Abstract
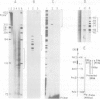
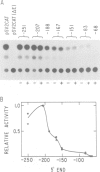
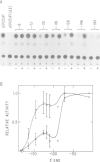
The acetylcholine receptor is a multimeric membrane protein whose expression is activated during muscle differentiation and upon denervation of adult muscle. To gain insight into the coordinate expression of receptor subunits during myogenesis we have analyzed the chick muscle receptor delta-subunit gene upstream region. The delta-subunit gene lacks canonical promoter elements (CCAAT and TATA boxes). Nuclease protection and primer extension analysis revealed that transcription starts at six major and several minor sites between -110 and -30 upstream of the translational initiation site; two sites, at positions -77 and -66, give rise to approximately 50% of all transcripts. Using nested deletions of the proximal 960 bp of the 5' flanking region of this gene we have identified a 62 bp sequence (-207 to -146) that activates transcription in a position independent manner. This enhancer-like element is activated during myotube formation; it contains two distinct functional moieties, each resembling the same 16 bp portion of the stage and tissue specific alpha-subunit gene enhancer which we have characterized previously [Wang et al. (1988) Neuron, 1, 527-534]. This common element, which also comprises several previously proposed skeletal muscle specific motifs [Buskin, J. N. and Hauschka, S. D. (1989) Mol. Cell Biol., 9, 2627-2640; Mar, J. H. and Ordahl, C. P. (1988) Proc. Natl. Acad. Sci. USA, 85, 6404-6408], may account for the coordinate expression of the two subunits. The cell specificity of the delta-subunit gene 5' flanking region is partly due to the enhancer, partly to an inhibitory element upstream of -207.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin T. J., Burden S. J. Isolation and characterization of the mouse acetylcholine receptor delta subunit gene: identification of a 148-bp cis-acting region that confers myotube-specific expression. J Cell Biol. 1988 Dec;107(6 Pt 1):2271–2279. doi: 10.1083/jcb.107.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin T. J., Burden S. J. Muscle-specific gene expression controlled by a regulatory element lacking a MyoD1-binding site. Nature. 1989 Oct 26;341(6244):716–720. doi: 10.1038/341716a0. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Buonanno A., Merlie J. P. Transcriptional regulation of nicotinic acetylcholine receptor genes during muscle development. J Biol Chem. 1986 Sep 5;261(25):11452–11455. [PubMed] [Google Scholar]
- Buskin J. N., Hauschka S. D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989 Jun;9(6):2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T. R., Dorotinsky C., Macy M., Hay R. Cell identity resolved. Nature. 1989 Jul 13;340(6229):106–106. doi: 10.1038/340106b0. [DOI] [PubMed] [Google Scholar]
- Crowder C. M., Merlie J. P. DNase I-hypersensitive sites surround the mouse acetylcholine receptor delta-subunit gene. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8405–8409. doi: 10.1073/pnas.83.21.8405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder C. M., Merlie J. P. Stepwise activation of the mouse acetylcholine receptor delta- and gamma-subunit genes in clonal cell lines. Mol Cell Biol. 1988 Dec;8(12):5257–5267. doi: 10.1128/mcb.8.12.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Simone V., Ciliberto G., Hardon E., Paonessa G., Palla F., Lundberg L., Cortese R. Cis- and trans-acting elements responsible for the cell-specific expression of the human alpha 1-antitrypsin gene. EMBO J. 1987 Sep;6(9):2759–2766. doi: 10.1002/j.1460-2075.1987.tb02570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Ishii S., Xu Y. H., Stratton R. H., Roe B. A., Merlino G. T., Pastan I. Characterization and sequence of the promoter region of the human epidermal growth factor receptor gene. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4920–4924. doi: 10.1073/pnas.82.15.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A., Daubas P., Bourachot B., Changeux J. P. A 5'-flanking region of the chicken acetylcholine receptor alpha-subunit gene confers tissue specificity and developmental control of expression in transfected cells. Mol Cell Biol. 1987 Feb;7(2):951–955. doi: 10.1128/mcb.7.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Mar J. H., Ordahl C. P. A conserved CATTCCT motif is required for skeletal muscle-specific activity of the cardiac troponin T gene promoter. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6404–6408. doi: 10.1073/pnas.85.17.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrogan M., Simonsen C. C., Smouse D. T., Farnham P. J., Schimke R. T. Heterogeneity at the 5' termini of mouse dihydrofolate reductase mRNAs. Evidence for multiple promoter regions. J Biol Chem. 1985 Feb 25;260(4):2307–2314. [PubMed] [Google Scholar]
- Nabel G. J., Gorka C., Baltimore D. T-cell-specific expression of interleukin 2: evidence for a negative regulatory site. Proc Natl Acad Sci U S A. 1988 May;85(9):2934–2938. doi: 10.1073/pnas.85.9.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef P., Mauron A., Stalder R., Alliod C., Ballivet M. Structure linkage, and sequence of the two genes encoding the delta and gamma subunits of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7975–7979. doi: 10.1073/pnas.81.24.7975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir U., Walker M. D., Rutter W. J. Regulation of rat insulin 1 gene expression: evidence for negative regulation in nonpancreatic cells. Proc Natl Acad Sci U S A. 1986 May;83(10):3180–3184. doi: 10.1073/pnas.83.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz-Ares J., Ghosal D., Wienand U., Peterson P. A., Saedler H. The regulatory c1 locus of Zea mays encodes a protein with homology to myb proto-oncogene products and with structural similarities to transcriptional activators. EMBO J. 1987 Dec 1;6(12):3553–3558. doi: 10.1002/j.1460-2075.1987.tb02684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezzementi L., Schmidt J. Ryanodine alters the rate of acetylcholine receptor synthesis in chick skeletal muscle cell cultures. J Biol Chem. 1981 Dec 25;256(24):12651–12654. [PubMed] [Google Scholar]
- Piette J., Klarsfeld A., Changeux J. P. Interaction of nuclear factors with the upstream region of the alpha-subunit gene of chicken muscle acetylcholine receptor: variations with muscle differentiation and denervation. EMBO J. 1989 Mar;8(3):687–694. doi: 10.1002/j.1460-2075.1989.tb03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds G. A., Basu S. K., Osborne T. F., Chin D. J., Gil G., Brown M. S., Goldstein J. L., Luskey K. L. HMG CoA reductase: a negatively regulated gene with unusual promoter and 5' untranslated regions. Cell. 1984 Aug;38(1):275–285. doi: 10.1016/0092-8674(84)90549-x. [DOI] [PubMed] [Google Scholar]
- Schimmel S. D., Kent C., Vagelos P. R. Isolation of plasma membranes from cultured muscle cells. Methods Cell Biol. 1977;15:289–301. doi: 10.1016/s0091-679x(08)60221-6. [DOI] [PubMed] [Google Scholar]
- Shieh B. H., Ballivet M., Schmidt J. Acetylcholine receptor synthesis rate and levels of receptor subunit messenger RNAs in chick muscle. Neuroscience. 1988 Jan;24(1):175–187. doi: 10.1016/0306-4522(88)90321-1. [DOI] [PubMed] [Google Scholar]
- Stratton M. R., Reeves B. R., Cooper C. S. Misidentified cell. Nature. 1989 Jan 26;337(6205):311–312. doi: 10.1038/337311c0. [DOI] [PubMed] [Google Scholar]
- Thanos D., Mavrothalassitis G., Papamatheakis J. Multiple regulatory regions on the 5' side of the mouse E alpha gene. Proc Natl Acad Sci U S A. 1988 May;85(9):3075–3079. doi: 10.1073/pnas.85.9.3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsay H. J., Schmidt J. Skeletal muscle denervation activates acetylcholine receptor genes. J Cell Biol. 1989 Apr;108(4):1523–1526. doi: 10.1083/jcb.108.4.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingron M., Nordheim A., Müller R. Anatomy of fos proteins. Oncogene Res. 1988;3(1):1–7. [PubMed] [Google Scholar]
- Wang Y., Xu H. P., Wang X. M., Ballivet M., Schmidt J. A cell type-specific enhancer drives expression of the chick muscle acetylcholine receptor alpha-subunit gene. Neuron. 1988 Aug;1(6):527–534. doi: 10.1016/0896-6273(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Winoto A., Baltimore D. Alpha beta lineage-specific expression of the alpha T cell receptor gene by nearby silencers. Cell. 1989 Nov 17;59(4):649–655. doi: 10.1016/0092-8674(89)90010-x. [DOI] [PubMed] [Google Scholar]
- Yang J. Q., Bauer S. R., Mushinski J. F., Marcu K. B. Chromosome translocations clustered 5' of the murine c-myc gene qualitatively affect promoter usage: implications for the site of normal c-myc regulation. EMBO J. 1985 Jun;4(6):1441–1447. doi: 10.1002/j.1460-2075.1985.tb03800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. Q., Remmers E. F., Marcu K. B. The first exon of the c-myc proto-oncogene contains a novel positive control element. EMBO J. 1986 Dec 20;5(13):3553–3562. doi: 10.1002/j.1460-2075.1986.tb04682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]