Abstract
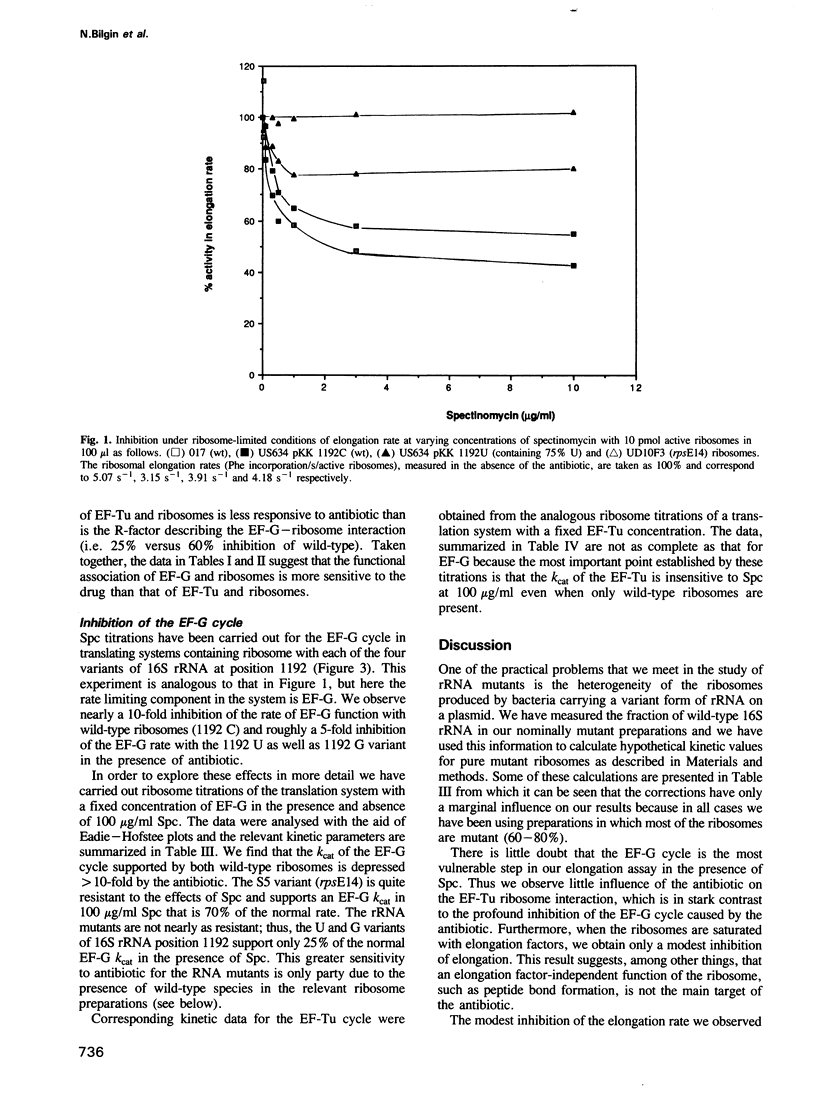
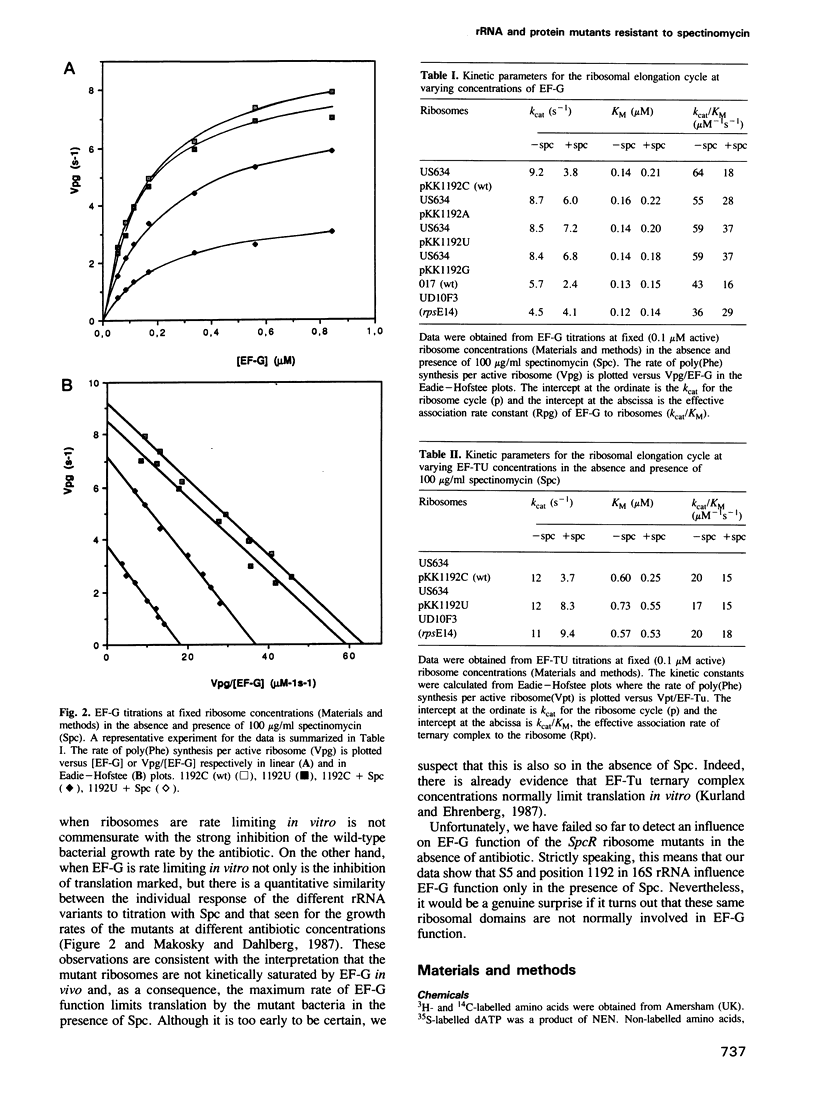
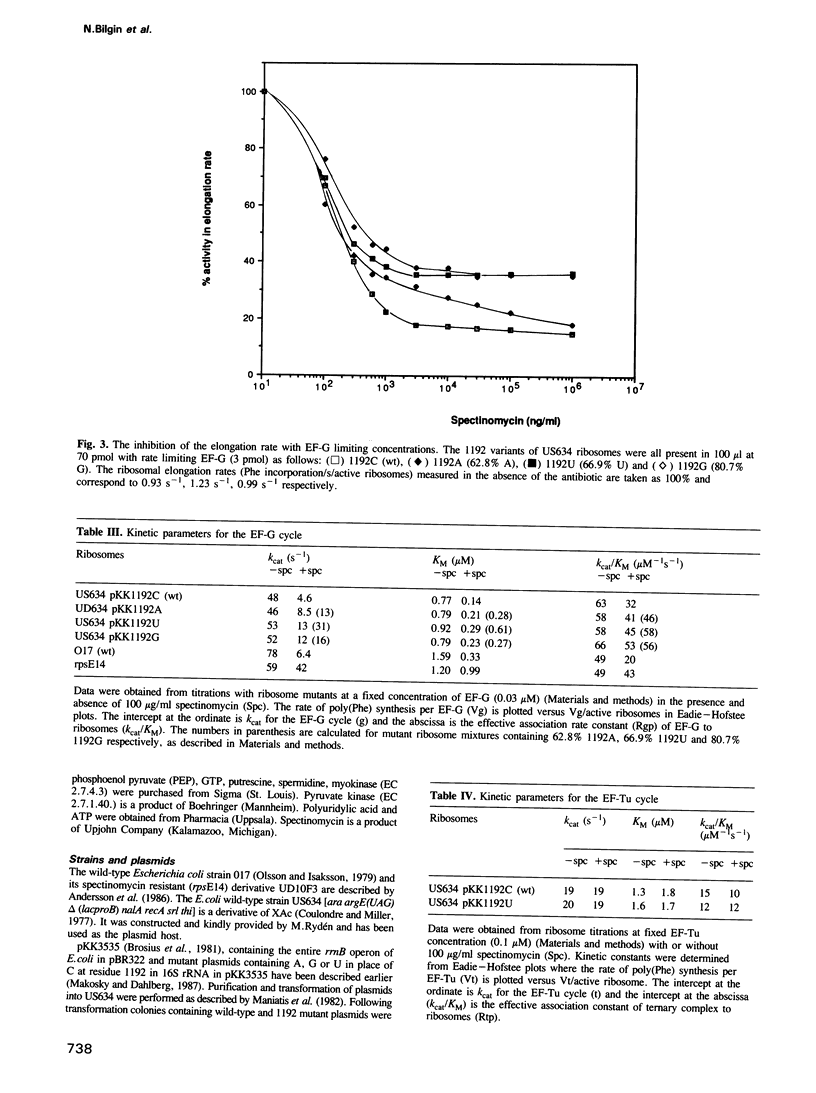
We have compared the influence of spectinomycin (Spc) on individual partial reactions during the elongation phase of translation in vitro by wild-type and mutant ribosomes. The data show that the antibiotic specifically inhibits the elongation factor G (EF-G) cycle supported by wild-type ribosomes. In addition, we have reproduced the in vivo Spc resistant phenotype of relevant ribosome mutants in our in vitro translation system. In particular, three mutants with alterations at position 1192 in 16S rRNA as well as an rpsE mutant with an alteration of protein S5 were analysed. All of these ribosomal mutants confer a degree of Spc resistance for the EF-G cycle in vitro that is correlated with the degree of growth rate resistance to the antibiotic in culture.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P., Davies J., Davis B. D. Effect of spectinomycin on polypeptide synthesis in extracts of Escherichia coli. J Mol Biol. 1967 Oct 14;29(1):203–215. doi: 10.1016/0022-2836(67)90191-x. [DOI] [PubMed] [Google Scholar]
- Andersson D. I., Andersson S. G., Kurland C. G. Functional interactions between mutated forms of ribosomal proteins S4, S5 and S12. Biochimie. 1986 May;68(5):705–713. doi: 10.1016/s0300-9084(86)80164-x. [DOI] [PubMed] [Google Scholar]
- Bilgin N., Kirsebom L. A., Ehrenberg M., Kurland C. G. Mutations in ribosomal proteins L7/L12 perturb EF-G and EF-Tu functions. Biochimie. 1988 May;70(5):611–618. doi: 10.1016/0300-9084(88)90244-1. [DOI] [PubMed] [Google Scholar]
- Bollen A., Davies J., Ozaki M., Mizushima S. Ribosomal protein conferring sensitivity to the antibiotic spectinomycin in Escherichia coli. Science. 1968 Jul 4;165(3888):85–86. [PubMed] [Google Scholar]
- Brosius J., Ullrich A., Raker M. A., Gray A., Dull T. J., Gutell R. R., Noller H. F. Construction and fine mapping of recombinant plasmids containing the rrnB ribosomal RNA operon of E. coli. Plasmid. 1981 Jul;6(1):112–118. doi: 10.1016/0147-619x(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Burns D. J., Cundliffe E. Bacterial-protein synthesis. A novel system for studying antibiotic action in vivo. Eur J Biochem. 1973 Sep 3;37(3):570–574. doi: 10.1111/j.1432-1033.1973.tb03020.x. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. III. Additional correlation of mutational sites with specific amino acid residues. J Mol Biol. 1977 Dec 15;117(3):525–567. doi: 10.1016/0022-2836(77)90056-0. [DOI] [PubMed] [Google Scholar]
- Gillam I., Millward S., Blew D., von Tigerstrom M., Wimmer E., Tener G. M. The separation of soluble ribonucleic acids on benzoylated diethylaminoethylcellulose. Biochemistry. 1967 Oct;6(10):3043–3056. doi: 10.1021/bi00862a011. [DOI] [PubMed] [Google Scholar]
- Jelenc P. C., Kurland C. G. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenc P. C. Rapid purification of highly active ribosomes from Escherichia coli. Anal Biochem. 1980 Jul 1;105(2):369–374. doi: 10.1016/0003-2697(80)90472-8. [DOI] [PubMed] [Google Scholar]
- Kurland C. G., Ehrenberg M. Growth-optimizing accuracy of gene expression. Annu Rev Biophys Biophys Chem. 1987;16:291–317. doi: 10.1146/annurev.bb.16.060187.001451. [DOI] [PubMed] [Google Scholar]
- Makosky P. C., Dahlberg A. E. Spectinomycin resistance at site 1192 in 16S ribosomal RNA of E. coli: an analysis of three mutants. Biochimie. 1987 Aug;69(8):885–889. doi: 10.1016/0300-9084(87)90216-1. [DOI] [PubMed] [Google Scholar]
- Moazed D., Stern S., Noller H. F. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol. 1986 Feb 5;187(3):399–416. doi: 10.1016/0022-2836(86)90441-9. [DOI] [PubMed] [Google Scholar]
- Olsson M. O., Isaksson L. A. Analysis of rpsD mutations in Escherichia coli. I. Comparison of mutants with various alterations in ribosomal protein S4. Mol Gen Genet. 1979 Feb 1;169(3):251–257. doi: 10.1007/BF00382271. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund C. D., Ettayebi M., Morgan E. A. Antibiotic resistance mutations in 16S and 23S ribosomal RNA genes of Escherichia coli. Nucleic Acids Res. 1984 Jun 11;12(11):4653–4663. doi: 10.1093/nar/12.11.4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. G., Jelenc P. C., Ehrenberg M., Kurland C. G. Rate of elongation of polyphenylalanine in vitro. Eur J Biochem. 1982 Feb;122(1):193–197. doi: 10.1111/j.1432-1033.1982.tb05866.x. [DOI] [PubMed] [Google Scholar]


