Abstract
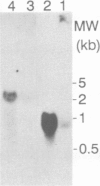
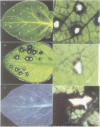
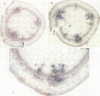
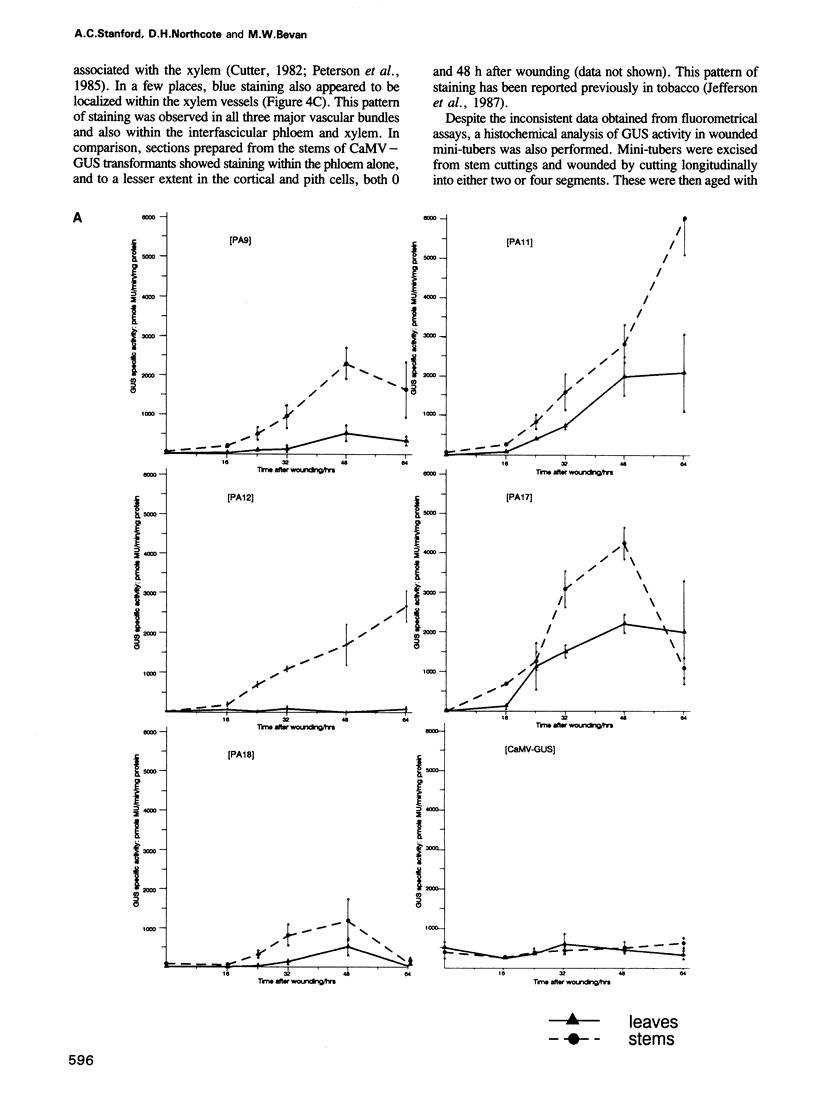
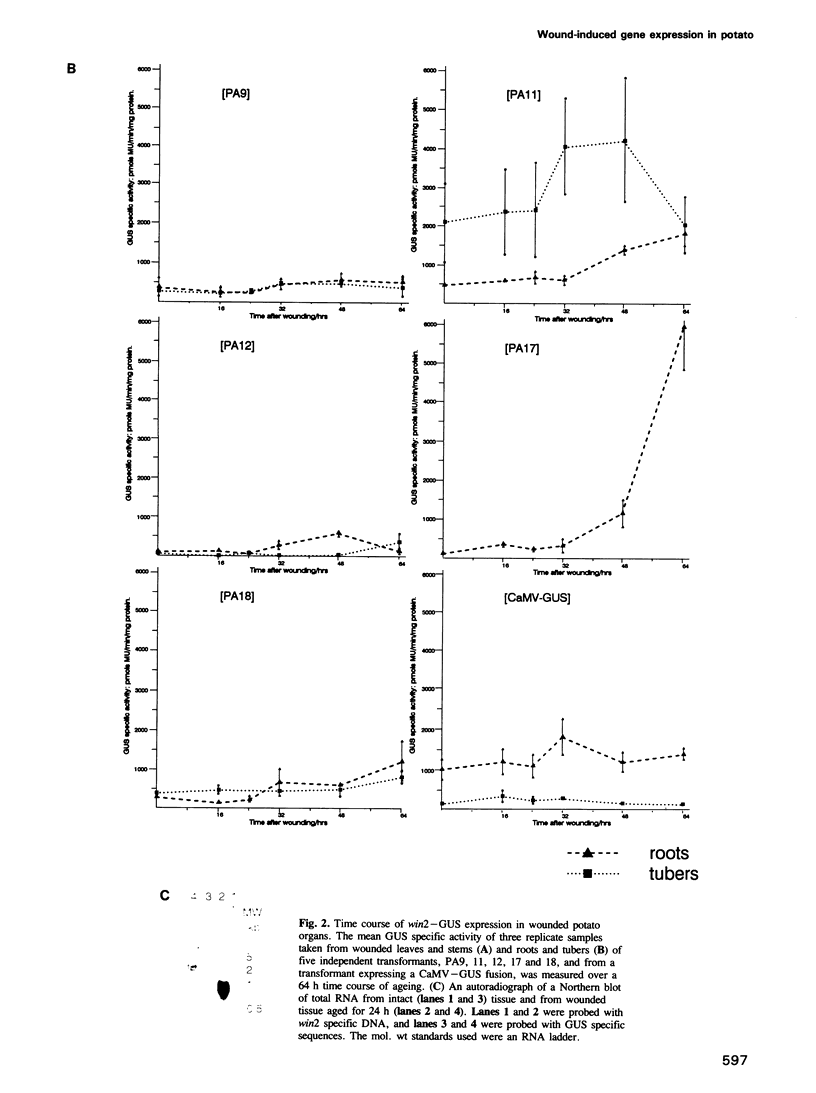
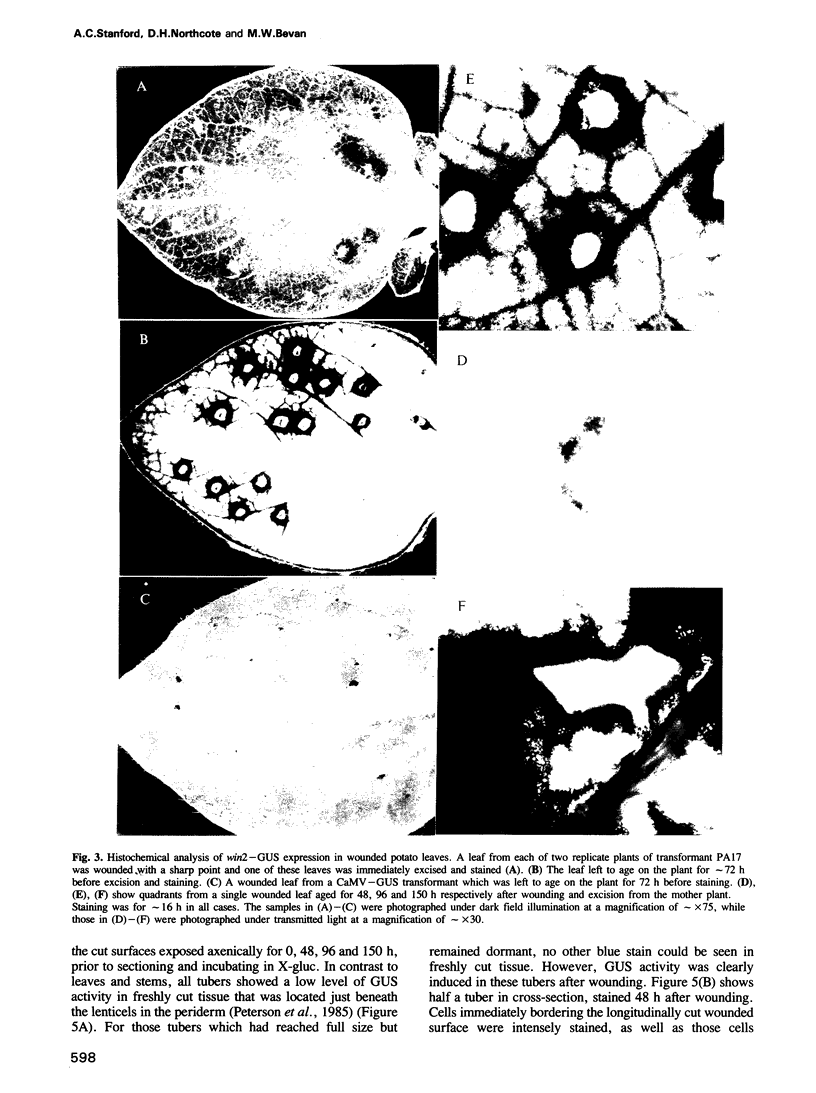
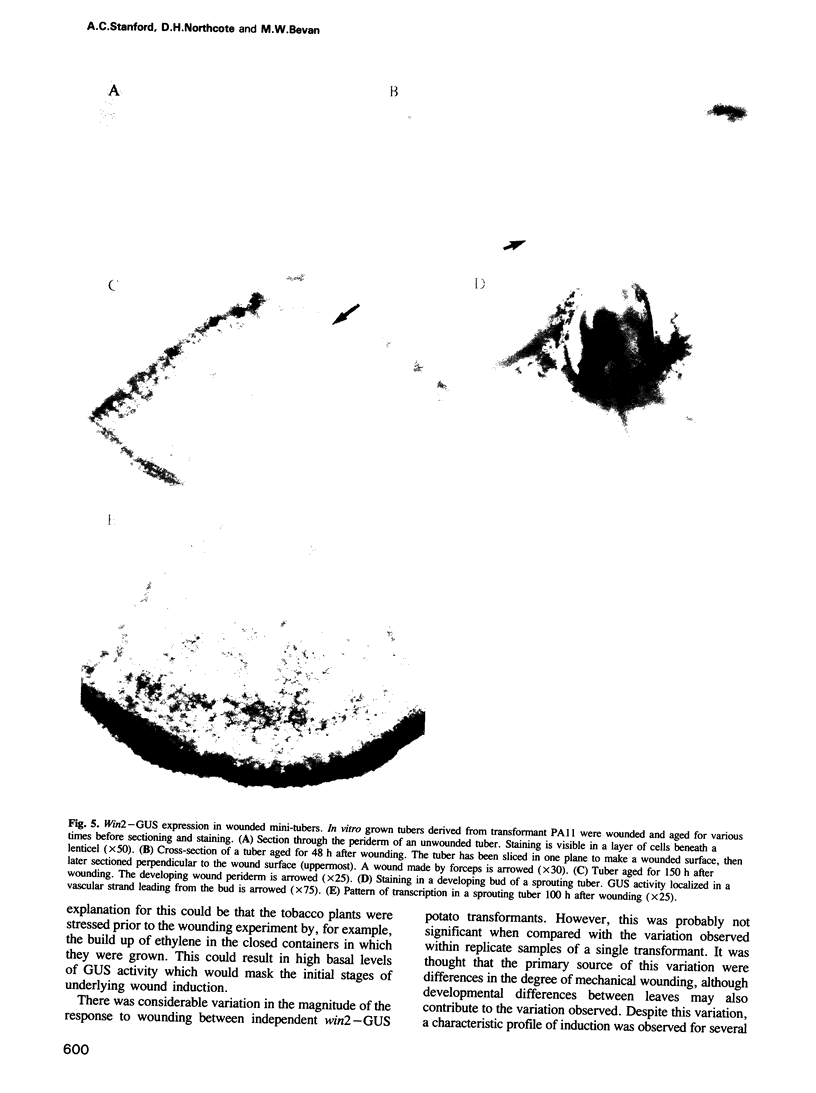
Transcriptional fusions between the gene encoding win2 from potato and the reporter gene encoding beta-glucuronidase (GUS) have been used to study the spatial and temporal patterns of wound induced gene activity in transgenic potato and tobacco plants. Gene fusions containing a full length win2 promoter were found to be correctly regulated in response to mechanical wounding in transgenic potato, but not in the heterologous host, tobacco. Sequences greater than 560 bp upstream of the transcription start site of win2 were shown to be important for wound inducibility. The dramatic induction of GUS activity detected using fluorometric assays of extracts of wounded and aged leaves of several independent win2--GUS transformants was consistent with the kinetics of win2 mRNA accumulation. Histochemical analysis of wounded leaves showed that transcription first occurred in cells immediately adjacent to the wound, and was then progressively induced in cells associated with the vascular system at a distance from the wound site. In tubers, a localized response to wounding was observed, and this only spread to other parts of the tuber if it had started to sprout. It was concluded that active vascular transport was necessary for the spread of wound response. Win2--GUS fusions were also expressed as part of normal plant development, as GUS activity was detected in the developing buds and in a layer of cells associated with the lenticels of unwounded tubers.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- A simple and general method for transferring genes into plants. Science. 1985 Mar 8;227(4691):1229–1231. doi: 10.1126/science.227.4691.1229. [DOI] [PubMed] [Google Scholar]
- An G., Mitra A., Choi H. K., Costa M. A., An K., Thornburg R. W., Ryan C. A. Functional analysis of the 3' control region of the potato wound-inducible proteinase inhibitor II gene. Plant Cell. 1989 Jan;1(1):115–122. doi: 10.1105/tpc.1.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J. N., Ryder T. B., Wingate V. P., Bailey J. A., Lamb C. J. Differential accumulation of plant defense gene transcripts in a compatible and an incompatible plant-pathogen interaction. Mol Cell Biol. 1986 May;6(5):1615–1623. doi: 10.1128/mcb.6.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 1984 Nov 26;12(22):8711–8721. doi: 10.1093/nar/12.22.8711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M., Shufflebottom D., Edwards K., Jefferson R., Schuch W. Tissue- and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. EMBO J. 1989 Jul;8(7):1899–1906. doi: 10.1002/j.1460-2075.1989.tb03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourque J. E., Miller J. C., Park W. D. Use of an in vitro tuberization system to study tuber protein gene expression. In Vitro Cell Dev Biol. 1987 May;23(5):381–386. doi: 10.1007/BF02620996. [DOI] [PubMed] [Google Scholar]
- Broglie K. E., Biddle P., Cressman R., Broglie R. Functional analysis of DNA sequences responsible for ethylene regulation of a bean chitinase gene in transgenic tobacco. Plant Cell. 1989 Jun;1(6):599–607. doi: 10.1105/tpc.1.6.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr J. P., Dixon D. C., Nikolau B. J., Voelkerding K. V., Klessig D. F. Synthesis and localization of pathogenesis-related proteins in tobacco. Mol Cell Biol. 1987 Apr;7(4):1580–1583. doi: 10.1128/mcb.7.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin D. R., Sauer N., Lamb C. J. Differential regulation of a hydroxyproline-rich glycoprotein gene family in wounded and infected plants. Mol Cell Biol. 1987 Dec;7(12):4337–4344. doi: 10.1128/mcb.7.12.4337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick S. A., Bell J. N., Boller T., Lamb C. J. Chitinase cDNA cloning and mRNA induction by fungal elicitor, wounding, and infection. Plant Physiol. 1988 Jan;86(1):182–186. doi: 10.1104/pp.86.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson R. A., Kavanagh T. A., Bevan M. W. GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987 Dec 20;6(13):3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil M., Sánchez-Serrano J. J., Willmitzer L. Both wound-inducible and tuber-specific expression are mediated by the promoter of a single member of the potato proteinase inhibitor II gene family. EMBO J. 1989 May;8(5):1323–1330. doi: 10.1002/j.1460-2075.1989.tb03512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton M. A., Lamb C. J. Transcriptional activation of plant defense genes by fungal elicitor, wounding, and infection. Mol Cell Biol. 1987 Jan;7(1):335–341. doi: 10.1128/mcb.7.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J., Lipphardt S., Lörz H., Häuser I., Willmitzer L., Schell J. 5' upstream sequences from the wun1 gene are responsible for gene activation by wounding in transgenic plants. Plant Cell. 1989 Jan;1(1):151–158. doi: 10.1105/tpc.1.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann J., Mayer J. E., Schell J., Willmitzer L. Differential expression of genes in potato tubers after wounding. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1136–1140. doi: 10.1073/pnas.85.4.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapid switching of plant gene expression induced by fungal elicitor. Science. 1985 Mar 8;227(4691):1240–1243. doi: 10.1126/science.227.4691.1240. [DOI] [PubMed] [Google Scholar]
- Ryder T. B., Hedrick S. A., Bell J. N., Liang X. W., Clouse S. D., Lamb C. J. Organization and differential activation of a gene family encoding the plant defense enzyme chalcone synthase in Phaseolus vulgaris. Mol Gen Genet. 1987 Dec;210(2):219–233. doi: 10.1007/BF00325687. [DOI] [PubMed] [Google Scholar]
- Sanchez-Serrano J. J., Keil M., O'Connor A., Schell J., Willmitzer L. Wound expression of a potato proteinase inhibitor II gene in transgenic tobacco plants. EMBO J. 1987 Feb;6(2):303–306. doi: 10.1002/j.1460-2075.1987.tb04754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somssich I. E., Schmelzer E., Bollmann J., Hahlbrock K. Rapid activation by fungal elicitor of genes encoding "pathogenesis-related" proteins in cultured parsley cells. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2427–2430. doi: 10.1073/pnas.83.8.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford A., Bevan M., Northcote D. Differential expression within a family of novel wound-induced genes in potato. Mol Gen Genet. 1989 Jan;215(2):200–208. doi: 10.1007/BF00339718. [DOI] [PubMed] [Google Scholar]
- Thornburg R. W., An G., Cleveland T. E., Johnson R., Ryan C. A. Wound-inducible expression of a potato inhibitor II-chloramphenicol acetyltransferase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1987 Feb;84(3):744–748. doi: 10.1073/pnas.84.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]