Abstract
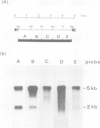
Northern blot and nucleotide sequence analyses of copia RNA from a transfectant made by introducing a genomic copia into copia-free cells showed that the 2 kb RNA, one of the major transcripts of copia, is generated through splicing. Using the polymerase chain reaction (PCR), we have also found that the position of the splice sites used in Drosophila larvae and cultured cells originally containing copia is the same as that used in the transfectant. To investigate the function of the 2 kb RNA, we constructed mutant copias which harboured a single point mutation at the splice site or approximately 3 kb deletion of the internal region corresponding to the spliced out sequence. Analyses of transfectants made by introducing these mutant copias into copia-free cells demonstrated that the spliced 2 kb RNA contains sufficient information to make copia virus-like particles (VLPs). Furthermore, when copia RNA corresponding to the spliced RNA was translated in vitro, the major VLP protein was found to be released autocatalytically from its own precursor. A single amino acid substitution at the putative protease active site in the precursor prevented the processing, and resulted in accumulation of the mutant precursor in vitro. From these results, we conclude that copia VLPs are produced through autocatalytic processing of the precursor polyprotein encoded by the spliced copia RNA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. E., Mellor J., Gull K., Sim R. B., Tuite M. F., Kingsman S. M., Kingsman A. J. The functions and relationships of Ty-VLP proteins in yeast reflect those of mammalian retroviral proteins. Cell. 1987 Apr 10;49(1):111–119. doi: 10.1016/0092-8674(87)90761-6. [DOI] [PubMed] [Google Scholar]
- Aoyagi T., Yagisawa Y., Kumagai M., Hamada M., Morishima H. Letter: New pepstatins, pepstatins Bu, Pr and Ac produced by Streptomyces. J Antibiot (Tokyo) 1973 Sep;26(9):539–541. doi: 10.7164/antibiotics.26.539. [DOI] [PubMed] [Google Scholar]
- Carlson M., Brutlag D. One of the copia genes is adjacent to satellite DNA in Drosophila melanogaster. Cell. 1978 Nov;15(3):733–742. doi: 10.1016/0092-8674(78)90259-3. [DOI] [PubMed] [Google Scholar]
- Debouck C., Gorniak J. G., Strickler J. E., Meek T. D., Metcalf B. W., Rosenberg M. Human immunodeficiency virus protease expressed in Escherichia coli exhibits autoprocessing and specific maturation of the gag precursor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8903–8906. doi: 10.1073/pnas.84.24.8903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar K. J., Moelling K. Biochemical properties of p15-associated protease in an avian RNA tumor virus. J Virol. 1978 Oct;28(1):106–118. doi: 10.1128/jvi.28.1.106-118.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echalier G., Ohanessian A. In vitro culture of Drosophila melanogaster embryonic cells. In Vitro. 1970 Nov-Dec;6(3):162–172. doi: 10.1007/BF02617759. [DOI] [PubMed] [Google Scholar]
- Emori Y., Shiba T., Kanaya S., Inouye S., Yuki S., Saigo K. The nucleotide sequences of copia and copia-related RNA in Drosophila virus-like particles. 1985 Jun 27-Jul 3Nature. 315(6022):773–776. doi: 10.1038/315773a0. [DOI] [PubMed] [Google Scholar]
- Falkenthal S., Lengyel J. A. Structure, translation, and metabolism of the cytoplasmic copia ribonucleic acid of Drosophila melanogaster. Biochemistry. 1980 Dec 9;19(25):5842–5850. doi: 10.1021/bi00566a028. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J., Rubin G. M., Young M. W., Hogness D. S. Repeated gene families in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1053–1063. doi: 10.1101/sqb.1978.042.01.106. [DOI] [PubMed] [Google Scholar]
- Finnegan D. J. Transposable elements in eukaryotes. Int Rev Cytol. 1985;93:281–326. doi: 10.1016/s0074-7696(08)61376-5. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Levis R., Simon M. A., Rubin G. M. The 5' termini of RNAs encoded by the transposable element copia. Nucleic Acids Res. 1981 Dec 11;9(23):6279–6291. doi: 10.1093/nar/9.23.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell A. J., Ruby S. W., Toole J. J., Roberts B. E., Rubin G. M. Translation and developmental regulation of RNA encoded by the eukaryotic transposable element copia. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7107–7111. doi: 10.1073/pnas.77.12.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989 Oct 6;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Graves M. C., Lim J. J., Heimer E. P., Kramer R. A. An 11-kDa form of human immunodeficiency virus protease expressed in Escherichia coli is sufficient for enzymatic activity. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2449–2453. doi: 10.1073/pnas.85.8.2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J., Billich S., Schulze T., Sukrow S., Moelling K. Partial purification and substrate analysis of bacterially expressed HIV protease by means of monoclonal antibody. EMBO J. 1988 Jun;7(6):1785–1791. doi: 10.1002/j.1460-2075.1988.tb03009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh I., Yasunaga T., Ikawa Y., Yoshinaka Y. Inhibition of retroviral protease activity by an aspartyl proteinase inhibitor. Nature. 1987 Oct 15;329(6140):654–656. doi: 10.1038/329654a0. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y., Ando Y., Shiba T. Unusual priming mechanism of RNA-directed DNA synthesis in copia retrovirus-like particles of Drosophila. 1986 Oct 30-Nov 5Nature. 323(6091):824–826. doi: 10.1038/323824a0. [DOI] [PubMed] [Google Scholar]
- Kräusslich H. G., Ingraham R. H., Skoog M. T., Wimmer E., Pallai P. V., Carter C. A. Activity of purified biosynthetic proteinase of human immunodeficiency virus on natural substrates and synthetic peptides. Proc Natl Acad Sci U S A. 1989 Feb;86(3):807–811. doi: 10.1073/pnas.86.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Mills J., Mous J. Active site mutagenesis of the AIDS virus protease and its alleviation by trans complementation. EMBO J. 1988 Aug;7(8):2547–2553. doi: 10.1002/j.1460-2075.1988.tb03103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Dunsmuir P., Rubin G. M. Terminal repeats of the Drosophila transposable element copia: nucleotide sequence and genomic organization. Cell. 1980 Sep;21(2):581–588. doi: 10.1016/0092-8674(80)90496-1. [DOI] [PubMed] [Google Scholar]
- Miller K., Rosenbaum J., Zbrzezna V., Pogo A. O. The nucleotide sequence of Drosophila melanogaster copia-specific 2.1-kb mRNA. Nucleic Acids Res. 1989 Mar 11;17(5):2134–2134. doi: 10.1093/nar/17.5.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T., Mae N., Shiba T., Kondo S. Production of virus-like particles by the transposable genetic element, copia, of Drosophila melanogaster. Mol Gen Genet. 1987 Apr;207(1):29–37. doi: 10.1007/BF00331487. [DOI] [PubMed] [Google Scholar]
- Mosna G., Dolfini S. Morphological and chromosomal characterization of three new continuous cell lines of Drosophila melanogaster. Chromosoma. 1972;38(1):1–9. doi: 10.1007/BF00319954. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M., Rubin G. M. Complete nucleotide sequence of the Drosophila transposable element copia: homology between copia and retroviral proteins. Mol Cell Biol. 1985 Jul;5(7):1630–1638. doi: 10.1128/mcb.5.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mous J., Heimer E. P., Le Grice S. F. Processing protease and reverse transcriptase from human immunodeficiency virus type I polyprotein in Escherichia coli. J Virol. 1988 Apr;62(4):1433–1436. doi: 10.1128/jvi.62.4.1433-1436.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Developmental expression of Drosophila melanogaster retrovirus-like transposable elements. EMBO J. 1987 Feb;6(2):419–424. doi: 10.1002/j.1460-2075.1987.tb04771.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz H. E., Lockett T. J., Young M. W. Analysis of transcripts from two families of nomadic DNA. J Mol Biol. 1982 May 5;157(1):49–68. doi: 10.1016/0022-2836(82)90512-5. [DOI] [PubMed] [Google Scholar]
- Shiba T., Saigo K. Retrovirus-like particles containing RNA homologous to the transposable element copia in Drosophila melanogaster. Nature. 1983 Mar 10;302(5904):119–124. doi: 10.1038/302119a0. [DOI] [PubMed] [Google Scholar]
- Torruella M., Gordon K., Hohn T. Cauliflower mosaic virus produces an aspartic proteinase to cleave its polyproteins. EMBO J. 1989 Oct;8(10):2819–2825. doi: 10.1002/j.1460-2075.1989.tb08428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Wight A., Eisenman R. In vitro cleavage of avian retrovirus gag proteins by viral protease p15. Virology. 1979 Oct 15;98(1):154–167. doi: 10.1016/0042-6822(79)90534-8. [DOI] [PubMed] [Google Scholar]
- von der Helm K. Cleavage of Rous sarcoma viral polypeptide precursor into internal structural proteins in vitro involves viral protein p15. Proc Natl Acad Sci U S A. 1977 Mar;74(3):911–915. doi: 10.1073/pnas.74.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]