Abstract
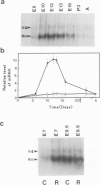
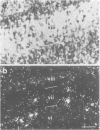
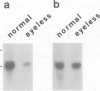
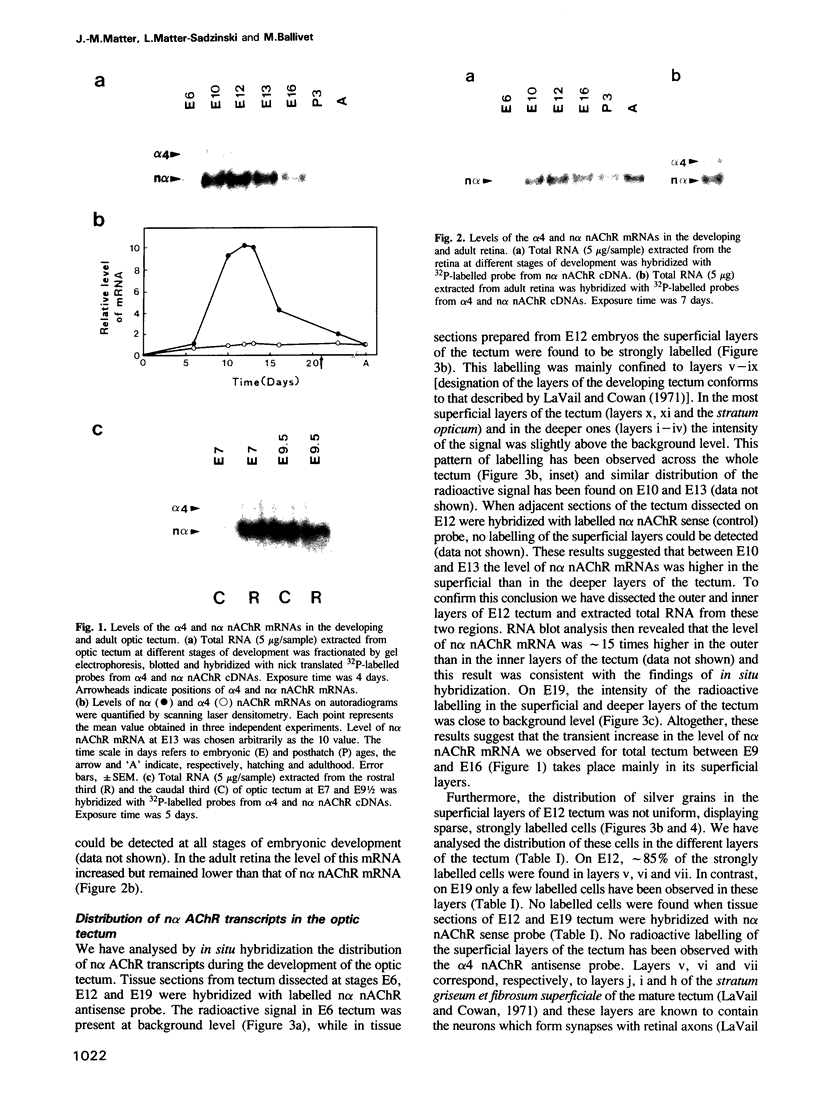
Expression of the neuronal non-alpha nicotinic acetylcholine receptor (n alpha nAChR) gene is transiently stimulated in the chick optic tectum between embryonic days 7 and 16 with a peak value reached around embryonic day 12. This stimulation takes place at the time when optic nerve axons are invading this region of the brain and proceeds along a rostral to caudal gradient. Transcripts of the n alpha nAChR gene are localized in the superficial layers of the tectum at the time when cells in these layers are forming synapses with retina axons. The transient expression of n alpha nAChR gene does not take place in the optic tectum of 'eyeless' embryos. The results of our study suggest that the neuronal n alpha nAChR gene may play a role in neurogenesis of retino-tectal connections.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballivet M., Nef P., Couturier S., Rungger D., Bader C. R., Bertrand D., Cooper E. Electrophysiology of a chick neuronal nicotinic acetylcholine receptor expressed in Xenopus oocytes after cDNA injection. Neuron. 1988 Nov;1(9):847–852. doi: 10.1016/0896-6273(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Boulter J., Connolly J., Deneris E., Goldman D., Heinemann S., Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulter J., Evans K., Goldman D., Martin G., Treco D., Heinemann S., Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. 1986 Jan 30-Feb 5Nature. 319(6052):368–374. doi: 10.1038/319368a0. [DOI] [PubMed] [Google Scholar]
- Boyd R. T., Jacob M. H., Couturier S., Ballivet M., Berg D. K. Expression and regulation of neuronal acetylcholine receptor mRNA in chick ciliary ganglia. Neuron. 1988 Aug;1(6):495–502. doi: 10.1016/0896-6273(88)90180-8. [DOI] [PubMed] [Google Scholar]
- Cauley K., Agranoff B. W., Goldman D. Identification of a novel nicotinic acetylcholine receptor structural subunit expressed in goldfish retina. J Cell Biol. 1989 Feb;108(2):637–645. doi: 10.1083/jcb.108.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Crossland W. J., Cowan W. M., Rogers L. A. Studies on the development of the chick optic tectum. IV. An autoradiographic study of the development of retino-tectal connections. Brain Res. 1975 Jun 20;91(1):1–23. doi: 10.1016/0006-8993(75)90463-1. [DOI] [PubMed] [Google Scholar]
- Deneris E. S., Connolly J., Boulter J., Wada E., Wada K., Swanson L. W., Patrick J., Heinemann S. Primary structure and expression of beta 2: a novel subunit of neuronal nicotinic acetylcholine receptors. Neuron. 1988 Mar;1(1):45–54. doi: 10.1016/0896-6273(88)90208-5. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Goldberg S. Studies on the mechanics of development of the visual pathways in the chick embryo. Dev Biol. 1974 Jan;36(1):24–43. doi: 10.1016/0012-1606(74)90188-2. [DOI] [PubMed] [Google Scholar]
- Goldman D., Deneris E., Luyten W., Kochhar A., Patrick J., Heinemann S. Members of a nicotinic acetylcholine receptor gene family are expressed in different regions of the mammalian central nervous system. Cell. 1987 Mar 27;48(6):965–973. doi: 10.1016/0092-8674(87)90705-7. [DOI] [PubMed] [Google Scholar]
- Henley J. M., Lindstrom J. M., Oswald R. E. Acetylcholine receptor synthesis in retina and transport to optic tectum in goldfish. Science. 1986 Jun 27;232(4758):1627–1629. doi: 10.1126/science.3715468. [DOI] [PubMed] [Google Scholar]
- Jacob M. H., Berg D. K., Lindstrom J. M. Shared antigenic determinant between the Electrophorus acetylcholine receptor and a synaptic component on chicken ciliary ganglion neurons. Proc Natl Acad Sci U S A. 1984 May;81(10):3223–3227. doi: 10.1073/pnas.81.10.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. P., Cowan W. M. Studies on the development of the chick optic tectum. 3. Effects of early eye removal. Brain Res. 1972 Jul 20;42(2):263–288. doi: 10.1016/0006-8993(72)90530-6. [DOI] [PubMed] [Google Scholar]
- Khandjian E. W. UV crosslinking of RNA to nylon membrane enhances hybridization signals. Mol Biol Rep. 1986;11(2):107–115. doi: 10.1007/BF00364822. [DOI] [PubMed] [Google Scholar]
- LaVail J. H., Cowan W. M. The development of the chick optic tectum. I. Normal morphology and cytoarchitectonic development. Brain Res. 1971 May 21;28(3):391–419. doi: 10.1016/0006-8993(71)90053-9. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McGraw C. F., McLaughlin B. J. Fine structural studies of synaptogenesis in the superficial layers of the chick optic tectum. J Neurocytol. 1980 Feb;9(1):79–93. doi: 10.1007/BF01205228. [DOI] [PubMed] [Google Scholar]
- McLoon S. C. Evidence for shifting connections during development of the chick retinotectal projection. J Neurosci. 1985 Oct;5(10):2570–2580. doi: 10.1523/JNEUROSCI.05-10-02570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nef P., Oneyser C., Alliod C., Couturier S., Ballivet M. Genes expressed in the brain define three distinct neuronal nicotinic acetylcholine receptors. EMBO J. 1988 Mar;7(3):595–601. doi: 10.1002/j.1460-2075.1988.tb02852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Leary D. D., Cowan W. M. Topographic organization of certain tectal afferent and efferent connections can develop normally in the absence of retinal input. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6131–6135. doi: 10.1073/pnas.80.19.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent P. B., Pike S. H., Nadel D. B., Lindstrom J. M. Nicotinic acetylcholine receptor-like molecules in the retina, retinotectal pathway, and optic tectum of the frog. J Neurosci. 1989 Feb;9(2):565–573. doi: 10.1523/JNEUROSCI.09-02-00565.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepfer R., Whiting P., Esch F., Blacher R., Shimasaki S., Lindstrom J. cDNA clones coding for the structural subunit of a chicken brain nicotinic acetylcholine receptor. Neuron. 1988 May;1(3):241–248. doi: 10.1016/0896-6273(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Swanson L. W., Lindstrom J., Tzartos S., Schmued L. C., O'Leary D. D., Cowan W. M. Immunohistochemical localization of monoclonal antibodies to the nicotinic acetylcholine receptor in chick midbrain. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4532–4536. doi: 10.1073/pnas.80.14.4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson L. W., Simmons D. M., Whiting P. J., Lindstrom J. Immunohistochemical localization of neuronal nicotinic receptors in the rodent central nervous system. J Neurosci. 1987 Oct;7(10):3334–3342. doi: 10.1523/JNEUROSCI.07-10-03334.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada E., Wada K., Boulter J., Deneris E., Heinemann S., Patrick J., Swanson L. W. Distribution of alpha 2, alpha 3, alpha 4, and beta 2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989 Jun 8;284(2):314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- Wada K., Ballivet M., Boulter J., Connolly J., Wada E., Deneris E. S., Swanson L. W., Heinemann S., Patrick J. Functional expression of a new pharmacological subtype of brain nicotinic acetylcholine receptor. Science. 1988 Apr 15;240(4850):330–334. doi: 10.1126/science.2832952. [DOI] [PubMed] [Google Scholar]
- Whiting P. J., Liu R., Morley B. J., Lindstrom J. M. Structurally different neuronal nicotinic acetylcholine receptor subtypes purified and characterized using monoclonal antibodies. J Neurosci. 1987 Dec;7(12):4005–4016. doi: 10.1523/JNEUROSCI.07-12-04005.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]