Abstract
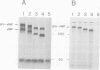
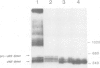
The precursor protein of von Willebrand factor (pro-vWF) consist of four repeated domains, denoted D1-D2-D'-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2. The domains D1 and D2 constitute the amino-terminal pro-polypeptide and the remaining domains mature vWF, generated upon proteolytic processing. We have shown previously that the pro-polypeptide of pro-vWF is obligatory for assembly of pro-vWF dimers into multimers, a process vital for efficient adhesion of platelets to an injured vessel wall. Here, we have employed full length vWF cDNA to construct a series of deletion mutants, based on the homology between the various domains. Specifically, the domains D', D3 or both were deleted and the multimeric pattern of the mutant vWF proteins was analysed after transient expression in COS-1 cells. It is demonstrated that in addition to the pro-polypeptide, both the D' and the D3 domain are required for multimer assembly. Furthermore, by analysing a construct containing only the domains D' and D3 next to the pro-polypeptide it is shown that this is the only part of the vWF protein involved in multimer assembly. Since, the formation of pro-vWF dimers relies on the carboxy-terminal area of mature vWF, it is concluded that multimer assembly is a process independent of dimerization.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonthron D. T., Handin R. I., Kaufman R. J., Wasley L. C., Orr E. C., Mitsock L. M., Ewenstein B., Loscalzo J., Ginsburg D., Orkin S. H. Structure of pre-pro-von Willebrand factor and its expression in heterologous cells. Nature. 1986 Nov 20;324(6094):270–273. doi: 10.1038/324270a0. [DOI] [PubMed] [Google Scholar]
- Fretto L. J., Fowler W. E., McCaslin D. R., Erickson H. P., McKee P. A. Substructure of human von Willebrand factor. Proteolysis by V8 and characterization of two functional domains. J Biol Chem. 1986 Nov 25;261(33):15679–15689. [PubMed] [Google Scholar]
- Girma J. P., Meyer D., Verweij C. L., Pannekoek H., Sixma J. J. Structure-function relationship of human von Willebrand factor. Blood. 1987 Sep;70(3):605–611. [PubMed] [Google Scholar]
- Hoyer L. W., Shainoff J. R. Factor VIII-related protein circulates in normal human plasma as high molecular weight multimers. Blood. 1980 Jun;55(6):1056–1059. [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of antihemophilic factor antigen by cultured human endothelial cells. J Clin Invest. 1973 Nov;52(11):2757–2764. doi: 10.1172/JCI107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loesberg C., Gonsalves M. D., Zandbergen J., Willems C., van Aken W. G., Stel H. V., Van Mourik J. A., De Groot P. G. The effect of calcium on the secretion of factor VIII-related antigen by cultured human endothelial cells. Biochim Biophys Acta. 1983 Sep 22;763(2):160–168. doi: 10.1016/0167-4889(83)90039-3. [DOI] [PubMed] [Google Scholar]
- Luthman H., Magnusson G. High efficiency polyoma DNA transfection of chloroquine treated cells. Nucleic Acids Res. 1983 Mar 11;11(5):1295–1308. doi: 10.1093/nar/11.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch D. C., Zimmerman T. S., Ling E. H., Browning P. J. An explanation for minor multimer species in endothelial cell-synthesized von Willebrand factor. J Clin Invest. 1986 Jun;77(6):2048–2051. doi: 10.1172/JCI112535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti T., Rösselet S. J., Titani K., Walsh K. A. Identification of disulfide-bridged substructures within human von Willebrand factor. Biochemistry. 1987 Dec 15;26(25):8099–8109. doi: 10.1021/bi00399a013. [DOI] [PubMed] [Google Scholar]
- Martin S. E., Marder V. J., Francis C. W., Barlow G. H. Structural studies of the functional heterogeneity of von Willebrand protein polymers. Blood. 1981 Feb;57(2):313–323. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Nachman R., Levine R., Jaffe E. A. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977 Oct;60(4):914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinders J. H., de Groot P. G., Dawes J., Hunter N. R., van Heugten H. A., Zandbergen J., Gonsalves M. D., van Mourik J. A. Comparison of secretion and subcellular localization of von Willebrand protein with that of thrombospondin and fibronectin in cultured human vascular endothelial cells. Biochim Biophys Acta. 1985 Mar 21;844(3):306–313. doi: 10.1016/0167-4889(85)90131-4. [DOI] [PubMed] [Google Scholar]
- Ruggeri Z. M., Zimmerman T. S. The complex multimeric composition of factor VIII/von Willebrand factor. Blood. 1981 Jun;57(6):1140–1143. [PubMed] [Google Scholar]
- Shelton-Inloes B. B., Titani K., Sadler J. E. cDNA sequences for human von Willebrand factor reveal five types of repeated domains and five possible protein sequence polymorphisms. Biochemistry. 1986 Jun 3;25(11):3164–3171. doi: 10.1021/bi00359a014. [DOI] [PubMed] [Google Scholar]
- Sporn L. A., Chavin S. I., Marder V. J., Wagner D. D. Biosynthesis of von Willebrand protein by human megakaryocytes. J Clin Invest. 1985 Sep;76(3):1102–1106. doi: 10.1172/JCI112064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn L. A., Marder V. J., Wagner D. D. Inducible secretion of large, biologically potent von Willebrand factor multimers. Cell. 1986 Jul 18;46(2):185–190. doi: 10.1016/0092-8674(86)90735-x. [DOI] [PubMed] [Google Scholar]
- Titani K., Kumar S., Takio K., Ericsson L. H., Wade R. D., Ashida K., Walsh K. A., Chopek M. W., Sadler J. E., Fujikawa K. Amino acid sequence of human von Willebrand factor. Biochemistry. 1986 Jun 3;25(11):3171–3184. doi: 10.1021/bi00359a015. [DOI] [PubMed] [Google Scholar]
- Verweij C. L., Diergaarde P. J., Hart M., Pannekoek H. Full-length von Willebrand factor (vWF) cDNA encodes a highly repetitive protein considerably larger than the mature vWF subunit. EMBO J. 1986 Aug;5(8):1839–1847. doi: 10.1002/j.1460-2075.1986.tb04435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., Hart M., Pannekoek H. Expression of variant von Willebrand factor (vWF) cDNA in heterologous cells: requirement of the pro-polypeptide in vWF multimer formation. EMBO J. 1987 Oct;6(10):2885–2890. doi: 10.1002/j.1460-2075.1987.tb02591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij C. L., Hart M., Pannekoek H. Proteolytic cleavage of the precursor of von Willebrand factor is not essential for multimer formation. J Biol Chem. 1988 Jun 15;263(17):7921–7924. [PubMed] [Google Scholar]
- Wagner D. D., Fay P. J., Sporn L. A., Sinha S., Lawrence S. O., Marder V. J. Divergent fates of von Willebrand factor and its propolypeptide (von Willebrand antigen II) after secretion from endothelial cells. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1955–1959. doi: 10.1073/pnas.84.7.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Marder V. J. Biosynthesis of von Willebrand protein by human endothelial cells: processing steps and their intracellular localization. J Cell Biol. 1984 Dec;99(6):2123–2130. doi: 10.1083/jcb.99.6.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. D., Mayadas T., Marder V. J. Initial glycosylation and acidic pH in the Golgi apparatus are required for multimerization of von Willebrand factor. J Cell Biol. 1986 Apr;102(4):1320–1324. doi: 10.1083/jcb.102.4.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R. J., Pittman D. D., Handin R. I., Kaufman R. J., Orkin S. H. The propeptide of von Willebrand factor independently mediates the assembly of von Willebrand multimers. Cell. 1988 Jan 29;52(2):229–236. doi: 10.1016/0092-8674(88)90511-9. [DOI] [PubMed] [Google Scholar]
- van Mourik J. A., Bolhuis P. A. Dispersity of human factor VIII--Von Willebrand factor. Thromb Res. 1978 Jul;13(1):15–24. doi: 10.1016/0049-3848(78)90105-6. [DOI] [PubMed] [Google Scholar]
- van Zonneveld A. J., Veerman H., Pannekoek H. Autonomous functions of structural domains on human tissue-type plasminogen activator. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4670–4674. doi: 10.1073/pnas.83.13.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]