Abstract
We tested the effect of systematic destruction of all three lac operators of the chromosomal lac operon of Escherichia coli on repression by Lac repressor. Absence of just one 'pseudo-operator' O2 or O3 decreases repression by wild-type tetrameric Lac repressor approximately 2- to 3-fold; absence of both 'pseudo-operators' decreases repression greater than 50-fold. O1 alone represses under these conditions only approximately 20-fold. Dimeric active Lac repressor (iadi) represses the wild-type lac operon to about the same low extent. This indicates that cooperative interaction between lac operators is due to DNA loop formation mediated by tetrameric Lac repressor. Under conditions where loop formation is impossible, occupation of O3 but not of O2 may lead to weak repression. This suggests that under these conditions CAP activation may be inhibited and that stopping transcription at O2 does not significantly contribute to repression.
Full text
PDF
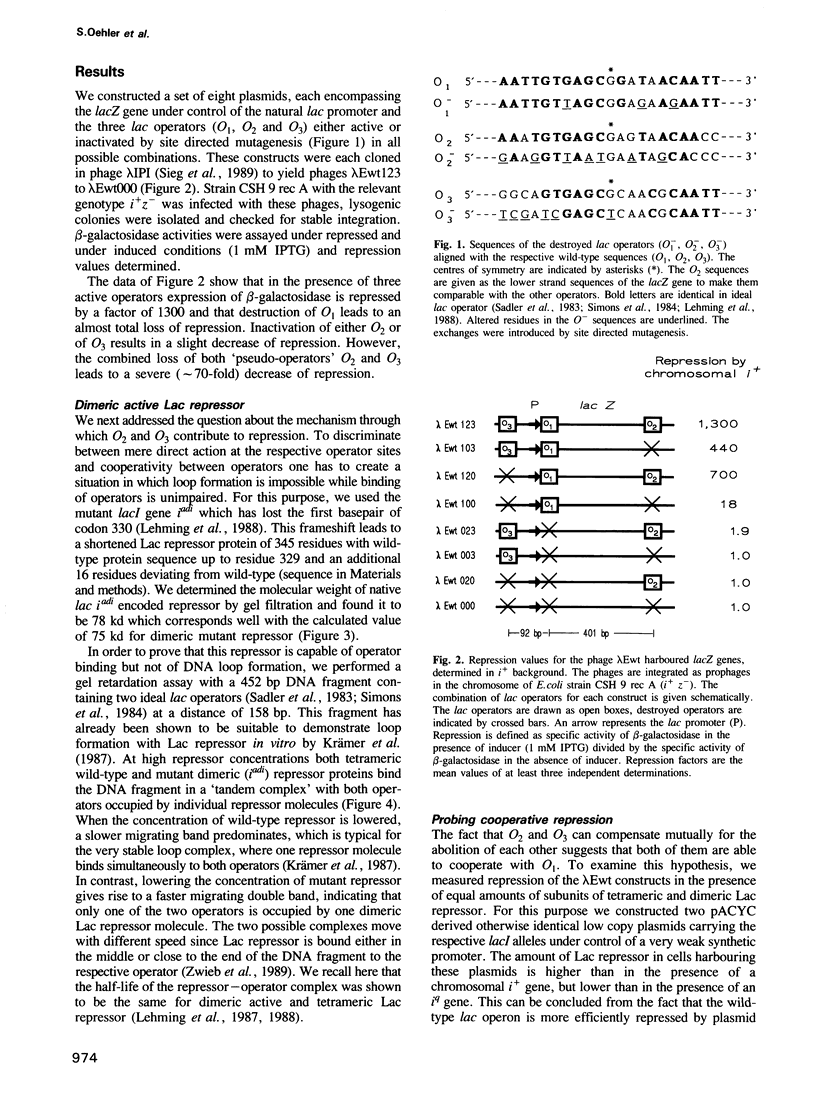



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. I. LA BIOSYNTH'ESE INDUITE DE LA GALACTOKINASE ET L'INDUCTION SIMULTAN'EE DE LA S'EQUENCE ENZYMATIQUE. J Mol Biol. 1963 Aug;7:164–182. doi: 10.1016/s0022-2836(63)80044-3. [DOI] [PubMed] [Google Scholar]
- Besse M., von Wilcken-Bergmann B., Müller-Hill B. Synthetic lac operator mediates repression through lac repressor when introduced upstream and downstream from lac promoter. EMBO J. 1986 Jun;5(6):1377–1381. doi: 10.1002/j.1460-2075.1986.tb04370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowiec J. A., Zhang L., Sasse-Dwight S., Gralla J. D. DNA supercoiling promotes formation of a bent repression loop in lac DNA. J Mol Biol. 1987 Jul 5;196(1):101–111. doi: 10.1016/0022-2836(87)90513-4. [DOI] [PubMed] [Google Scholar]
- Calos M. P. DNA sequence for a low-level promoter of the lac repressor gene and an 'up' promoter mutation. Nature. 1978 Aug 24;274(5673):762–765. doi: 10.1038/274762a0. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Culard F., Maurizot J. C. Lac repressor - lac operator interaction. Circular dichroism study. Nucleic Acids Res. 1981 Oct 10;9(19):5175–5184. doi: 10.1093/nar/9.19.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandanell G., Valentin-Hansen P., Larsen J. E., Hammer K. Long-range cooperativity between gene regulatory sequences in a prokaryote. 1987 Feb 26-Mar 4Nature. 325(6107):823–826. doi: 10.1038/325823a0. [DOI] [PubMed] [Google Scholar]
- Dunn T. M., Hahn S., Ogden S., Schleif R. F. An operator at -280 base pairs that is required for repression of araBAD operon promoter: addition of DNA helical turns between the operator and promoter cyclically hinders repression. Proc Natl Acad Sci U S A. 1984 Aug;81(16):5017–5020. doi: 10.1073/pnas.81.16.5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eismann E., von Wilcken-Bergmann B., Müller-Hill B. Specific destruction of the second lac operator decreases repression of the lac operon in Escherichia coli fivefold. J Mol Biol. 1987 Jun 20;195(4):949–952. doi: 10.1016/0022-2836(87)90499-2. [DOI] [PubMed] [Google Scholar]
- Flashner Y., Gralla J. D. Dual mechanism of repression at a distance in the lac operon. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8968–8972. doi: 10.1073/pnas.85.23.8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz H. J., Bicknäse H., Gleumes B., Heibach C., Rosahl S., Ehring R. Characterization of two mutations in the Escherichia coli galE gene inactivating the second galactose operator and comparative studies of repressor binding. EMBO J. 1983;2(12):2129–2135. doi: 10.1002/j.1460-2075.1983.tb01713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galas D. J., Schmitz A. DNAse footprinting: a simple method for the detection of protein-DNA binding specificity. Nucleic Acids Res. 1978 Sep;5(9):3157–3170. doi: 10.1093/nar/5.9.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gho D., Miller J. H. Deletions fusing the i and lac regions of the chromosome in E. coli: isolation and mapping. Mol Gen Genet. 1974;131(2):137–146. doi: 10.1007/BF00266149. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. The lac operator is DNA. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2415–2421. doi: 10.1073/pnas.58.6.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. J., Burns P. A., Fix D. F., Yatagai F., Allen F. L., Horsfall M. J., Halliday J. A., Gray J., Bernelot-Moens C., Glickman B. W. Missense mutation in the lacI gene of Escherichia coli. Inferences on the structure of the repressor protein. J Mol Biol. 1988 Mar 20;200(2):239–251. doi: 10.1016/0022-2836(88)90237-9. [DOI] [PubMed] [Google Scholar]
- Haber R., Adhya S. Interaction of spatially separated protein-DNA complexes for control of gene expression: operator conversions. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9683–9687. doi: 10.1073/pnas.85.24.9683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia H. C., Lebkowski J. S., Leong P. M., Calos M. P., Miller J. H. Comparison of ultraviolet irradiation-induced mutagenesis of the lacI gene in Escherichia coli and in human 293 cells. J Mol Biol. 1989 Jan 5;205(1):103–113. doi: 10.1016/0022-2836(89)90368-9. [DOI] [PubMed] [Google Scholar]
- Irani M. H., Orosz L., Adhya S. A control element within a structural gene: the gal operon of Escherichia coli. Cell. 1983 Mar;32(3):783–788. doi: 10.1016/0092-8674(83)90064-8. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Johnson A. D., Meyer B. J., Ptashne M. Interactions between DNA-bound repressors govern regulation by the lambda phage repressor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5061–5065. doi: 10.1073/pnas.76.10.5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. D., Poteete A. R., Lauer G., Sauer R. T., Ackers G. K., Ptashne M. lambda Repressor and cro--components of an efficient molecular switch. Nature. 1981 Nov 19;294(5838):217–223. doi: 10.1038/294217a0. [DOI] [PubMed] [Google Scholar]
- Kania J., Brown D. T. The functional repressor parts of a tetrameric lac repressor-beta-galactosidase chimaera are organized as dimers. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3529–3533. doi: 10.1073/pnas.73.10.3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kania J., Müller-Hill B. Construction, isolation and implications of repressor-galactosidase - beta-galactosidase hybrid molecules. Eur J Biochem. 1977 Oct 3;79(2):381–386. doi: 10.1111/j.1432-1033.1977.tb11819.x. [DOI] [PubMed] [Google Scholar]
- Kuhnke G., Krause A., Heibach C., Gieske U., Fritz H. J., Ehring R. The upstream operator of the Escherichia coli galactose operon is sufficient for repression of transcription initiated at the cyclic AMP-stimulated promoter. EMBO J. 1986 Jan;5(1):167–173. doi: 10.1002/j.1460-2075.1986.tb04192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehming N., Sartorius J., Niemöller M., Genenger G., v Wilcken-Bergmann B., Müller-Hill B. The interaction of the recognition helix of lac repressor with lac operator. EMBO J. 1987 Oct;6(10):3145–3153. doi: 10.1002/j.1460-2075.1987.tb02625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehming N., Sartorius J., Oehler S., von Wilcken-Bergmann B., Müller-Hill B. Recognition helices of lac and lambda repressor are oriented in opposite directions and recognize similar DNA sequences. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7947–7951. doi: 10.1073/pnas.85.21.7947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal N., Su W., Haber R., Adhya S., Echols H. DNA looping in cellular repression of transcription of the galactose operon. Genes Dev. 1990 Mar;4(3):410–418. doi: 10.1101/gad.4.3.410. [DOI] [PubMed] [Google Scholar]
- Mossing M. C., Record M. T., Jr Upstream operators enhance repression of the lac promoter. Science. 1986 Aug 22;233(4766):889–892. doi: 10.1126/science.3090685. [DOI] [PubMed] [Google Scholar]
- O'Gorman R. B., Dunaway M., Matthews K. S. DNA binding characteristics of lactose repressor and the trypsin-resistant core repressor. J Biol Chem. 1980 Nov 10;255(21):10100–10106. [PubMed] [Google Scholar]
- Pfahl M. Characteristics of tight binding repressors of the lac operon. J Mol Biol. 1981 Mar 25;147(1):1–10. doi: 10.1016/0022-2836(81)90075-9. [DOI] [PubMed] [Google Scholar]
- Pfahl M., Gulde V., Bourgeois S. "Second" and "third operator" of the lac operon: an investigation of their role in the regulatory mechanism. J Mol Biol. 1979 Jan 25;127(3):339–344. doi: 10.1016/0022-2836(79)90333-4. [DOI] [PubMed] [Google Scholar]
- Reitzer L. J., Magasanik B. Transcription of glnA in E. coli is stimulated by activator bound to sites far from the promoter. Cell. 1986 Jun 20;45(6):785–792. doi: 10.1016/0092-8674(86)90553-2. [DOI] [PubMed] [Google Scholar]
- Reznikoff W. S., Winter R. B., Hurley C. K. The location of the repressor binding sites in the lac operon. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2314–2318. doi: 10.1073/pnas.71.6.2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler J. R., Sasmor H., Betz J. L. A perfectly symmetric lac operator binds the lac repressor very tightly. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6785–6789. doi: 10.1073/pnas.80.22.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasse-Dwight S., Gralla J. D. Probing co-operative DNA-binding in vivo. The lac O1:O3 interaction. J Mol Biol. 1988 Jul 5;202(1):107–119. doi: 10.1016/0022-2836(88)90523-2. [DOI] [PubMed] [Google Scholar]
- Schleif R. Gene regulation: why should DNA loop? Nature. 1987 Jun 4;327(6121):369–370. doi: 10.1038/327369a0. [DOI] [PubMed] [Google Scholar]
- Sieg K., Kun J., Pohl I., Scherf A., Müller-Hill B. A versatile phage lambda expression vector system for cloning in Escherichia coli. Gene. 1989 Feb 20;75(2):261–270. doi: 10.1016/0378-1119(89)90272-2. [DOI] [PubMed] [Google Scholar]
- Simons A., Tils D., von Wilcken-Bergmann B., Müller-Hill B. Possible ideal lac operator: Escherichia coli lac operator-like sequences from eukaryotic genomes lack the central G X C pair. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1624–1628. doi: 10.1073/pnas.81.6.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straney S. B., Crothers D. M. Lac repressor is a transient gene-activating protein. Cell. 1987 Dec 4;51(5):699–707. doi: 10.1016/0092-8674(87)90093-6. [DOI] [PubMed] [Google Scholar]
- Vidal-Ingigliardi D., Raibaud O. The mac promoters: functional hybrid promoters activated by the malT product and repressed by the lacI product. Nucleic Acids Res. 1985 Feb 25;13(4):1163–1172. doi: 10.1093/nar/13.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitson P. A., Hsieh W. T., Wells R. D., Matthews K. S. Influence of supercoiling and sequence context on operator DNA binding with lac repressor. J Biol Chem. 1987 Oct 25;262(30):14592–14599. [PubMed] [Google Scholar]
- Whitson P. A., Hsieh W. T., Wells R. D., Matthews K. S. Supercoiling facilitates lac operator-repressor-pseudooperator interactions. J Biol Chem. 1987 Apr 15;262(11):4943–4946. [PubMed] [Google Scholar]
- Winter R. B., von Hippel P. H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 2. The Escherichia coli repressor--operator interaction: equilibrium measurements. Biochemistry. 1981 Nov 24;20(24):6948–6960. doi: 10.1021/bi00527a029. [DOI] [PubMed] [Google Scholar]
- Zwieb C., Kim J., Adhya S. DNA bending by negative regulatory proteins: Gal and Lac repressors. Genes Dev. 1989 May;3(5):606–611. doi: 10.1101/gad.3.5.606. [DOI] [PubMed] [Google Scholar]