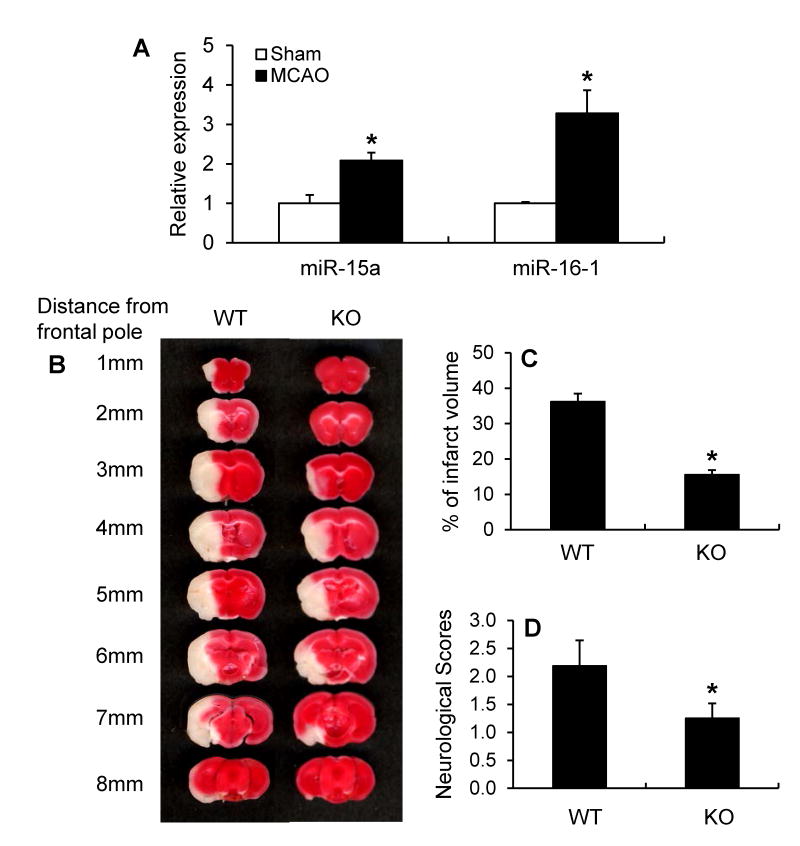
In order to explore the function of the miR-15a/16-1 cluster in ischemic neurovascular injury, we first evaluated the expression of cerebral miR-15a/16-1 in a mouse ischemic stroke model. As shown in Figure 1A, miR-15a and miR-16-1 displayed 2-3 fold increased expression in the ipsilateral cerebral cortex after 1h MCAO followed by 72h reperfusion. These data suggest the dysregulation of the miR-15a/16-1 profile correlated with the pathogenesis of ischemic neurovascular injury.
Figure 1.
MiR-15a/16-1 expression in mouse brains after MCAO and the effect of miR-15a/16-1 genetic deficiency on ischemic brain infarct and neurological outcomes. (A) Quantitative PCR data indicates that miR-15a/16-1 expression levels were significantly increased in the ipsilateral cerebral cortex of mice after 1h MCAO and 72h reperfusion (n=3). (B) MiR-15a/16-1 knockout mice (KO) and littermate wild-type control (WT) mice were subjected to 1h MCAO and 72 h reperfusion, and brains were stained with 2% TTC and cut into coronal sections from the posterior to frontal pole. (C, D) Quantitative analysis was performed to measure infarct volume (C) and neurological deficits (D) in mice after stroke. Compared to WT mice, miR-15a/16-1 KO mice exhibited smaller ischemia-induced brain infarct (n=8) and improved neurological outcomes (n=8). Data are expressed as mean ± SEM. *p < 0.05 vs Sham or WT group.
Genetic deletion or pharmacological inhibition of the miR-15a/16-1 cluster reduced brain infarction and improved neurological deficits in mice after cerebral ischemia
Next, we employed miR-15a/16-1 knockout mice (Supplemental Figure I) to observe whether of the absence of miR-15a/16-1 expression affects stroke outcomes. Compared to miR-15a/16-1 wild-type controls, genetic deletion of the miR-15a/16-1 cluster resulted in smaller brain infarct volume (Fig. 1B, C) and improved neurological outcomes (Fig. 1D) in mice 72h after MCAO. These results indicate that expression of miR-15a/16-1 cluster is a functional aspect of stroke injury.
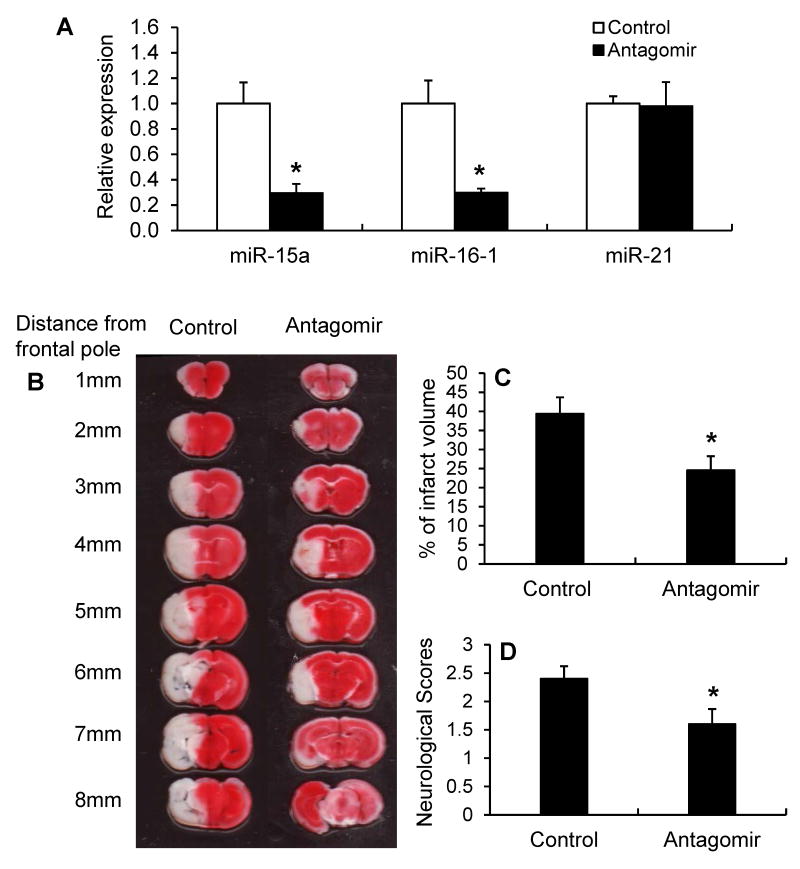
To determine if inhibition of the miR-15a/16-1 cluster activity affects ischemic brain injury using a more translatable approach, we performed intravenous (tail vein) injection of miR-15a/16-1 antagomir or its scramble control (each at final concentration of 30 pmol/g) into mice at the onset of 1h MCA occlusion and allowed the animals to survive for 72h of reperfusion. Compared to the scramble controls, miR-15a/16-1 antagomir effectively inhibited miR-15a and miR-16-1 expression in mouse cerebral cortex (Fig. 2A). Administration of miR-15a/16-1 antagomir had no effect on the expression of another microRNA, miR-21, supporting the specific targeting of the miR-15a/16-1 antamoir. Compared to miR-15a/16-1 antagomir control, treatment with miR-15a/16-1 antagomir significantly attenuated ischemic brain infarct (Fig. 2B, C), and improved neurological deficits (Fig. 2D) in mice 72h after focal cerebral ischemia. Systematic administration of miR-15a/16-1 antagomir at 2h reperfusion after 1h MCAO also provided similar brain protection in stroke mice (Supplemental Figure II).
Figure 2.
The effect of miR-15a/16-1 antagomir on ischemic brain infarct and neurological outcome. C57BL/6J mice were subjected to 1h MCAO and 72h reperfusion. Mice were also subjected to intravenous (tail vein) injection of the miR-15a/16-1 specific antagomir (30 pmol/g) or scramble control (30 pmol/g) immediately after onset of MCAO. (A) Quantitative PCR data demonstrates that miR-15a and miR-16-1 expression were significantly inhibited in the cerebral cortex of mice after 1h MCAO and 72h reperfusion (n=3). Systematic treatment with miR-15a/16-1 antagomir had no effects on cerebral miR-21 expression. (B) 2% TTC-stained coronal sections are shown at different brain levels from posterior to the frontal pole from scramble-control treated ischemic mice and ischemic mice injected with miR-15a/16-1 antigomir. Infarct volume (C) and neurological deficits (D) were quantitatively assessed in mice after cerebral ischemia. Compared to antagomir control group, miR-15a/16-1 antagomir-treated mice showed smaller ischemia-induced brain infarct volume (n=10) and improved neurological outcomes (n=10). Data are expressed as mean ± SEM. *p<0.05 vs antagomir control group.
Also we noticed that regional CBF did not differ by either miR-15a/16-1 genetic deletion or pharmacological inhibition at 15 min before ischemia, 15 minutes after ischemia, and 15 minutes after reperfusion (Supplemental Figure III and IV). These findings verify that the observed protection is not attributed to decreased ischemic induction/duration or variations in blood flow due to possible cerebrovascular structural (collateral) changes in the miR-15a/16-1 KO or miR-15a/16-1 antagomir-treated mice. Taken together, these data suggest that the presence and activity of the miR-15a/16-1 cluster significantly contributes to ischemic brain injury.
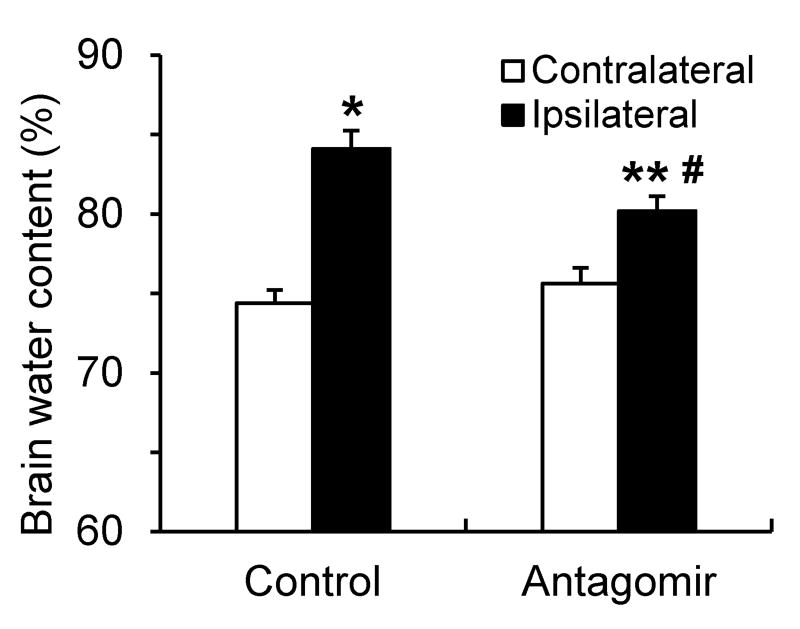
Intravenous delivery of miR-15a/16-1 antagomir reduced brain edema in stroke mice
We also investigated the effects of inhibitory miR-15a/16-1 activity in vivo on water content in mouse brain subjected to cerebral ischemia. Following 72h of reperfusion, brain water content from ischemic mice treated with the miR-15a/16-1 antagomir was significantly smaller than in antagomir control-treated mice (Fig.3).
Figure 3.
Brain water content in stroke mice after intravenous injection of miR-15a/16-1 antagomir. C57BL/6J mice were treated with miR-15a/16-1 antagomir or antagomir control (n=8) and subjected to 1h MCAO and 72h reperfusion. Brains were dissected out and water content was measured by wet/dry weight method. Cerebral ischemia caused a significant increase in water content in the ipsilateral hemisphere compared to contralateral hemisphere. Systematic delivery of miR-15a/16-1 antagomir significantly reduced brain water content in MCAO mice compared to antagomir control group. Data are expressed as mean ± SEM. *p<0.05, **p<0.01 vs contralateral (non-ischemic) side, #p<0.05 vs MCAO + antagomir control group.
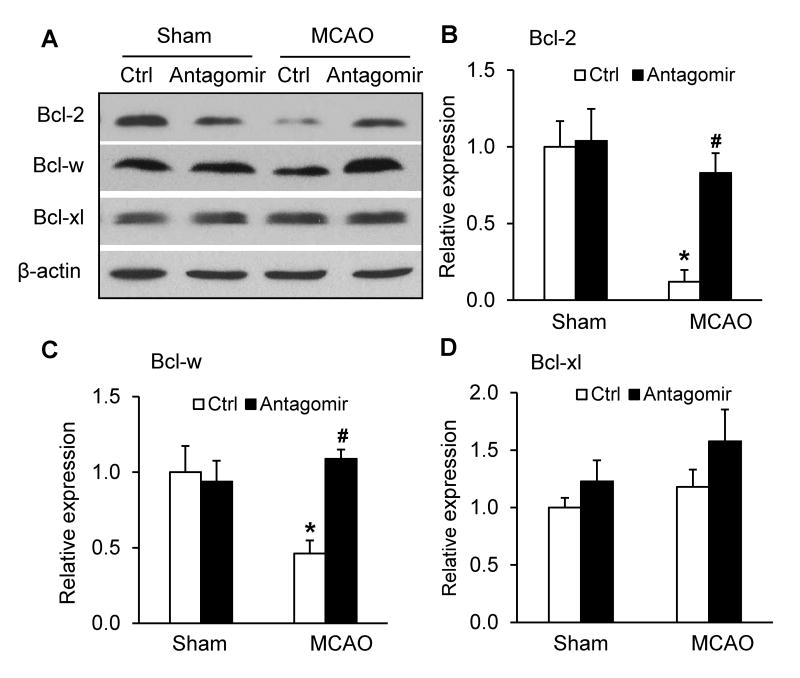
The effects of miR-15a/16-1 antagomir on the expression of anti-apoptotic proteins
Our previous study reports that miR-15a directly binds to 3′-UTRs of anti-apoptotic bcl-2 gene and translationally suppresses its activities in cultured cerebrovascular endothelial cells after OGD.7 To investigate the potential mechanisms of the miR-15a/16-1 cluster in the regulation of ischemic brain injury, we examined the cortical expression levels of several key anti-apoptotic proteins, Bcl-2, Bcl-w and Bcl-xl by western blotting. Knockdown of cerebral miR-15a/16-1 levels via intravenous systematic delivery of miR-15a/16-1 antagomirs effectively enhanced Bcl-2/Bcl-w protein levels in cortical ischemic regions after focal cerebral ischemia (Fig. 4A-D, Supplemental Figure V). Interestingly, treatment of miR-15a/16-1 antagomir had no effect on the expression of Bcl-2 and Bcl-w in sham-operated mice, nor on the expression of the anti-apoptotic protein bcl-xl in stroke mice, implying that miR-15a/16-1 antagomirs may specifically increase Bcl-2 and Bcl-w protein levels under ischemic conditions. These data suggest that the miR-15a/16-1 cluster promotes ischemic brain injury by negatively regulating anti-apoptotic mediators, Bcl-2 and Bcl-w.
Figure 4.
Ischemia-induced reduction of cortical Bcl-2 and Bcl-w protein levels are reversed by intravenous delivery of miR-15a/16-1 antagomir. C57BL/6J mice were treated with miR-15a/16-1 antagomir or antagomir control (Ctrl) and subjected to 1h MCAO and 72h reperfusion. Ischemic brain regions were dissected out and anti-apoptotic protein (Bcl-2, Bcl-w and Bcl-xl) levels were measured by western blotting (A) and quantitatively analyzed (B-D). Bcl-2 and Bcl-w were downregulated following 72h reperfusion after 1h MCAO (A-C). Intravenous administration of miR-15a/16-1 antagomir significantly enhanced Bcl-2 and Bcl-w protein levels (A-C), whereas the expression levels of Bcl-xl, another member of the bcl-2 family, was not affected in the ischemic cerebral cortex after treatment with the miR-15a/16-1 antagomir (A, D). Data are expressed as mean ± SEM from 3 separate experiments. *p<0.05 vs Sham group. #p<0.05 vs MCAO + antagomir control.
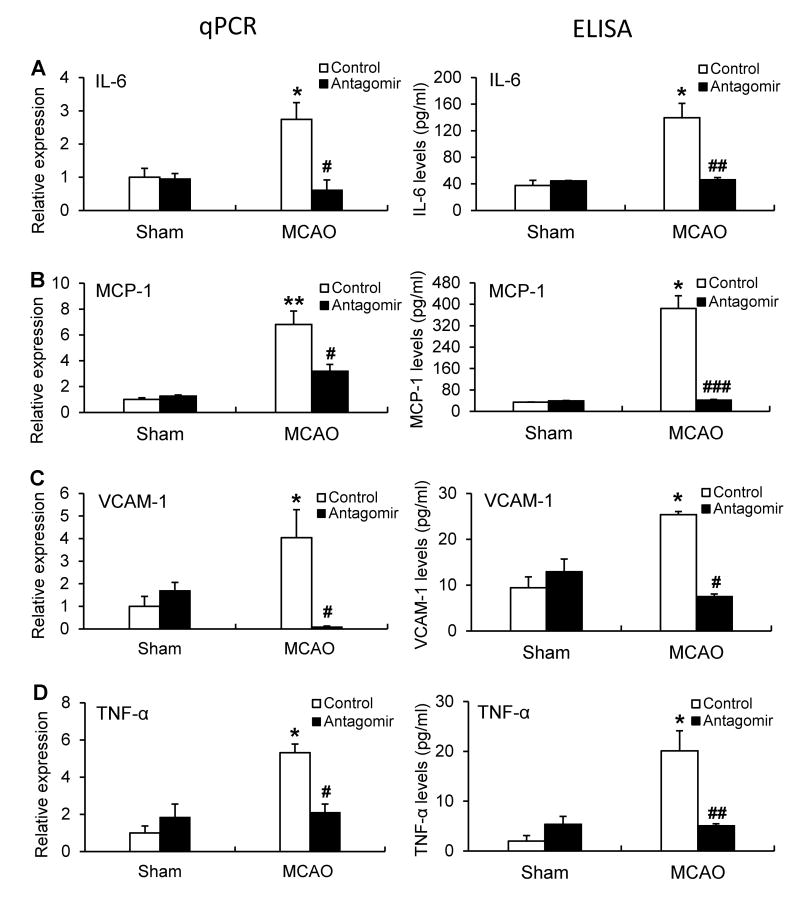
MiR-15a/16-1 antagomir reduced the expression of pro-inflammatory factors in mouse brain after cerebral ischemia
There is increasing evidence that pro-inflammatory cytokines are involved in the pathogenesis of focal cerebral ischemia.19, 20 To determine if manipulation of miR-15a/16-1 alters the inflammatory response to ischemic injury, we assessed inflammatory signaling in ischemic mice treated with the miR-15a/16-1 antagomir or its control. Ischemic injury induced a significant increase in the expression of IL-6, MCP-1, VCAM-1 and TNF-α at both the mRNA and protein levels in ischemic cortex compared to sham controls (Fig. 5, Supplemental Figure VI). Administration of the miR-15a/16-1 antagomirs significantly reduced mRNA levels of these pro-inflammatory cytokines in the ischemic cortex 24h or 72h after MCAO compared to the scramble control (Supplemental Figure VI, Fig. 5). No significant differences of these pro-inflammatory cytokines were observed between the sham-operated groups treated with either miR-15a/16-1 antagomir or scramble control. Consistent with changes in the mRNA level, ELISA data further confirmed that antagomir treatment also attenuated the ischemic induction of the protein levels of these pro-inflammatory cytokines in comparison with the antagomir controls (Fig. 5).
Figure 5.
Effects of miR-15a/16-1 antagomir on mRNA and protein levels of ischemia-induced pro-inflammatory cytokines in mouse brains. Quantitative PCR (qPCR) and ELISA data showing that miR-15a/16-1 antagomir significantly reduced pro-inflammation cytokines, IL-6 (A), MCP-1 (B), VCAM-1 (C), and TNF-α (D), mRNA and protein expression in the cerebral cortex of mice after 1h MCAO and 72h reperfusion (n=3). Data are expressed as mean ± SEM. *p<0.05, **p<0.01 vs Sham group, #p<0.05, ##p<0.01, ###p<0.001 vs MCAO + antagomir control group.
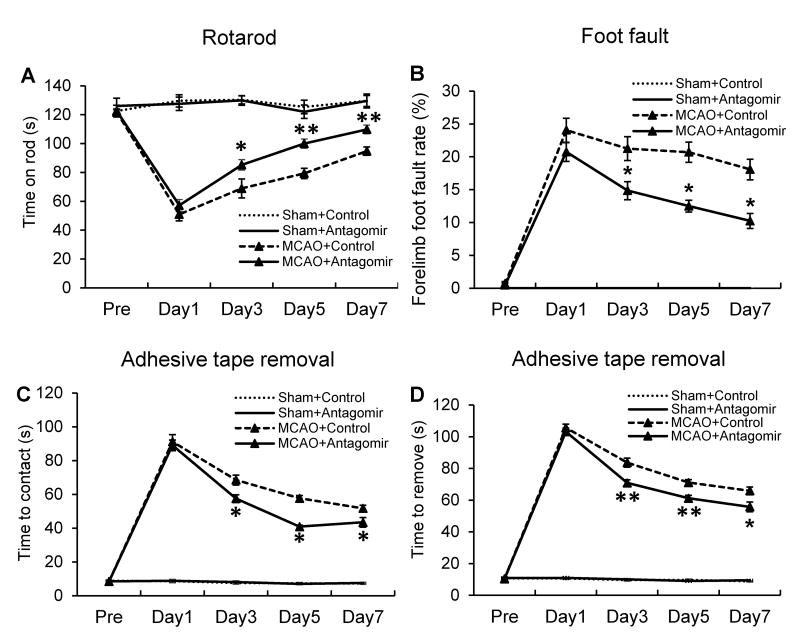
MiR-15a/16-1 antagomir improved neurobehavioral performance in mice after cerebral ischemia
Next, we performed three neurobehavioral tests18 (rotarod, foot fault and adhesive tape removal) over 7 days following MCAO to examine the effect of the miR-15a/16-1 antagomir on the sensorimotor recovery of affected limbs in MCAO mice. Using the rotarod test, we found that miR-15a/16-1 antagomir-treated stroke mice were able to staying on the rod significantly longer compared to the scramble control-treated stroke mice, beginning 3 days following MCAO (Fig. 6A). Similar to rotarod test, the percentage of foot faults was reduced in stroke mice with intravenous injections of miR-15a/16-1 antagomir compared to stroke mice injected with scramble control beginning 3 days after cerebral ischemia (Fig. 6B). The adhesive tape removal test demonstrated improved recovery in the miR-15a/16-1 antagomir-treated ischemic mice both in the time to contact and remove adhesive tapes on the foot compared to antagomir control in mice, beginning 3 days and continuing through 7 days after ischemic stroke (Fig. 6C and 6D). These data suggest that inhibition of cerebral miR-15a/16-1 activity effectively improves sensorimotor deficits in mice after ischemic stroke.
Figure 6.
MiR-15a/16-1 antagomir improves sensorimotor functions of mice after cerebral ischemia. C57BL/6J mice were treated with miR-15a/16-1 antagomir or antagomir control (n=10) via tail vein injections and subjected to 1h MCAO followed by reperfusion, then tested every other day for 7 days. Ischemic mice were subjected to rotarod (A), foot fault (B), and adhesive tape removal tests (C, time to contact; D, time to remove) for examination of sensorimotor functions. In comparison with the antagomir control group, mice treated with miR-15a/16-1 antagomir showed improved duration of time on the rotarod, reduced percentage of foot fault steps, and improvement in the touch time and removal time of the adhesive tape compared to control ischemic mice at 3-7 days after MCAO. Data are expressed as mean ± SEM. *p<0.05, **p <0.01 vs MCAO + antagomir control group.
Discussion
In this study, we found increased expression of cerebral miR-15a/16-1 in the mouse ischemic stroke model, and that genetic deletion of this miR cluster significantly reduced brain infarction and improved neurological score in stroke mice. To explore the translational value of miR-15a/16-1 manipulation in ischemic stroke, we delivered synthetic miR-15a/16-1 antagomir by tail vein injection to effectively inhibit miR-15a/16-1 activity and then observed how this systematic treatment affects stroke outcomes in mice. Pharmacological intervention of cerebral miR-15a/16-1 significantly reduced brain infarct, decreased brain edema and improved neurobehavioral outcomes in mice after ischemic stroke. We further demonstrated that treatment of miR-15a/16-1 antagomir offered increased expression of anti-apoptotic proteins and suppressed expression of pro-inflammatory molecules in ischemic brain regions, thus supporting a protective role for miR-15a/16-1 inhibition in stroke mice.
Accumulating evidence has demonstrated altered cerebral miR profiles in ischemia models, which play a critical role in the regulation of gene expression underlying the cellular response to ischemic stroke.9, 21-23 Ischemic preconditioning changes miR expression, including miR-132, the miR-200 family, miR-182 family, and others, which may promote ischemic tolerance via neuroprotective signaling pathways.9, 24 Altered miR levels were also found in blood samples of rodent stroke models 5 and stroke patients12, 25 and may serve as potential biomarkers. We and others were among the first to identify the function of individual miRs in stroke pathology. 9, 21-23 Previously we have reported that inhibition of endothelial miR-15a contributes to the PPARδ-mediated cerebral vasoprotective role in stroke.7 The functional role of the miR-15a/16-1 cluster in the brain after cerebral ischemic stroke was unknown. In this study, we have provided the first evidence that genetic deletion and pharmacological inhibition of cerebral miR-15a/16-1 activity appears to reduce ischemic brain damage and improve neurological outcomes through anti-apoptotic and anti-inflammatory effects. Our novel findings here have unveiled the miR-15a/16-1 cluster as a novel pro-apoptotic/pro-inflammatory regulator and contributes to the pathogenesis of focal cerebral ischemia.
During cerebral ischemia, inflammatory reactions in various neurovascular cells (cerebral vascular cells, microglia, astrocytes, neurons, and others) substantially contribute to the pathogenesis of the disease. Increasing evidence suggests that pro-inflammatory cytokines are closely involved in the pathophysiology of focal cerebral ischemia.19, 20 Indeed, cerebral neural cells are able to mediate cerebrovascular and brain parenchyma inflammation by producing and secreting pro-inflammatory cytokines to initiate primary and delayed neuronal death after cerebral ischemia.19, 20 These pro-inflammatory responses are suggested to be regulated by microRNAs. In our study, systematic administration of miR-15a/16-1 antagomir significantly reduced ischemia-triggered pro-inflammatory cytokines IL-6, MCP-1, VCAM-1 and TNF-α levels in mouse ischemic brain regions. This suggests that inhibition of miR-15a/16-1 activity may play protective roles by inhibiting inflammatory responses induced by ischemic stroke.
Extensive studies have shown that microRNAs play important roles in apoptosis by regulating pro- and anti-apoptotic genes in apoptotic signaling pathways.26 For example, several miRs such as miR-15a, miR-16 and miR-29 have been reported as pro-apoptotic molecules in various cancer cells.27, 28 By contrast, miR-21 and the miR-17-92 cluster represent another class of miRs that inhibit apoptosis.29, 30 In our previous study,7 we found OGD-induced elevation in miR-15a expression levels in mouse cerebrovascular endothelial cell (CEC) cultures is associated with increased CEC death. In vitro loss- or gain-of-miR-15a function results in reduced or increased cell death caspase-3 activity and Golgi fragmentation, respectively. Moreover, we have demonstrated that bcl-2 is a translationally repressed target of miR-15a, suggest that upregulated miR-15a may inhibit bcl-2 gene expression and play a detrimental role in OGD-induced CEC apoptosis. Extending our previous finding, we have shown in this study that intravenous delivery of miR-15a/16-1 antagomir effectively increased bcl-2 and bcl-w expression in ischemic mouse cerebral cortex, suggesting a negative regulation of miR-15a/16-1 on the translation of these two anti-apoptotic genes. Taken together, our data unveils the miR-15a/16-1 cluster as a novel pro-apoptotic regulator and contributor to the pathogenesis of focal cerebral ischemia.
MiR-based therapeutics have been tested for the potential treatment of many diseases, including liver disease and cardiovascular disease.14 Systemic administration of antagomirs has been used to silence miR-122 in the liver of mice 31 and miR-133 in mouse heart.32 In addition, anti-miR-122 locked nucleic acid (LNA) has been used to treat chronic viral hepatitis in chimpanzees.33 Several companies are now developing miR-based therapeutics. Santaris Pharmaceuticals (http://www.santaris.com) has launched a phase II clinical trial for the treatment of hepatitis C using liver-specific anti-miR-122 LNA. Several groups, including ours, have also employed intracerebroventricular (i.c.v.) delivery of miR antagomirs (inhibitors) to define the functional role of specific miR in stroke outcomes in mice,9 showing a significant neuroprotective role in rodent experimental stroke models. However, i.c.v. delivery of antagomir has the limitation of not being practical for patients with stroke. Currently a few groups have reported that intravenous (i.v.) injection of antagomirs targeted for specific miRs may be an effective therapeutic route in experimental stroke models. For instance, Caballero-Garrido et al. reported that i.v. injection of miR-155 inhibitor after cerebral ischemia enhances brain angiogenesis, reduces brain tissue damage and improves long-term functional recovery in mice.34 Another group reported that post-ischemic treatment with a miR-181a antagomir by intravenous injection has neuroprotective effects against ischemic neuronal damage and neurological impairment in mice.16 Moreover, Liu et al. reported that miR-122 mimic treatment by i.v. injections effectively improves stroke outcomes via downregulating miR-122 target genes in blood leukocytes in rats.35
MiR-15a/16-1 inhibition by i.v. injection has proven to be effective in ischemic heart disease.13 Ongoing preclinical studies using anti-miR-15/195, miR-92, and miR-143/145 LNA at miRagen Therapeutics are designed to develop anti-miR inhibitors for the improvement of post-myocardial infarction remodeling and treatment of peripheral artery disease and vascular disease.9 We have previously reported that PPARδ inhibition of miR-15a activity may prevent ischemia-induced cerebrovascular dysfunction.7 But whether global inhibition of the miR-15a/16-1 cluster is effective in the regulation of ischemic brain injury of experimental stroke model has been unknown. In this study, we provided novel evidence that intravenous delivery of miR-15a/16-1 antagomir significantly reduced brain infarct volume, and improved neurological outcome in mice after cerebral ischemia. Mechanistically, treatment of miR-15a/16-1 antagomir decreased the expression of several major pro-inflammatory cytokines and increased anti-apoptotic Bcl-2 and Bcl-w protein levels in the ischemic brain regions. Thus, the miR-15a/16-1 cluster may serve as a potential target for the development of microRNA-based therapeutics for ischemic stroke.
Supplementary Material
Acknowledgments
Source of Funding: This work was supported by the National Institutes of Health Grants: NS091175 (K.J. Yin and J. Chen), and NS094930, NS086820 (K.J. Yin)
Footnotes
Conflicts of Interest/Disclosures: None
References
- 1.Bartel DP. Micrornas: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 2.Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, et al. A microrna feedback circuit in midbrain dopamine neurons. Science (New York, N Y. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, et al. Loss of microrna cluster mir-29a/b-1 in sporadic alzheimer's disease correlates with increased bace1/beta-secretase expression. Proc Natl Acad Sci U S A. 2008;105:6415–6420. doi: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral micrornaome. J Cereb Blood Flow Metab. 2009;29:675–687. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, et al. Brain and blood microrna expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lusardi TA, Farr CD, Faulkner CL, Pignataro G, Yang T, Lan J, et al. Ischemic preconditioning regulates expression of micrornas and a predicted target, mecp2, in mouse cortex. J Cereb Blood Flow Metab. 2010;30:744–756. doi: 10.1038/jcbfm.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin KJ, Deng Z, Hamblin M, Xiang Y, Huang H, Zhang J, et al. Peroxisome proliferator-activated receptor δ regulation of mir-15a in ischemia-induced cerebral vascular endothelial injury. The Journal of Neuroscience. 2010;30:6398–6408. doi: 10.1523/JNEUROSCI.0780-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, et al. Mir-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiology of disease. 2010;38:17–26. doi: 10.1016/j.nbd.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin KJ, Hamblin M, Eugene Chen Y. Angiogenesis-regulating micrornas and ischemic stroke. Current vascular pharmacology. 2015;13:352–365. doi: 10.2174/15701611113119990016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, et al. Mir-15a and mir-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein U, Lia M, Crespo M, Siegel R, Shen Q, Mo T, et al. The dleu2/mir-15a/16-1 cluster controls b cell proliferation and its deletion leads to chronic lymphocytic leukemia. Cancer Cell. 2010;17:28–40. doi: 10.1016/j.ccr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 12.Tan KS, Armugam A, Sepramaniam S, Lim KY, Setyowati KD, Wang CW, et al. Expression profile of micrornas in young stroke patients. PLoS ONE. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hullinger TG, Montgomery RL, Seto AG, Dickinson BA, Semus HM, Lynch JM, et al. Inhibition of mir-15 protects against cardiac ischemic injury. Circ Res. 2012;110:71–81. doi: 10.1161/CIRCRESAHA.111.244442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Rooij E, Purcell AL, Levin AA. Developing microrna therapeutics. Circ Res. 2012;110:496–507. doi: 10.1161/CIRCRESAHA.111.247916. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Tang X, Liu K, Hamblin MH, Yin KJ. Long noncoding rna malat1 regulates cerebrovascular pathologies in ischemic stroke. J Neurosci. 2017;37:1797–1806. doi: 10.1523/JNEUROSCI.3389-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu LJ, Ouyang YB, Xiong X, Stary CM, Giffard RG. Post-stroke treatment with mir-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Exp Neurol. 2015;264:1–7. doi: 10.1016/j.expneurol.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hara H, Huang PL, Panahian N, Fishman MC, Moskowitz MA. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient mca occlusion. J Cereb Blood Flow Metab. 1996;16:605–611. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Shi Y, Zhang L, Zhang F, Hu X, Zhang W, et al. Omega-3 polyunsaturated fatty acids enhance cerebral angiogenesis and provide long-term protection after stroke. Neurobiol Dis. 2014;68:91–103. doi: 10.1016/j.nbd.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: New opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Becker KJ. Inflammation and acute stroke. Curr Opin Neurol. 1998;11:45–49. doi: 10.1097/00019052-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Saugstad JA. Micrornas as effectors of brain function with roles in ischemia and injury, neuroprotection, and neurodegeneration. J Cereb Blood Flow Metab. 2010;30:1564–1576. doi: 10.1038/jcbfm.2010.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vemuganti R. The micrornas and stroke: No need to be coded to be counted. Transl Stroke Res. 2010;1:158–160. doi: 10.1007/s12975-010-0030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin KJ, Hamblin M, Chen YE. Non-coding rnas in cerebral endothelial pathophysiology: Emerging roles in stroke. Neurochemistry international. 2014;77:9–16. doi: 10.1016/j.neuint.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dharap A, Vemuganti R. Ischemic pre-conditioning alters cerebral micrornas that are upstream to neuroprotective signaling pathways. J Neurochem. 113:1685–1691. doi: 10.1111/j.1471-4159.2010.06735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai PC, Liao YC, Wang YS, Lin HF, Lin RT, Juo SH. Serum microrna-21 and microrna-221 as potential biomarkers for cerebrovascular disease. J Vasc Res. 2013;50:346–354. doi: 10.1159/000351767. [DOI] [PubMed] [Google Scholar]
- 26.Jovanovic M, Hengartner MO. Mirnas and apoptosis: Rnas to die for. Oncogene. 2006;25:6176–6187. doi: 10.1038/sj.onc.1209912. [DOI] [PubMed] [Google Scholar]
- 27.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, et al. Mir-15 and mir-16 induce apoptosis by targeting bcl2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mott JL, Kobayashi S, Bronk SF, Gores GJ. Mir-29 regulates mcl-1 protein expression and apoptosis. Oncogene. 2007;26:6133–6140. doi: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. Programmed cell death 4 (pdcd4) is an important functional target of the microrna mir-21 in breast cancer cells. J Biol Chem. 2008;283:1026–1033. doi: 10.1074/jbc.M707224200. [DOI] [PubMed] [Google Scholar]
- 30.Hayashita Y, Osada H, Tatematsu Y, Yamada H, Yanagisawa K, Tomida S, et al. A polycistronic microrna cluster, mir-17-92, is overexpressed in human lung cancers and enhances cell proliferation. Cancer Res. 2005;65:9628–9632. doi: 10.1158/0008-5472.CAN-05-2352. [DOI] [PubMed] [Google Scholar]
- 31.Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, et al. Silencing of micrornas in vivo with ‘antagomirs'. Nature. 2005;438:685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 32.Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, et al. Microrna-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- 33.Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, et al. Therapeutic silencing of microrna-122 in primates with chronic hepatitis c virus infection. Science (New York, N Y. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caballero-Garrido E, Pena-Philippides JC, Lordkipanidze T, Bragin D, Yang Y, Erhardt EB, et al. In vivo inhibition of mir-155 promotes recovery after experimental mouse stroke. J Neurosci. 2015;35:12446–12464. doi: 10.1523/JNEUROSCI.1641-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu da Z, Jickling GC, Ander BP, Hull H, Zhan X, Cox C, et al. Elevating microrna-122 in blood improves outcomes after temporary middle cerebral artery occlusion in rats. J Cereb Blood Flow Metab. 2016;36:1374–1383. doi: 10.1177/0271678X15610786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.