Abstract
Potassium channels importantly contribute to the regulation of vascular smooth muscle (VSM) contraction and growth. They are the dominant ion conductance of the VSM cell membrane and importantly determine and regulate membrane potential. Membrane potential, in turn, regulates the open-state probability of voltage-gated Ca2+ channels (VGCC), Ca2+ influx through VGCC, intracellular Ca2+ and VSM contraction. Membrane potential also affects release of Ca2+ from internal stores and the Ca2+ sensitivity of the contractile machinery such that K+ channels participate in all aspects of regulation of VSM contraction. Potassium channels also regulate proliferation of VSM cells through membrane potential-dependent and membrane potential-independent mechanisms. Vascular smooth muscle cells express multiple isoforms of at least five classes of K+ channels contribute to the regulation of contraction and cell proliferation (growth). This review will examine the structure, expression and function of large-conductance, Ca2+-activated K+ (BKCa) channels, intermediate-conductance Ca2+-activated K+ (KCa3.1) channels, multiple isoforms of voltage-gated K+ (KV) channels, ATP-sensitive K+ (KATP) channels, and inward-rectifier K+ (KIR) channels in both contractile and proliferating VSM cells.
Keywords: Potassium channels, vascular smooth muscle, membrane potential, vasoconstriction, vasodilation, proliferation, phenotypic modulation
Introduction
Potassium channels importantly contribute to the regulation of vascular smooth muscle (VSM) contraction and growth. They are the dominant ion conductance of the VSM cell membrane and importantly determine and regulate VSM cell membrane potential (Jackson, 2000, 2005). Membrane potential, in turn, regulates the open-state probability of voltage-gated Ca2+ channels (VGCC), Ca2+ influx through these channels, intracellular Ca2+ and VSM contraction (Jackson, 2000, 2005). Membrane potential also affects release of Ca2+ from internal stores and the Ca2+ sensitivity of the contractile machinery such that K+ channels participate in all aspects of regulation of VSM contraction (del Valle-Rodriguez, Lopez-Barneo, & Urena, 2003; Fernández-Tenorio et al., 2010; Fernandez-Tenorio et al., 2011; V. Y. Ganitkevich & Isenberg, 1993; Kukuljan, Rojas, Catt, & Stojilkovic, 1994; Q. H. Liu et al., 2009; Mahaut-Smith, Martinez-Pinna, & Gurung, 2008; Okada, Yanagisawa, & Taira, 1993; Urena, del Valle-Rodriguez, & Lopez-Barneo, 2007; Yamagishi, Yanagisawa, & Taira, 1992; Yamamura, Ohya, Muraki, & Imaizumi, 2012; Yanagisawa, Yamagishi, & Okada, 1993). Potassium channels also contribute to the regulation of proliferation of VSM cells through membrane potential-dependent (Bi et al., 2013; Miguel-Velado et al., 2005; Miguel-Velado et al., 2010) and membrane potential-independent mechanisms (Cidad et al., 2012; Cidad et al., 2015; Jimenez-Perez et al., 2016).
Vascular smooth muscle cells express multiple isoforms of at least five classes of K+ channels that participate in the regulation of contraction and cell proliferation (growth). These include large-conductance, Ca2+-activated K+ (BKCa) channels, intermediate-conductance Ca2+-activated K+ (KCa3.1) channels, multiple isoforms of voltage-gated K+ (KV) channels, ATP-sensitive K+ (KATP) channels, inward-rectifier K+ (KIR) channels, and members of the two-pore K+ (K2P) channel family of K+ channels. Subsequent sections of this review will examine the function of K+ channels in the regulation of VSM cell contraction and proliferation. The expression and function of K2P channels in VSM cells will not be addressed and the reader is referred to the literature for information on these channels (Feliciangeli, Chatelain, Bichet, & Lesage, 2015; Gurney & Manoury, 2009; O’Connell, Morton, & Hunter, 2002; Renigunta, Schlichthorl, & Daut, 2015; Sepulveda, Pablo Cid, Teulon, & Niemeyer, 2015).
Potassium channels and regulation of VSM contraction
Setting the stage
VSM cells, in small arteries and arterioles that develop myogenic tone when pressurized, are relatively depolarized, with membrane potentials on the order of −45 to −30 mV (Burns, Cohen, & Jackson, 2004; Emerson & Segal, 2000; Knot & Nelson, 1998; Siegl, Koeppen, Wolfle, Pohl, & de Wit, 2005; Welsh, Jackson, & Segal, 1998). At physiological ion concentrations (3–5 mM K+ extracellular, 140 mM K+ intracellular), the electrochemical gradient for K+ (the driving force for movement of K+ through a K+ channel) is outward. This means that opening of K+ channels will lead to K+ diffusion out of the cell, loss of positive charge and membrane hyperpolarization (M. T. Nelson, Patlak, Worley, & Standen, 1990). Conversely, closure of open K+ channels will result in a decrease in this hyperpolarizing current, and membrane depolarization. The resistance of the plasma membrane of VSM cells to current flow is very high, on the order of 1–10 GΩ (M. T. Nelson, Patlak, et al., 1990). This means that very small currents produced by only a few active K+ channels can have very large effects on membrane potential (M. T. Nelson, Patlak, et al., 1990). Voltage-gated Ca2+ channels contribute substantially to the regulation of intracellular Ca2+ and contraction of VSM cells in differentiated, contractile VSM cells, particularly in resistance arteries and arterioles (M. T. Nelson, Patlak, et al., 1990). Voltage-dependent activation (depolarization) and deactivation (hyperpolarization) of these channels importantly regulates VSM contraction (M. T. Nelson, Patlak, et al., 1990).
The structure of the ion-conducting pore of K+ channels is thought to be similar across all of the channels based on studies of KcsA, a two transmembrane (TM) domain K+ channel from Streptomyces lividans (Kuang, Purhonen, & Hebert, 2015) (Figure 2). As shown in Figures 2B and 2C, the pore is formed by TM2 and the P-loop, which connects TM1 and TM2. Conserved residues in the P-loop (Thr-Val-Gly-Tyr-Gly) comprise the K+ selectivity filter (red highlighted segment of the P-loop in Figure 2B) (Kuang et al., 2015). In BKCa channels and KV channels, segment 6 (S6) and the P-loop between S5 and S6 form the channels’ pores (Figures 3 and 4) (Kuang et al., 2015).
Figure 2.
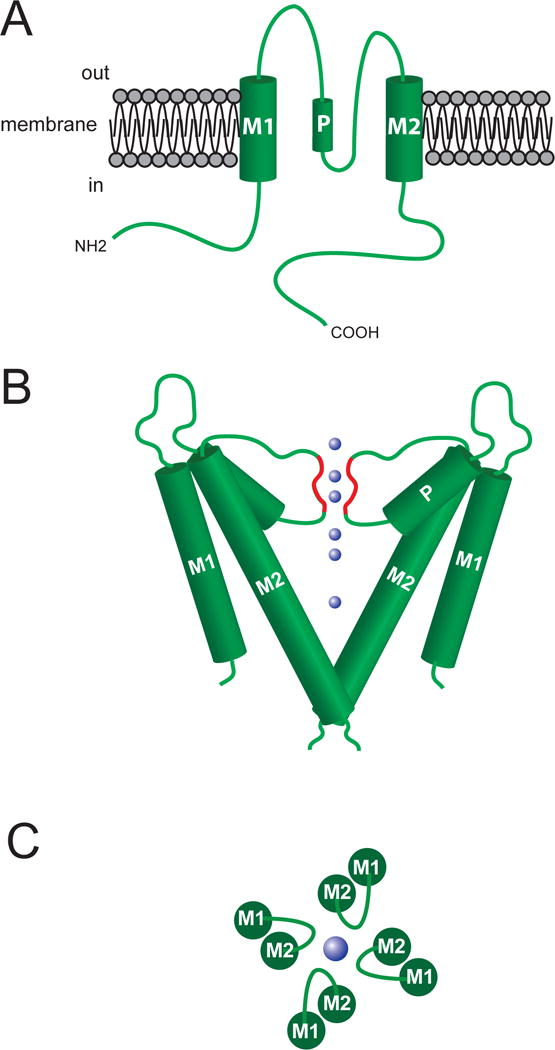
Structure of the ion conducting pore of K+ channels. Top panel (A) shows a schematic representation of a two membrane spanning domain (M1 and M2) K+ channel. Functional channels are formed from a tetramer of these units, with the ion-conducting pore being formed by M2 and the P-loop domain that connects M1 and M2 (P in figure refers to the pore helical domain). Middle panel (B) shows approximate orientation of two sets of the M1, M2 and P-loops forming the channel. The blue spheres represent K+ ions, and the red highlighted regions of the P-loops represent the selectivity filter of the channel’s pore. The bottom panel (C) shows a top view of the channel subunits and the P-loop forming the K+ ion-conducting pore. See text for references.
Figure 3.
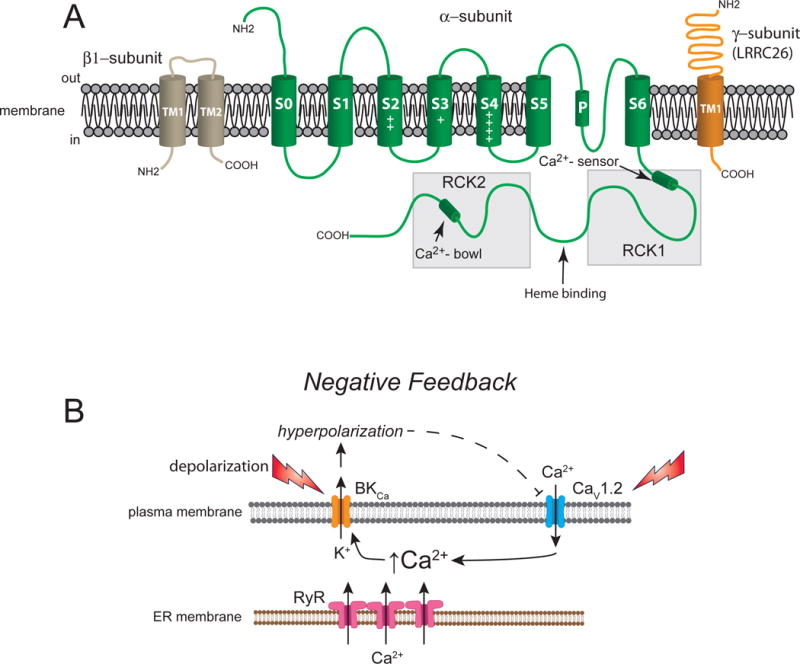
Vascular BKCa channels. Panel A shows a β1- subunit with two membrane-spanning domains, one pore-forming α-subunit with seven membrane-spanning domains and a γ-subunit (LRRC26, for example) with one membrane-spanning domain. Panel B shows a schematic of the primary negative feedback role for BKCa channels in contractile VSM. Membrane depolarization (due to activation of other membrane channels, not shown), or increases in intracellular Ca2+ in the vicinity of BKCa channels due to release of Ca2+ from ryanodine receptors (RyR, Ca2+ sparks), or influx of Ca2+ through L-type voltage gated Ca2+ channels (CaV1.2), results in activation and opening of BKCa channels. The efflux of K+ through these channels leads to membrane hyperpolarization and closure of CaV1.2 channels, negative feedback regulation of VSM excitability.
Figure 4.
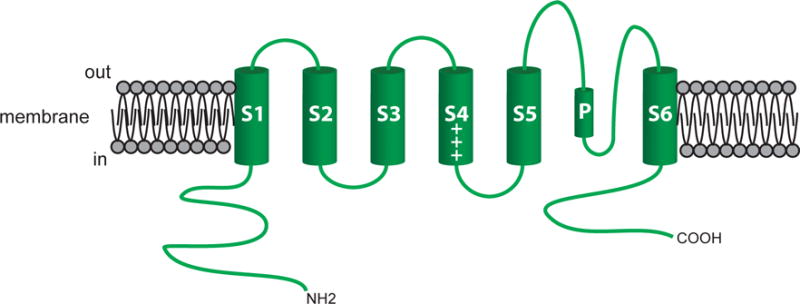
The pore-forming α-subunit of KV channels. Shown is a schematic of the 6 membrane spanning domains of a typical KV channel. The length and composition of the carboxy (COOH) and amino (NH2) varies among the large number of KV channel isoforms expressed in VSM cells. See text for more information.
BKCa channels and VSM contraction
Vascular smooth muscle cells typically express a high density of BKCa channels in their plasma membranes that importantly contribute to the negative-feedback regulation of vascular tone (Figure 3). A homo-tetramer of α-subunits encoded by the KCNMA1 gene composes the channels (Butler, Tsunoda, McCobb, Wei, & Salkoff, 1993; Pallanck & Ganetzky, 1994) (Figure 3A). Segment 6 and the P-loop connecting S5 and S6 form the ion-conducting pore (Meera, Wallner, Song, & Toro, 1997) (Figure 3A). Two regulator of K+ conductance (RCK) domains (RCK1 and RCK2) in the C-terminus of the α-subunits contain the channel’s Ca2+ sensors (Hoshi, Pantazis, & Olcese, 2013) (Figure 3A). Positively charged residues in transmembrane domains S2, S3 and S4 serve as the channel’s voltage sensors (Hoshi, Pantazis, et al., 2013) (Figure 3A).
Accessory β1-subunits (locus: KCNMB1) slow gating kinetics, increase the Ca2+ sensitivity, and affect the pharmacology of the channels (McManus et al., 1995; Meera, Wallner, Jiang, & Toro, 1996; Tseng-Crank et al., 1996) (Figure 2). Activation of VSM BKCa channels by 17β-estradiol, lithocholate, dehydroepiandrosterone. dehydrosoyasaponin-I and docosahexaenoic acid (DHA) requires the presence of β1-subunits (Hoshi, Tian, Xu, Heinemann, & Hou, 2013; Hou, Heinemann, & Hoshi, 2009). These subunits also contribute to dynamic trafficking of α-subunits to plasma membrane (Leo et al., 2014). The degree of coupling between the α-subunits and the β1-subunits may account for the high Ca2+-setpoint observed in arteriolar BKCa channels (Yang et al., 2009; Yang, Sohma, et al., 2013).
In addition to the β1-subunits, leucine-rich-repeat-containing proteins (LRRCs), such as LRRC26, have been proposed as γ-subunits of VSM BKCa channels (Evanson, Bannister, Leo, & Jaggar, 2014). These subunits interact with BKCa channels, increasing both their voltage-sensitivity and channel activation by agents such as NS1619 (Evanson et al., 2014).
Membrane depolarization and increases in intracellular Ca2+ activate BKCa channels (Figure 3B). In many resistance arteries and arterioles that develop pressure-induced myogenic tone, ex vivo, BKCa channels are active and contribute to resting VSM membrane potential. However, the source of Ca2+ responsible for BKCa channel activation may differ dependent on the anatomical origin of the vessel. Calcium released from groups of ryanodine receptors (RyR) in the sub-plasma membrane endoplasmic reticulum, in the form of Ca2+ sparks, control the activity of overlying BKCa channels in many resistance arteries (Brenner et al., 2000; Bychkov, Gollasch, Ried, Luft, & Haller, 1997; Furstenau et al., 2000; Gollasch et al., 2000; Jaggar, Porter, Lederer, & Nelson, 2000; Jaggar, Stevenson, & Nelson, 1998; Jaggar, Wellman, et al., 1998; Knot, Standen, & Nelson, 1998; M.T. Nelson et al., 1995; M. T. Nelson & Quayle, 1995; Perez, Bonev, & Nelson, 2001; Perez, Bonev, Patlak, & Nelson, 1999; Porter et al., 1998; Wellman et al., 2002; Wellman & Nelson, 2003). In contrast, Ca2+ influx through VGCC may activate BKCa channels in other vessels including hamster and mouse cremaster arterioles (Westcott, Goodwin, Segal, & Jackson, 2012; Westcott & Jackson, 2011), rabbit coronary arteries (Guia, Wan, Courtemanche, & Leblanc, 1999) and mouse mesenteric arteries (Y. Suzuki, Yamamura, Ohya, & Imaizumi, 2013). In striated muscle resistance arteries, both RyR-based Ca2+ sparks and VGCC Ca2+-influx contribute to activation of BKCa channels (Westcott et al., 2012; Westcott & Jackson, 2011) suggesting that both mechanisms may be active in some cells. In cerebral VSM cells, Ca2+ influx through T-type, CaV3.2 VGCC stimulates RyR-based Ca2+ sparks contributing to the negative feedback regulation of myogenic tone (Harraz et al., 2014; Harraz et al., 2015).
Vasoconstrictors have been reported to both activate (Berczi, Stekiel, Contney, & Rusch, 1992; Brayden & Nelson, 1992; V. Ganitkevich & Isenberg, 1990; Hashemzadeh-Gargari & Rembold, 1992; Jackson & Blair, 1998; M.T. Nelson et al., 1995; M. T. Nelson & Quayle, 1995; Rusch & Liu, 1997; Wakatsuki, Nakaya, & Inoue, 1992) and inhibit (Lange, Gebremedhin, Narayanan, & Harder, 1997; Scornik & Toro, 1992; Toro, Amador, & Stefani, 1990; Wesselman, Schubert, VanBavel, Nilsson, & Mulvany, 1997) BKCa channels. Activation of BKCa channels would tend to hyperpolarize VSM cells, deactivate VGCC and limit VSM contraction, essentially preventing vasospasm (Figure 3B). This activation results from both vasoconstrictor-induced depolarization and increases in intracellular Ca2+ (Figure 3B). In contrast, inhibition of BKCa channels would promote depolarization and would enhance VSM contraction, in a positive feedback fashion. Protein kinase C (PKC), which is commonly activated by vasoconstrictors that activate Gq/11-coupled, heptihelical receptors, may be involved in this process in some blood vessels (Lange et al., 1997; Minami, Fukuzawa, & Nakaya, 1993), and may involve internalization and degradation of BKCa channels (Leo et al., 2015). Despite the evidence for inhibition of BKCa channel activity, the dominant effect of vasoconstrictors in most blood vessels is to activate BKCa channels.
Vasodilators that act through receptors coupled to the guanine nucleotide binding protein, Gαs, and formation of cAMP activate BKCa channels, as part of their mechanism of action (Kume, Graziano, & Kotlikoff, 1992; Kume, Takai, Tokuno, & Tomita, 1989; Sadoshima, Akaike, Kanaide, & Nakamura, 1988). Activation of BKCa channels may result from a number of mechanisms. Interaction of Gαs with BKCa channels, independent from cAMP and protein kinase A (PKA), may increase channel activity (Kume et al., 1992; Kume, Hall, Washabau, Takagi, & Kotlikoff, 1994; Scornik, Codina, Birnbaumer, & Toro, 1993). Protein kinase A-dependent phosphorylation of the α-subunits also can activate BKCa channels (Nara, Dhulipala, Wang, & Kotlikoff, 1998; Tian et al., 2004; Tian et al., 2001). Increased trafficking of the β1-subunits to the plasma membrane may also contribute to the mechanism of action of cAMP-related agonists (Matsumoto, Szasz, Tostes, & Webb, 2012). Vasodilators that act via cAMP and PKA increase BKCa channel activity by increasing Ca2+ spark activity (Porter et al., 1998; Wellman, Santana, Bonev, & Nelson, 2001; Yamaguchi, Kajita, & Madison, 1995). In addition, exchange proteins activated by cAMP (EPACs) participate in cAMP-related activation of BKCa channels in VSM (Roberts, Kamishima, Barrett-Jolley, Quayle, & Dart, 2013). Thus, cAMP-related vasodilators may activate BKCa channels by a number of mechanisms.
Endothelium-derived NO, nitrovasodilators that release NO and other vasodilators that act through the cGMP-protein kinase G (PKG) signaling cascade also have been proposed to activate BKCa channels (Fujino et al., 1991; P. L. Li, Zou, & Campbell, 1997; Robertson, Schubert, Hescheler, & Nelson, 1993; Taniguchi, Furukawa, & Shigekawa, 1993; Williams, Katz, Roy-Contancin, & Reuben, 1988; Winquist et al., 1985; Winquist, Faison, & Nutt, 1984). This may occur through modulation of Ca2+ sparks (Jewell, Saundry, Bonev, Tranmer, & Wellman, 2004; Mandala, Heppner, Bonev, & Nelson, 2007; Yuill, McNeish, Kansui, Garland, & Dora, 2010), by phosphorylation of the channels by PKG (Alioua, Huggins, & Rousseau, 1995; Swayze & Braun, 2001) or altered channel trafficking (Leo et al., 2014). Activation of BKCa channels by NO, independent from cGMP has also been proposed (Ahern, Hsu, & Jackson, 1999; Bolotina, Najibi, Palacino, Pagano, & Cohen, 1994; Buxton, Kaiser, Malmquist, & Tichenor, 2001; Lang, Harvey, McPhee, & Klemm, 2000; Lang, Harvey, & Mulholland, 2003; P. L. Li, Jin, & Campbell, 1998; Lovren & Triggle, 2000; Mistry & Garland, 1998; Plane, Hurrell, Jeremy, & Garland, 1996; Plane, Sampson, Smith, & Garland, 2001; Yu, Sun, Maier, Harder, & Roman, 2002; Zhang, Tazzeo, Chu, & Janssen, 2006). In contrast, there are also a number of studies that have failed to demonstrate participation of BKCa channels in the mechanism of action of NO (Armstead, 1997; Bialecki & Stinson-Fisher, 1995; Brayden, 1990; Cooke, Rossitch, Andon, Loscalzo, & Dzau, 1991; H. Dong, Waldron, Galipeau, Cole, & Triggle, 1997; Fukami et al., 1998; Garland & McPherson, 1992; Ghisdal, Gomez, & Morel, 2000; Hansen & Olesen, 1997; Hernanz et al., 1999; Kilpatrick & Cocks, 1994; Plane & Garland, 1993; Plane, Wiley, Jeremy, Cohen, & Garland, 1998; Taguchi, Heistad, Kitazono, & Faraci, 1995; Wellman & Bevan, 1995; Zhu, Beny, Flammer, Luscher, & Haefliger, 1997). Thus, there may be regional or species differences that account for the presence or lack of effect of NO on BKCa channel activity.
Carbon monoxide (Abraham & Kappas, 2008; Jaggar et al., 2002; Jaggar et al., 2005; A. Li et al., 2008; R. Wang, Wang, & Wu, 1997; R. Wang & Wu, 1997; R. Wang, Wu, & Wang, 1997; Xi et al., 2004; Xi et al., 2010), epoxyeicosatrienoic acids (EETs) (Campbell, Gebremedhin, Pratt, & Harder, 1996; Earley, Heppner, Nelson, & Brayden, 2005; Eckman, Hopkins, McBride, & Keef, 1998), H2O2 (Barlow & White, 1998) (Cheranov & Jaggar, 2006; Thengchaisri & Kuo, 2003), and H2S (Jackson-Weaver et al., 2013; Liang, Xi, Leffler, & Jaggar, 2012) all may activate BKCa channels. Effects of CO on BKCa channels may be direct via associated heme proteins that interact with the C-terminal domain of the α-subunits, between RCK1 and RCK2 (Jaggar et al., 2005) or by interaction with histidine residues in RCK1 (Hou et al., 2009) (Figure 3A). In addition, CO augments Ca2+ spark frequency and their coupling to BKCa channels (Jaggar et al., 2002; A. Li et al., 2008; Xi et al., 2010). Epoxyeicosatrienoic acids also may stimulate Ca2+ sparks to activate BKCa channels through actions of EETs on transient receptor potential (TRP) V4 channels (Earley et al., 2005). Stimulation of Ca2+ sparks also may underlie the activation of BKCa channels by H2S (Jackson-Weaver et al., 2013; Liang et al., 2012).
Diseases and VSM BKCa channels
The effects of disease states on BKCa channel expression and function is complex. The activity of BKCa channels appears to be depressed in obesity (Borbouse et al., 2009; Borbouse et al., 2010; Frisbee, Maier, & Stepp, 2002; Nystoriak et al., 2014; Ozkor et al., 2011; Rusch, 2009), diabetes (L. Dong et al., 2009; Fernandez-Velasco, Ruiz-Hurtado, Gomez, & Rueda, 2014; Y. Liu & Gutterman, 2002; McGahon et al., 2007; Mokelke, Dietz, Eckman, Nelson, & Sturek, 2005; Nystoriak et al., 2014; Y. Wang et al., 2010; Yi et al., 2014; W. Zhou, Wang, Lamping, & Lee, 2006) and some models of aging (Albarwani, Al-Siyabi, Baomar, & Hassan, 2010; Marijic et al., 2001). In diabetes, the impaired function of BKCa channels involves reduced expression and function of the β1-subunits (McGahon et al., 2007; Nystoriak et al., 2014; Yi et al., 2014). Diabetes and hyperglycemia results in increased proteolytic degradation of β1-subunits via Nuclear Factor (NF)-κB-dependent expression and function of the muscle RING finger protein 1 (MuRF1) ubiquitin ligase (Yi et al., 2014). The down-regulation of the β1-subunit in diabetes also may involve activation of calcineurin/nuclear factor of activated T-cells, cytoplasmic 3 (NFATC3) signaling that is facilitated by A-kinase anchoring protein 150 (AKAP150) (Nystoriak et al., 2014).
However, the effects of hypertension on BKCa channel function are not clear, because both increased (Asano, Masuzawa-Ito, & Matsuda, 1993; Asano, Matsuda, Hayakawa, Ito, & Ito, 1993; Y. Liu, Pleyte, Knaus, & Rusch, 1997; Paterno, Heistad, & Faraci, 1997; Rusch, Delucena, Wooldridge, England, & Cowley, 1992; Rusch & Liu, 1997; Zhang, Gao, Zuo, Lee, & Janssen, 2005) and reduced (Amberg, Bonev, Rossow, Nelson, & Santana, 2003; Amberg & Santana, 2003; Ambroisine et al., 2007; Bratz, Dick, Partridge, & Kanagy, 2005; Bratz, Swafford, Kanagy, & Dick, 2005; Callera, Yogi, Tostes, Rossoni, & Bendhack, 2004; Z. Li, Lu, & Shi, 2014; Moreno-Dominguez, Cidad, Miguel-Velado, Lopez-Lopez, & Perez-Garcia, 2009; Nieves-Cintron, Amberg, Nichols, Molkentin, & Santana, 2007; Yang, Li, et al., 2013) function has been reported. Differences in hypertension models, duration of hypertension, type of blood vessel and the species studied may account for the lack of consensus on the effects of hypertension on BKCa channel function.
KV channels and VSM contraction
Vascular smooth muscle cells express a diverse array of KV channels that include members of the KV1 (loci: KCNA2-6)(Cox, 2005), KV2 (loci: KCNB2-3) (Cox, 2005), KV3 (loci: KCNC2-4)(Cox, 2005), KV4 (loci: KCND1-3) (Cox, 2005), KV6.3 (locus: KCNG3) (Moreno-Dominguez et al., 2009), KV7 (loci:KCNQ1, KCNQ4-5) (Greenwood & Ohya, 2009; Jepps, Olesen, & Greenwood, 2013; Mackie & Byron, 2008) and KV9.3 (locus: KCNS3) (Cox, 2005) families of KV channels. They consist of homo- or heterotetramers of α-subunits (Figure 4) (Kuang et al., 2015). Segment 6 (S6) and the P-loop between S5 and S6 forms the channel’s pore, as noted above (Kuang et al., 2015). Positively charged residues in S4 confer voltage sensitivity to the channels (Figure 4) (Kuang et al., 2015). Modulatory accessory subunits accompany many KV channels, affecting channel membrane expression, gating kinetics, and voltage sensitivity (Gutman et al., 2005).
Membrane depolarization activates KV channels, and, in general, they participate in the negative feedback regulation of VSM contraction along with BKCa channels. Consistent with this negative-feedback role, block of KV channels potentiates VSM contraction induced by vasoconstrictors (Chadha et al., 2014; Cheong, Dedman, & Beech, 2001; Cheong, Dedman, Xu, & Beech, 2001; Cook, 1989; Hald et al., 2012; Martinez et al., 2009; Pagan et al., 2009; Shimizu, Yokoshiki, Sperelakis, & Paul, 2000). Voltage-gated K+ channels are active at the resting membrane potential of VSM cells in blood vessels displaying myogenic tone; closure of these channels leads to membrane depolarization and vasoconstriction (Cox, 2005; Jackson, 2000, 2005; M. T. Nelson & Quayle, 1995).
Vasoconstrictors, including phenylephrine (Mistry & Garland, 1999), 5-HT (Bae et al., 2006; Ko, Park, Firth, Hong, et al., 2010; Sung et al., 2013) and angiotensin II (Clement-Chomienne, Walsh, & Cole, 1996) all inhibit KV channels, probably acting through PKC (Clement-Chomienne et al., 1996; Hayabuchi, Standen, & Davies, 2001; Ko, Park, Firth, Hong, et al., 2010), Src tyrosine kinase (Sung et al., 2013), Rho kinase (Luykenaar, Brett, Wu, Wiehler, & Welsh, 2004; Luykenaar, El-Rahman, Walsh, & Welsh, 2009) and/or increased intracellular Ca+ (Cox & Petrou, 1999; Gelband, Ishikawa, Post, Keef, & Hume, 1993; Ishikawa, Hume, & Keef, 1993). This closure of KV channels may contribute to vasoconstrictor-induced VSM cell membrane depolarization and the mechanism of action of vasoconstrictors.
Vasodilators acting through the cAMP-PKA pathway activate KV channels and contribute to their mechanism of action (E. A. Aiello, Malcolm, Walsh, & Cole, 1998; E.A. Aiello, Walsh, & Cole, 1994; E. A. Aiello, Walsh, & Cole, 1995; Berwick et al., 2010; Chadha et al., 2014; Chadha et al., 2012; Dick et al., 2008; H. Dong, Waldron, Cole, & Triggle, 1998; Heaps & Bowles, 2002; Heaps, Tharp, & Bowles, 2005; Khanamiri et al., 2013; H. Li, Chai, Gutterman, & Liu, 2003; Moore, Nelson, Parelkar, Rusch, & Rhee, 2014; Satake, Shibata, & Shibata, 1996). Nitric oxide (Dick et al., 2008; Sobey & Faraci, 1999; Stott, Barrese, Jepps, Leighton, & Greenwood, 2015; Tanaka et al., 2006), H2S (Cheang et al., 2010; Martelli et al., 2013; Rogers, Chilian, Bratz, Bryan, & Dick, 2007; Schleifenbaum et al., 2010), hypoxia (Hedegaard et al., 2014), acidosis (Berger, Vandier, Bonnet, Jackson, & Rusch, 1998) and anticontractile substances release by perivascular adipose tissue (Tano, Schleifenbaum, & Gollasch, 2014; Zavaritskaya et al., 2013) also may activate VSM KV channels in some blood vessels.
Disease and VSM KV channels
The expression and function of KV channels is reduced in diabetes (Bubolz, Li, Wu, & Liu, 2005; Chai, Liu, & Chen, 2005; Chai et al., 2007; Ko, Park, Firth, Kim, et al., 2010; H. Li et al., 2003) that may be mediated by elevated glucose (H. Li, Gutterman, Rusch, Bubolz, & Liu, 2004; Y. Liu, Terata, Rusch, & Gutterman, 2001) and channel nitration (H. Li et al., 2004). The reduced KV channel function may contribute to the increased VSM contractile function that is observed in diabetes.
However, in hypertension and obesity, the impact on KV channels is not as clear. Increased (Cox, Folander, & Swanson, 2001; Cox, Fromme, Folander, & Swanson, 2008), decreased (Bratz, Dick, et al., 2005; Bratz, Swafford, et al., 2005; Cox, 1996; Cox, Lozinskaya, & Dietz, 2001; Y. Liu, Hudetz, Knaus, & Rusch, 1998; Martens & Gelband, 1996; Tobin et al., 2009) or no change (Y. Liu, Jones, & Sturek, 1994; Y. Liu et al., 1997) in KV channel function in hypertension has been reported. There also is no clear effect of obesity on KV channel function, with decreased (Berwick et al., 2012; Dick & Tune, 2010; Nieves-Cintron et al., 2015; Yang, Jones, Thomas, & Rubin, 2007) and increased (J. Jiang, Thoren, Caligiuri, Hansson, & Pernow, 1999; Ko, Park, Firth, Hong, et al., 2010) function reported. Specific effects may depend on the vascular bed studied, the duration and severity of the pathology and the species studied.
KATP Channels and VSM Contraction
Vascular smooth muscle cells express KATP channels that consist of tetramers of pore-forming KIR6.1 subunits (locus: KCNJ8) (Aziz et al., 2014; A. Li et al., 2013; Miki et al., 2002; Miura et al., 2003; M. Suzuki et al., 2001; Yamada et al., 1997), associated with an equal number of accessory sulphonylurea receptors (SUR) 2B (locus: ABCC9) (Adebiyi, McNally, & Jaggar, 2011; Miura et al., 2003; Quayle, Nelson, & Standen, 1997) (Figure 5). These channels were named because millimolar intracellular ATP closes the channels (Foster & Coetzee, 2016). However, their modulation by vasodilator substances is probably more important for their physiological function. Sensitivity to ATP is conferred by the KIR6.1 subunit, whereas sensitivity to channel blockade by sulphonylureas, such as glibenclamide, and activation by agonists such pinacidil and cromakalim resides in the SUR2B subunit (Foster & Coetzee, 2016).
Figure 5.
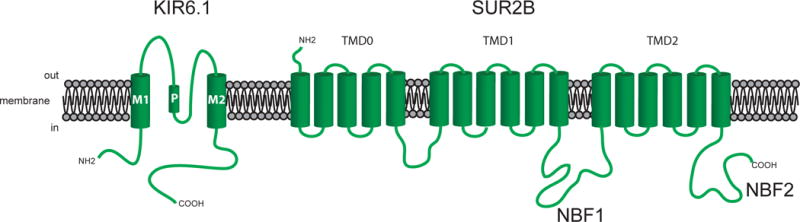
Subunits of KATP channels. Shown are the KIR6.1 and SUR2B subunits that are thought to comprise VSM KATP channels. The KIR6.1 subunits have two membrane spanning domains, whereas the SUR2B subunits have seventeen membrane spanning domains clustered into three groups (TMD0, TMD1, TMD2), as shown. Functional channels are formed from a hetero-octamer of these two subunits. See text for more information and references.
Vascular KATP channels appear to be active under resting conditions in coronary (Berwick et al., 2010; Dankelman, Van der Ploeg, & Spaan, 1994; Duncker, van Zon, Pavek, Herrlinger, & Bache, 1995; Farouque & Meredith, 2007; Farouque, Worthley, & Meredith, 2004; Farouque, Worthley, Meredith, Skyrme-Jones, & Zhang, 2002; Imamura et al., 1992; Jackson, Konig, Dambacher, & Busse, 1993; Merkus et al., 2003; Merkus, Sorop, Houweling, Hoogteijling, & Duncker, 2006; Mori et al., 1995; Randall, 1995; Richmond, Tune, Gorman, & Feigl, 1999, 2000; Samaha, Heineman, Ince, Fleming, & Balaban, 1992; Sharifi-Sanjani et al., 2013; Stepp, Kroll, & Feigl, 1997; X. Zhou, Teng, Tilley, Ledent, & Mustafa, 2014), skin (Abbink et al., 2002; Cankar & Strucl, 2008; Hojs, Strucl, & Cankar, 2009) and renal (Duncker, Oei, Hu, Stubenitsky, & Verdouw, 2001; Holdsworth et al., 2015) circulations, at rest. The resting activity of KATP channels is not as clear in skeletal muscle, because there is evidence both for (Jackson, 1993; Kosmas, Levy, & Hussain, 1995; Saito, McKay, Eraslan, & Hester, 1996; Vanelli, Chang, Gatensby, & Hussain, 1994; Vanelli & Hussain, 1994) and against (Banitt, Smits, Williams, Ganz, & Creager, 1996; Bank, Sih, Mullen, Osayamwen, & Lee, 2000; Bijlstra et al., 1996; Duncker et al., 2001; Farouque & Meredith, 2003a, 2003b, 2003c; Hammer, Ligon, & Hester, 2001; Holdsworth et al., 2015; Murrant & Sarelius, 2002) resting activity of these channels. In the cerebral circulation, KATP channels appear to be closed at rest (Faraci & Heistad, 1998; Horinaka et al., 1997; Leffler et al., 2011; Lindauer, Vogt, Schuh-Hofer, Dreier, & Dirnagl, 2003; Nnorom et al., 2014; Toyoda et al., 1997; Wei & Kontos, 1999).
Vasoconstrictors that activate PKC, close VSM KATP channels (Bonev & Nelson, 1996; Chrissobolis & Sobey, 2002; Cole, Malcolm, Walsh, & Light, 2000; Hayabuchi, Davies, & Standen, 2001; Quinn, Cui, Giblin, Clapp, & Tinker, 2003; Sampson, Davies, Barrett-Jolley, Standen, & Dart, 2007) and cause channel internalization (Jiao, Garg, Yang, Elton, & Hu, 2008). Vasoconstrictor-induced increases in intracellular Ca2+ also lead to KATP channel closure through activation of protein phosphatase 2b (calcineurin) (Wilson, Jabr, & Clapp, 2000). Vasoconstrictor-induced activation of Gi/o signaling also inhibits KATP channels through inhibition of adenylate cyclase, reduced cAMP and decreased channel phosphorylation (Hayabuchi, Davies, et al., 2001).
As with BKCa channels and KV channels, vasodilators that signal through the cAMP signaling pathway activate KATP channels (Akatsuka et al., 1994; Bouchard, Dumont, & Lamontagne, 1994; Dart & Standen, 1993; Eguchi et al., 2007; Jackson, 1993; Kitazono, Heistad, & Faraci, 1993b; Kleppisch & Nelson, 1995; Ming, Parent, & Lavallee, 1997; Nakashima & Vanhoutte, 1995; C. P. Nelson et al., 2011; M. T. Nelson, Huang, Brayden, Hescheler, & Standen, 1990; Quayle, Bonev, Brayden, & Nelson, 1994; Randall, 1995; Sawmiller, Ashtari, Urueta, Leschinsky, & Henning, 2006; Wellman, Quayle, & Standen, 1998; Yang et al., 2008). This may involve phosphorylation of both KIR6.1 (Quinn, Giblin, & Tinker, 2004) and SUR2B (Shi et al., 2008; Shi et al., 2007) subunits. Hydrogen sulfide (Cheng, Ndisang, Tang, Cao, & Wang, 2004; Leffler et al., 2011; Liang et al., 2011; Mustafa et al., 2011; Zhao, Zhang, Lu, & Wang, 2001), acidosis (Faraci, Breese, & Heistad, 1994; Heintz, Damm, Brand, Koch, & Deussen, 2008; Lindauer et al., 2003) and hypoxia (Daut et al., 1990; Loutzenhiser & Parker, 1994; Marshall, Thomas, & Turner, 1993; Nakhostine & Lamontagne, 1993, 1994; Taguchi, Heistad, Kitazono, & Faraci, 1994; Tomiyama, Brian, & Todd, 1999; von Beckerath, Cyrys, Dischner, & Daut, 1991) may act, in part, in some vascular beds, by activation of VSM KATP channels.
Disease and VSM KATP channels
The function of VSM KATP channels appears to be decreased in obesity (Erdos, Miller, & Busija, 2002; Erdos, Simandle, Snipes, Miller, & Busija, 2004; Hodnett, Xiang, Dearman, Carter, & Hester, 2008; Irat, Aslamaci, Karasu, & Ari, 2006; Lu et al., 2013; Miller, Tulbert, Puskar, & Busija, 2002; Spallarossa et al., 2001) and diabetes (Bouchard, Dumont, & Lamontagne, 1999; Kamata, Miyata, & Kasuya, 1989; Kinoshita et al., 2006; S. S. Li et al., 2015; Mayhan, 1994; Mayhan & Faraci, 1993; Miura et al., 2003). However, in hypertension KATP channel function has been reported to be decreased (Ghosh, Hanna, Wang, & McNeill, 2004; Kalliovalkama et al., 1999; Kam, Pfaffendorf, & van Zwieten, 1994; Kawata et al., 1998; Kitazono, Heistad, & Faraci, 1993a; Ohya et al., 1996; Tajada, Cidad, Moreno-Dominguez, Perez-Garcia, & Lopez-Lopez, 2012; Takaba et al., 1996; Van de Voorde, Vanheel, & Leusen, 1992), increased (Furspan & Webb, 1993; Miyata, Tsuchida, & Otomo, 1990) or not changed (Blanco-Rivero et al., 2008; Hutri-Kahonen et al., 1999; Kolias, Chai, & Webb, 1993).
KIR channels and VSM contraction
Vascular smooth muscle cells, particularly those in small resistance arteries and arterioles, also express one or more members of the strong inward rectifier K+ channels, with KIR2.1 (locus: KCNJ2) being the dominant isoform expressed (Longden & Nelson, 2015). These channels are formed from a tetramer of two-membrane spanning domain KIR channel subunits (Kuang et al., 2015) (Figure 6A). Block of the channel pore by intracellular polyamines and Mg2+ is responsible for the strong, voltage-dependent inward current rectification that is characteristic of these channels (Kuang et al., 2015) (Figure 6B).
Figure 6.
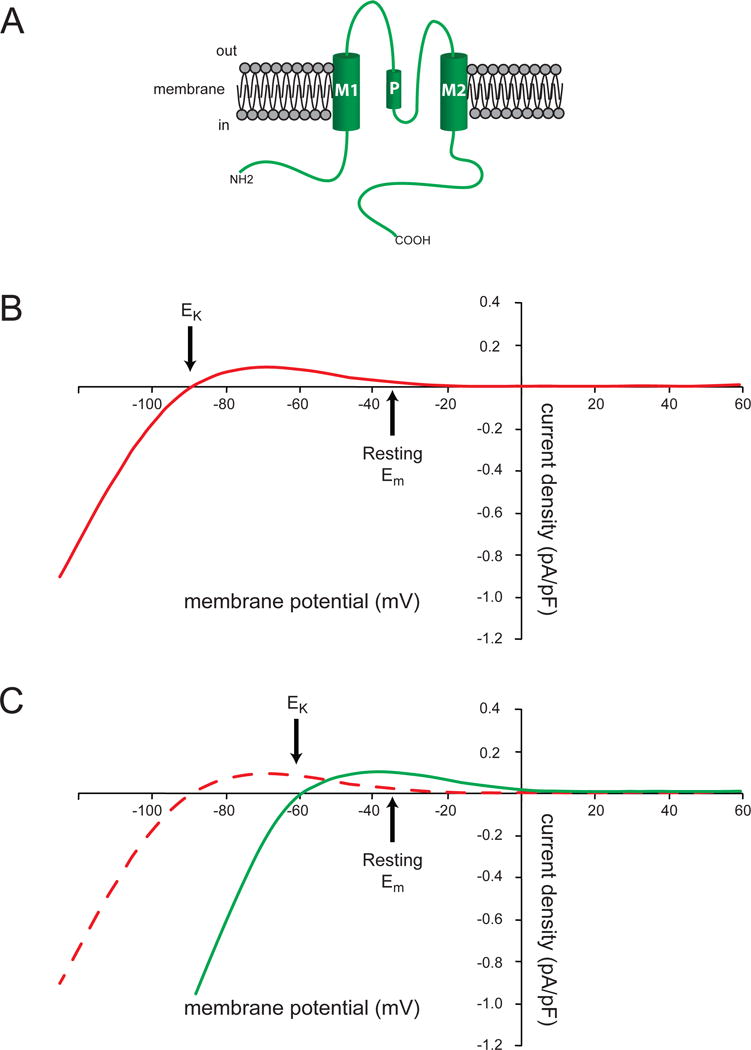
Vascular KIR channels and their currents. Panel A shows the topology of KIR channels with two membrane spanning domains. Functional channels are composed of a tetramer these subunits (see Figure 2). Panel B shows a schematic of the current-voltage-relationship for VSM KIR channels for a cell with 5 mM K+ in the extracellular solution (140 mM K+ intracellular) and is based on data from (Filosa et al., 2006). At membrane potentials more negative than the K+ equilibrium potential (EK, ~−90 mV in 5 mM K+), the channel conducts K+ into the cells, as shown by the negative current density values. At potentials more positive than EK up to ~−30 mV, KIR channels conduct K+ ions out of the cell, and contribute to the resting membrane potential as denoted by the small positive currents at the assumed resting membrane potential of −35 mV. Note that anything that hyperpolarizes the membrane will recruit outward, positive current through KIR channels, effectively amplifying the initial hyperpolarization. Panel C demonstrates the effects of increasing extracellular K+ from 5 mM (red dashed curve) to 15 mM K+ (green solid curve): increased extracellular K+ shifts the EK from −90 mV to ~−60 mV. Note that there is now an elevated outward K+ current at the original resting membrane potential. This enhanced outward K+ current will hyperpolarize the VSM cell membrane from its resting value toward EK, leading to vasodilation.
While these channels derive their name from the inward currents that they conduct at membrane potentials more negative than the K+ equilibrium potential (EK), it is the small, outward “hump” in the current-voltage relationship at potentials positive to EK that contributes to their physiology (Longden & Nelson, 2015; Quayle et al., 1997) (Figure 6B).
Current through KIR channels contributes to the resting membrane potential in a number of vascular beds (Burns et al., 2004; Chilton et al., 2008; Chilton & Loutzenhiser, 2001; Chilton, Smirnov, Loutzenhiser, Wang, & Loutzenhiser, 2011; Edwards & Hirst, 1988; Edwards, Hirst, & Silverberg, 1988; Jantzi et al., 2006; Z. G. Jiang, Si, Lasarev, & Nuttall, 2001; Johnson, Marrelli, Steenberg, Childres, & Bryan, 1998; McCarron & Halpern, 1990; Smith et al., 2008; Troncoso Brindeiro, Fallet, Lane, & Carmines, 2008; Wu et al., 2007). Importantly, because of the shape of the current-voltage relationship, anything that hyperpolarizes the membrane will recruit outward current through KIR channels (See Figure 6B). Thus, these channels act to amplify hyperpolarization induced by opening of other K+ channels or other cellular processes, such as the Na+/K+ ATPase, and thus, may contribute to the mechanism of action of a number of vasodilators (Jackson, 2005; Jantzi et al., 2006; Longden & Nelson, 2015; Smith et al., 2008; Sonkusare, Dalsgaard, Bonev, & Nelson, 2016).
Increases in extracellular K+ also activate KIR channels, allowing these channels to contribute to functional hyperemia in electrically active tissues such as the brain (Filosa et al., 2006; Girouard et al., 2010; Paisansathan, Xu, Vetri, Hernandez, & Pelligrino, 2010; Vetri, Xu, Paisansathan, & Pelligrino, 2012) and skeletal muscle (Armstrong, Dua, & Murrant, 2007; Crecelius, Kirby, Luckasen, Larson, & Dinenno, 2013; Crecelius, Luckasen, Larson, & Dinenno, 2014) (Figure 6C). These channels may also be activated by K+ released through other VSM or endothelial cell K+ channels, another means by which KIR channels can amplify the effects of vasodilators (Busse et al., 2002; Haddy, Vanhoutte, & Feletou, 2006; Longden & Nelson, 2015).
Vasoconstrictors may close KIR channels through mechanisms involving PKC (Henry, Pearson, & Nichols, 1996; Park, Han, Kim, Youm, et al., 2005; Park et al., 2006; Zitron et al., 2004) or tyrosine kinases (Wischmeyer, Doring, & Karschin, 1998; Zitron et al., 2008), although this has not been well studied in blood vessels. Vasodilators that act through cAMP signaling may activate KIR channels in some blood vessels (Paisansathan et al., 2010; Park, Han, Kim, Ko, et al., 2005; Son et al., 2005). However, it is unclear whether this is due to PKA-dependent phosphorylation of KIR channels, or due to activation of other K+ channels and amplification of hyperpolarization initiated by the opening of the other channel, as noted above.
Diseases and VSM KIR channels
The effects of hypertension on KIR channel function are not clear; increases (Nakahata et al., 2006), decreases (Seo et al., 2014), or no change in function (Tajada et al., 2012) have been reported. Similarly, diabetes has been reported to increase (Troncoso Brindeiro et al., 2008; Troncoso Brindeiro, Lane, & Carmines, 2012) or decrease (Matsushita & Puro, 2006; Mayhan, Mayhan, Sun, & Patel, 2004; Vetri et al., 2012) KIR channel function in different models. Regional, species or model dependent differences could be responsible for this heterogeneity.
Obesity (de Kreutzenberg et al., 2003; Haddock et al., 2011; Vigili de Kreutzenberg, Kiwanuka, Tiengo, & Avogaro, 2003), stress (Longden, Dabertrand, Hill-Eubanks, Hammack, & Nelson, 2014), and ischemia (Bastide et al., 1999; Bastide et al., 2003; Marrelli, Johnson, Khorovets, Childres, & Bryan, 1998; Povlsen, Longden, Bonev, Hill-Eubanks, & Nelson, 2016) all are associated with decreased KIR channel function. Membrane cholesterol and hypercholesterolemia strongly suppresses KIR channel function in other systems (Fang et al., 2006). However, the effects of hypercholesterolemia on VSM KIR channel expression and function have not been directly studied.
K+ channels and VSM proliferation
Remodeling of blood vessels after injury or due to diseases, such as atherosclerosis, results in phenotypic modulation of VSM cells from a quiescent, non-dividing, contractile phenotype into proliferating cells. Potassium channels importantly contribute to the proliferative phenotype in VSM cells. An increase in K+ channel expression and function is required for cells to proliferate (Neylon, 2002; Pardo, 2004; Urrego, Tomczak, Zahed, Stuhmer, & Pardo, 2014; Wonderlin & Strobl, 1996). Inhibition of K+ channel function attenuates proliferation of VSM (Kohler et al., 2003; Miguel-Velado et al., 2005; Neylon, 2002; Tharp & Bowles, 2009; Tharp, Wamhoff, Turk, & Bowles, 2006) and other cells (Pardo, 2004; Urrego et al., 2014; Wonderlin & Strobl, 1996). Potassium channels are required for cells to progress through the cell cycle, as during proliferation (Urrego et al., 2014). They participate in this process by several mechanisms including membrane potential regulation, cell volume regulation, and ion-permeation-independent mechanisms (Urrego et al., 2014). Potassium channels also participate in apoptosis, a required component of vascular remodeling after injury (Kondratskyi, Kondratska, Skryma, & Prevarskaya, 2015).
In quiescent, contractile VSM cells, Ca2+ influx through high-voltage-activated, L-type VGCC importantly contributes to cell Ca2+ regulation and contractile function (Jackson, 2000, 2005) (Figures 1 and 3B). In this setting, activation of K+ channels leads to membrane hyperpolarization, closure of VGCC and decreases in intracellular Ca2+ (Jackson, 2000, 2005) (Figures 1 and 3B). These cells also express a number of members of the transient receptor potential (TRP) family of ion channels including TRPC1, TRPC3, TRPC4, TRPC5, TRPC6, TRPM4, and TRPV4 (Earley & Brayden, 2015). These channels serve as store-operated channels (SOC; TRPC1, TRPC4, TRPC5), receptor operated channels (ROC; TRPC3, TRPC6, TRPM4, TRPV4), and stretch-activated channels (SAC; TRPC6, TRPM4) that contribute to agonist and pressure-induced contraction of native VSM cells (Earley & Brayden, 2015). However, in proliferating VSM cells, there is significant ion channel remodeling: expression of L-type VGCC is reduced, whereas expression of T-type VGCC, TRPC1, TRPC6 and SOC composed of ORAI and the endoplasmic reticulum Ca2+-sensing protein, STIM is increased (Beech, 2007; House, Potier, Bisaillon, Singer, & Trebak, 2008; Munoz et al., 2013; Trebak, 2012; Tzeng et al., 2012) (Figure 7). Importantly, there are also changes in K+ channel expression that are essential for VSM cells to progress through the cell cycle and proliferate (Figure 7, and sections below). During proliferation, increased expression and activation of a K+ channel will either hyperpolarize the membrane to increase or maintain the electrochemical gradient for Ca2+ entry through TRP channels and ORAI/STIM-based SOC (e.g., Figure 8), which will increase or sustain Ca2+ influx through these channels (Bi et al., 2013; Munoz et al., 2013; Urrego et al., 2014). Increased intracellular Ca2+ concentration is an important signal for cell proliferation (Bi et al., 2013; Munoz et al., 2013; Urrego et al., 2014). As noted above, other roles for K+ channels are also possible (Cidad et al., 2012; Cidad et al., 2015; Urrego et al., 2014).
Figure 1.
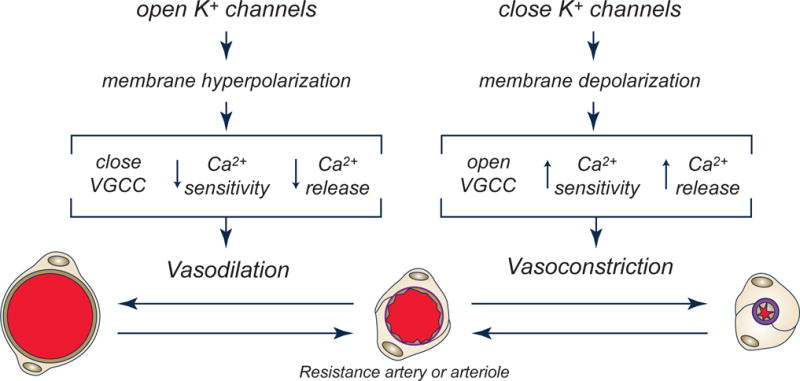
Potassium channels regulate vascular smooth muscle contraction. Schematic diagram outlining the effects of K+ channel opening and closing on the membrane potential of VSM cells, which, in turn, affects processes that lead to relaxation or contraction of VSM leading to vasodilation or vasoconstriction. Voltage-gated Ca2+ channels (VGCC). See text for more information. Modified from (Jackson, 2005).
Figure 7.
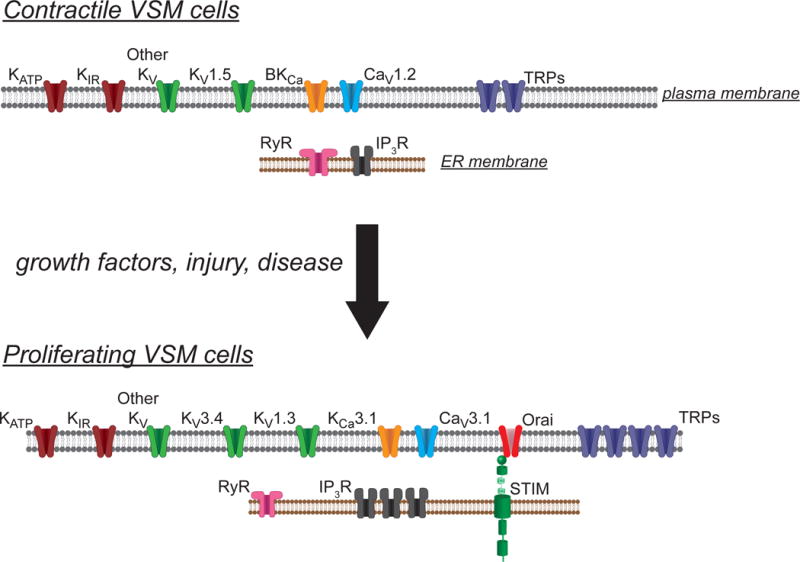
Ion channel remodeling in proliferating VSM cells. Schematic summary of the ion channels expressed in contractile VSM cells and those expressed in proliferating VSM cells. Contractile VSM cells express predominantly L-type voltage-gated Ca2+ channels (CaV1.2), BKCa channels, and KV1.5, in addition to KATP, KIR and several additional types of KV channels. A number of transient receptor potential channels (TRPs) also are expressed that contribute to store-operated, receptor operated and stretch-activated cation channels. Intracellular ryanodine receptors (RyR) and IP3 receptors (IP3R) also are expressed and functional in these cells. In response to growth factors, injury or disease, the pattern of expression of ion channels is remodeled. Proliferating VSM cells lose expression of CaV1.2, BKCa and KV1.5 channels. In their place, T-type Ca2+ (CaV3.1), KCa3.1, KV1.3 and KV3.4 channels are expressed. Proliferating cells also upregulate expression and function of store-operated channels (Orai/STIM), TRP channels and IP3R. See text for more information.
Figure 8.
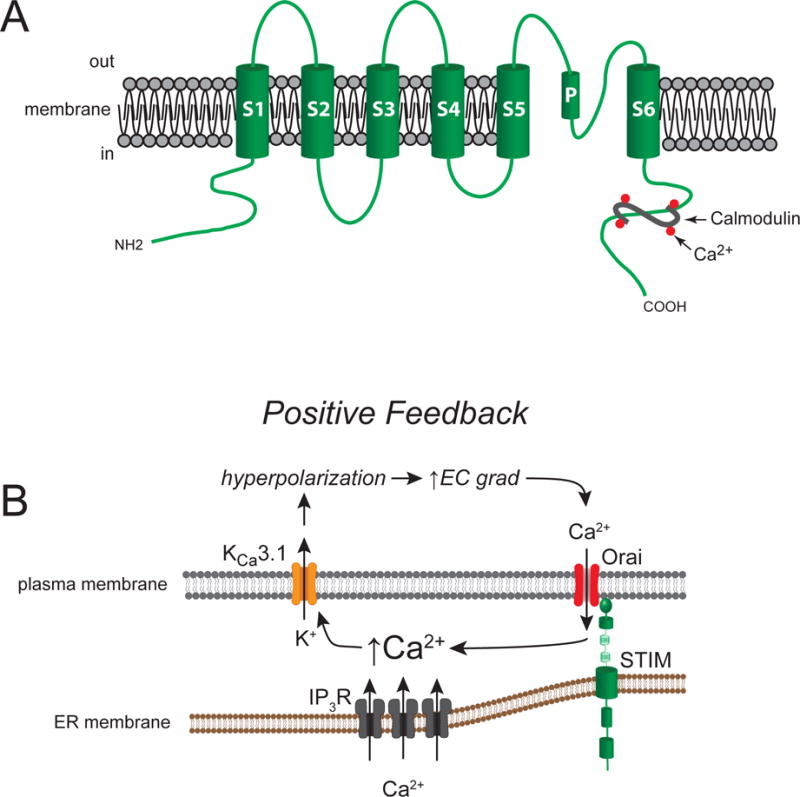
Vascular KCa3.1 channels and their function in proliferating VSM cells. Panel A shows a schematic of an α-subunit demonstrating the typical six-membrane spanning domain structure of KCa3.1 channels. Calcium sensitivity is conferred by the Ca2+-binding protein, calmodulin, that binds to the channel’s C-terminus. Panel B shows a schematic of the role played by KCa3.1 channels in proliferating VSM cells. In proliferating cells, store-operated Ca2+ channels, composed of Orai proteins and the Ca2+ -sensing protein, STIM, are up-regulated, as are IP3R receptors. Increases in intracellular Ca2+ produced by increased activity of Orai/STIM and IP3R, activates KCa3.1 channels, leading membrane hyperpolarization. This hyperpolarization increases the electrochemical (EC) gradient (grad) for Ca2+ diffusion into the cells, augmenting Ca2+ influx through Orai/STIM and other non-voltage-gated Ca2+ channels in proliferating cells, leading to an increase in intracellular Ca2+. This is a positive-feedback system, in contrast to the negative-feedback system that is found in contractile VSM cells (see Figure 3B).
KCa3.1 and VSM proliferation
The intermediate-conductance Ca2+-activated K+ channel, KCa3.1 (sK4, IK1, locus: KCNN4) has consistently been shown to play an important role in proliferation of VSM (Gole, Tharp, & Bowles, 2014; Kohler et al., 2003; Neylon, 2002; Tharp et al., 2006; Toyama et al., 2008) and other cells (Urrego et al., 2014) (Figure 8). These K+ channels are voltage insensitive and use calmodulin as the Ca2+ sensor (Fanger et al., 1999). Calmodulin interacts with the intracellular C-terminus of the channel to gate channel opening (Fanger et al., 1999) (Figure 8A). The concentration of free Ca2+ required for 50% of maximal activation of KCa3.1 is on the order of 300 nM, with the threshold for activity at approximately 100 nM and maximal activity at 1 μM (Ishii et al., 1997).
Growth factors upregulate expression of KCa3.1 in cultured VSM cells (Gole et al., 2014; Kohler et al., 2003; Neylon, 2002; Tharp et al., 2006; Toyama et al., 2008). In porcine coronary artery VSM cells, NADPH oxidase 5 (NOX5)-related increases in reactive oxygen species appear to mediate the upregulation of KCa3.1 expression that results from simulation by basic fibroblast growth factor (Gole et al., 2014). Growth factors regulate expression of KCa3.1 both by decreasing the activity of repressor element 1-silencing transcription factor (REST) (Cheong et al., 2005) and by increasing activity of the AP-1 transcription factor (Bi et al., 2013; Ghanshani et al., 2000). Nucleoside diphosphate kinase B-dependent phosphorylation of KCa3.1 may contribute to activation of these channels in a mouse model of vascular injury (X. B. Zhou et al., 2015).
Selective inhibition of KCa3.1 reduces VSM cell growth and proliferation (Kohler et al., 2003; Neylon, 2002; Tharp et al., 2006; Toyama et al., 2008). Importantly, inhibition of these channels lessens restenosis after balloon injury in rat (Kohler et al., 2003) and pig (Tharp et al., 2008), and limits VSM proliferation in a mouse model of atherosclerosis (Toyama et al., 2008). These ion channels also have been implicated in the VSM proliferation that occurs after organ transplantation (Chen, Lam, Gregory, Schrepfer, & Wulff, 2013) and in chronic kidney disease (Huang, Pollock, & Chen, 2015). The pro-proliferative effect of KCa3.1 is mediated by increases in intracellular Ca2+ (Bi et al., 2013), likely due to increased influx of extracellular Ca2+ driven by KCa3.1-induced hyperpolarization and the increased electrochemical gradient for Ca2+ influx through TRP channels and ORAI/STIM-based SOC (Figure 8B). The pro-proliferative effect of increases in intracellular Ca2+ involves phosphorylated cAMP-response element-binding protein (CREB), c-Fos, and neuron-derived orphan receptor-1 (NOR-1) in human coronary VSM cells (Bi et al., 2013). Unexpectedly, activators of Kca3.1 such as EBIO, SKA-31 and NS309 attenuated platelet-derived-growth-factor (PDGF)-induced proliferation of these cells (Bi et al., 2013). The inhibition of PDGF-induced proliferation appeared to arise from strong suppression of KCa3.1 expression by the activators, and the resultant reduction in intracellular Ca2+ signaling (Bi et al., 2013).
KV channels and VSM proliferation
In addition to KCa3.1, there are also KV channels that contribute to VSM cell proliferation. The KV channel, KV3.4 (locus: KCNC4) is upregulated during proliferation of human uterine artery VSM cells, and selective inhibition of KV3.4 blocks proliferation (Miguel-Velado et al., 2005; Miguel-Velado et al., 2010) and prevents progression of the cells through the G1 phase of the cell cycle (Miguel-Velado et al., 2010). The inhibitory effects of Kv3.4 blockade on proliferation could be mimicked by incubation of cells with elevated extracellular K+ to produce depolarization equivalent to that produced by KV3.4 blockade (Miguel-Velado et al., 2010). These data suggest that the proliferative effects of KV3.4 may be related to the channel’s impact on membrane potential (hyperpolarization), similar to the proposed mechanism for KCa3.1 channel stimulation of Ca2+ influx (Figure 8B).
In contrast, balloon-injury of mouse arteries results in upregulation of KV1.3 (locus: KCNE3) (Cidad et al., 2010) and downregulation of KV1.5 (Cidad et al., 2012; Cidad et al., 2015; Cidad et al., 2014). Proliferation and migration of VSM cells in this model can be attenuated by selective blockade of KV1.3 channels (Cheong et al., 2011; Cidad et al., 2010). Studies in human VSM cells also confirm a role for KV1.3 in proliferation (Cheong et al., 2011; Cidad et al., 2015). Interestingly, KV1.3 may contribute to VSM proliferation by ion-permeation-independent mechanisms (Cidad et al., 2012; Cidad et al., 2015; Jimenez-Perez et al., 2016). The pro-proliferative effects of KV1.3 are mediated by voltage-dependent exposure of key residues in the channel’s C-terminus (Tyr-447 and Ser-459) (Jimenez-Perez et al., 2016). These KV channels may act as scaffolding proteins that recruit signaling proteins into signalplexes to promote the proliferative phenotype, independent from K+ diffusion through the pore of the channel and changes in membrane potential (Cahalan & Chandy, 2009; Jimenez-Perez et al., 2016; Schwab, Hanley, Fabian, & Stock, 2008). In human coronary VSM cells, this may involve mitogen-activated protein kinase kinase (MEK)/extracellular signal–regulated kinase (ERK) and phospholipase Cγ signaling pathways (Cidad et al., 2015). This may provide additional targets to combat vascular proliferative diseases, in addition to the Phosphatidylinositol-4,5-bisphosphate 3-kinase/mammalian target of rapamycin (mTOR) pathway targeted by current therapies (Cidad et al., 2015).
Summary and questions for the future
While we have learned much about the expression and function of K+ channels in the regulation of VSM contraction and proliferation in the past 30 years, there remain several outstanding questions. First, why do VSM cells express so many different KV channels? Is this simply a matter of redundancy, or does the pattern of expression of these channels tune the electrophysiology of VSM cells in different vascular beds in ways that are not yet clear (Zhong et al., 2010)? Second, while it is clear that K+ channels, like all ion channels, exist in multi-protein signaling domains (Abriel, Rougier, & Jalife, 2015; Kim & Oh, 2016; Levitan, 2006), our understanding of the regional heterogeneity in the nature and composition of these signaling domains in different vascular beds is incomplete. Finally, our understanding of the regulation of expression and function of K+ channels in major cardiovascular disease states also remains incomplete, particularly as they relate to different vascular beds around the body. These are research areas where single cell transcriptome studies, high resolution proteomics and informatics along with detailed electrophysiology and mechanical studies would aid in providing a clearer picture of the expression and function of the diverse array of K+ channels that contribute to the regulation of VSM contraction and proliferation in health and disease.
Acknowledgments
Supported by the National Heart, Lung and Blood Institute of the National Institutes of Health, under award numbers RO1 HL32469 and P01 HL070687. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- EBIO
1-Ethyl-2-benzimidazolinone
- NS1619
1,3-Dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one
- NS309
6,7-Dichloro-1H-indole-2,3-dione 3-oxime
- AKAP
A-kinase anchoring protein
- NH2
amino
- KATP
ATP-sensitive K+
- CREB
cAMP-response element-binding protein
- CO
Carbon monoxide
- COOH
carboxy
- EC
electrochemical
- EETs
epoxyeicosatrienoic acids
- ERK
extracellular signal–regulated kinase
- grad
gradient
- H2S
Hydrogen sulfide
- KCa3.1, sK4, IK1
intermediate-conductance Ca2+-activated K+ channel
- IP3
inositol-1,4,5-trisphosphate
- KIR
inward-rectifier K+
- IP3R
IP3 receptors
- EK
K+ equilibrium potential
- CaV1.2
L-type voltage-gated Ca2+ channels
- BKCa
large-conductance Ca2+-activated K+
- LRRCs
leucine-rich-repeat-containing proteins
- mTOR
mammalian target of rapamycin
- MEK
mitogen-activated protein kinase kinase
- MuRF1
muscle RING finger protein 1
- NOX5
NADPH oxidase 5
- SKA-31
Naphtho[1,2-d]thiazol-2-ylamine
- NOR-1
neuron-derived orphan receptor-1
- NO
Nitric oxide
- NF
Nuclear Factor
- NFATC3
nuclear factor of activated T-cells, cytoplasmic 3
- PDGF
platelet-derived-growth-factor
- PKA
protein kinase A
- PKC
Protein kinase C
- PKG
protein kinase G
- ROC
receptor operated channels
- RCK
regulator of K+ conductance
- REST
repressor element 1-silencing transcription factor
- RyR
ryanodine receptors
- SOC
store-operated channels
- SAC
stretch-activated channels
- SUR
sulphonylurea receptors
- TRP
transient receptor potential
- TM
transmembrane
- VSM
vascular smooth muscle
- VGCC
voltage-gated Ca2+ channels
- KV
voltage-gated K+
Footnotes
Conflict of Interest Statement:
The author has no conflicts of interest to declare.
References
- Abbink EJ, Wollersheim H, Netten PM, Russel FG, Lutterman JA, Smits P. Microcirculatory effects of KATP channel blockade by sulphonylurea derivatives in humans. Eur J Clin Invest. 2002;32(3):163–171. doi: 10.1046/j.1365-2362.2002.00964.x. [DOI] [PubMed] [Google Scholar]
- Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60(1):79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- Abriel H, Rougier JS, Jalife J. Ion channel macromolecular complexes in cardiomyocytes: roles in sudden cardiac death. Circ Res. 2015;116(12):1971–1988. doi: 10.1161/CIRCRESAHA.116.305017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebiyi A, McNally EM, Jaggar JH. Vasodilation induced by oxygen/glucose deprivation is attenuated in cerebral arteries of SUR2 null mice. Am J Physiol Heart Circ Physiol. 2011;301(4):H1360–1368. doi: 10.1152/ajpheart.00406.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern GP, Hsu SF, Jackson MB. Direct actions of nitric oxide on rat neurohypophysial K+ channels. J Physiol. 1999;520(Pt 1):165–176. doi: 10.1111/j.1469-7793.1999.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello EA, Malcolm AT, Walsh MP, Cole WC. Beta-adrenoceptor activation and PKA regulate delayed rectifier K+ channels of vascular smooth muscle cells. Am J Physiol. 1998;275(2 Pt 2):H448–459. doi: 10.1152/ajpheart.1998.275.2.H448. [DOI] [PubMed] [Google Scholar]
- Aiello EA, Walsh MP, Cole WC. Isoproterenol and forskolin increase and PKI inhibits delayed rectifier K+ current in vascular myocytes isolated from rabbit coronary artery and portal vein. Can J Physiol Pharmacol. 1994;72:47. [Google Scholar]
- Aiello EA, Walsh MP, Cole WC. Phosphorylation by protein kinase A enhances delayed rectifier K+ current in rabbit vascular smooth muscle cells. Am J Physiol. 1995;268(2 Pt 2):H926–934. doi: 10.1152/ajpheart.1995.268.2.H926. [DOI] [PubMed] [Google Scholar]
- Akatsuka Y, Egashira K, Katsuda Y, Narishige T, Ueno H, Shimokawa H, Takeshita A. ATP sensitive potassium channels are involved in adenosine A2 receptor mediated coronary vasodilatation in the dog. Cardiovasc Res. 1994;28(6):906–911. doi: 10.1093/cvr/28.6.906. [DOI] [PubMed] [Google Scholar]
- Albarwani S, Al-Siyabi S, Baomar H, Hassan MO. Exercise training attenuates ageing-induced BKCa channel downregulation in rat coronary arteries. Exp Physiol. 2010;95(6):746–755. doi: 10.1113/expphysiol.2009.051250. [DOI] [PubMed] [Google Scholar]
- Alioua A, Huggins JP, Rousseau E. PKG-I alpha phosphorylates the alpha-subunit and upregulates reconstituted GKCa channels from tracheal smooth muscle. Am J Physiol. 1995;268(6 Pt 1):L1057–1063. doi: 10.1152/ajplung.1995.268.6.L1057. [DOI] [PubMed] [Google Scholar]
- Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca(2+) activated K(+) channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112(5):717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg GC, Santana LF. Downregulation of the BK channel beta1 subunit in genetic hypertension. Circ Res. 2003;93(10):965–971. doi: 10.1161/01.RES.0000100068.43006.36. [DOI] [PubMed] [Google Scholar]
- Ambroisine ML, Favre J, Oliviero P, Rodriguez C, Gao J, Thuillez C, Delcayre C. Aldosterone-induced coronary dysfunction in transgenic mice involves the calcium-activated potassium (BKCa) channels of vascular smooth muscle cells. Circulation. 2007;116(21):2435–2443. doi: 10.1161/CIRCULATIONAHA.107.722009. [DOI] [PubMed] [Google Scholar]
- Armstead WM. Role of activation of calcium-sensitive K+ channels in NO- and hypoxia-induced pial artery vasodilation. American Journal of Physiology-Heart and Circulatory Physiology. 1997;272(4):H1785–H1790. doi: 10.1152/ajpheart.1997.272.4.H1785. [DOI] [PubMed] [Google Scholar]
- Armstrong ML, Dua AK, Murrant CL. Potassium initiates vasodilatation induced by a single skeletal muscle contraction in hamster cremaster muscle. J Physiol. 2007;581(Pt 2):841–852. doi: 10.1113/jphysiol.2007.130013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M, Masuzawa-Ito K, Matsuda T. Charybdotoxin-sensitive K+ channels regulate the myogenic tone in the resting state of arteries from spontaneously hypertensive rats. Br J Pharmacol. 1993;108(1):214–222. doi: 10.1111/j.1476-5381.1993.tb13465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano M, Matsuda T, Hayakawa M, Ito KM, Ito K. Increased resting Ca2+ maintains the myogenic tone and activates K+ channels in arteries from young spontaneously hypertensive rats. Eur J Pharmacol. 1993;247(3):295–304. doi: 10.1016/0922-4106(93)90198-i. [DOI] [PubMed] [Google Scholar]
- Aziz Q, Thomas AM, Gomes J, Ang R, Sones WR, Li Y, Tinker A. The ATP-sensitive potassium channel subunit, Kir6.1, in vascular smooth muscle plays a major role in blood pressure control. Hypertension. 2014;64(3):523–529. doi: 10.1161/HYPERTENSIONAHA.114.03116. [DOI] [PubMed] [Google Scholar]
- Bae YM, Kim A, Kim J, Park SW, Kim TK, Lee YR, Cho SI. Serotonin depolarizes the membrane potential in rat mesenteric artery myocytes by decreasing voltage-gated K+ currents. Biochem Biophys Res Commun. 2006;347(2):468–476. doi: 10.1016/j.bbrc.2006.06.116. [DOI] [PubMed] [Google Scholar]
- Banitt PF, Smits P, Williams SB, Ganz P, Creager MA. Activation of ATP-sensitive potassium channels contributes to reactive hyperemia in humans. Am J Physiol. 1996;271(4 Pt 2):H1594–1598. doi: 10.1152/ajpheart.1996.271.4.H1594. [DOI] [PubMed] [Google Scholar]
- Bank AJ, Sih R, Mullen K, Osayamwen M, Lee PC. Vascular ATP-dependent potassium channels, nitric oxide, and human forearm reactive hyperemia. Cardiovasc Drugs Ther. 2000;14(1):23–29. doi: 10.1023/a:1007835003493. [DOI] [PubMed] [Google Scholar]
- Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275(4 Pt 2):H1283–1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- Bastide M, Bordet R, Pu Q, Robin E, Puisieux F, Dupuis B. Relationship between inward rectifier potassium current impairment and brain injury after cerebral ischemia/reperfusion. J Cereb Blood Flow Metab. 1999;19(12):1309–1315. doi: 10.1097/00004647-199912000-00003. [DOI] [PubMed] [Google Scholar]
- Bastide M, Gele P, Petrault O, Pu Q, Caliez A, Robin E, Bordet R. Delayed cerebrovascular protective effect of lipopolysaccharide in parallel to brain ischemic tolerance. J Cereb Blood Flow Metab. 2003;23(4):399–405. doi: 10.1097/01.WCB.0000050064.57184.F2. [DOI] [PubMed] [Google Scholar]
- Beech DJ. Ion channel switching and activation in smooth-muscle cells of occlusive vascular diseases. Biochem Soc Trans. 2007;35(Pt 5):890–894. doi: 10.1042/BST0350890. [DOI] [PubMed] [Google Scholar]
- Berczi V, Stekiel WJ, Contney SJ, Rusch NJ. Pressure-induced activation of membrane K+ current in rat saphenous artery. Hypertension. 1992;19(6 Pt 2):725–729. doi: 10.1161/01.hyp.19.6.725. [DOI] [PubMed] [Google Scholar]
- Berger MG, Vandier C, Bonnet P, Jackson WF, Rusch NJ. Intracellular acidosis differentially regulates KV channels in coronary and pulmonary vascular muscle. Am J Physiol. 1998;275(4 Pt 2):H1351–1359. doi: 10.1152/ajpheart.1998.275.4.H1351. [DOI] [PubMed] [Google Scholar]
- Berwick ZC, Dick GM, Moberly SP, Kohr MC, Sturek M, Tune JD. Contribution of voltage-dependent K(+) channels to metabolic control of coronary blood flow. J Mol Cell Cardiol. 2012;52(4):912–919. doi: 10.1016/j.yjmcc.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick ZC, Payne GA, Lynch B, Dick GM, Sturek M, Tune JD. Contribution of adenosine A(2A) and A(2B) receptors to ischemic coronary dilation: role of K(V) and K(ATP) channels. Microcirculation. 2010;17(8):600–607. doi: 10.1111/j.1549-8719.2010.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi D, Toyama K, Lemaitre V, Takai J, Fan F, Jenkins DP, Miura H. The intermediate conductance calcium-activated potassium channel KCa3.1 regulates vascular smooth muscle cell proliferation via controlling calcium-dependent signaling. J Biol Chem. 2013;288(22):15843–15853. doi: 10.1074/jbc.M112.427187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialecki RA, Stinson-Fisher C. KCa channel antagonists reduce NO donor-mediated relaxation of vascular and tracheal smooth muscle. Am J Physiol. 1995;268(1 Pt 1):L152–159. doi: 10.1152/ajplung.1995.268.1.L152. [DOI] [PubMed] [Google Scholar]
- Bijlstra PJ, den Arend JA, Lutterman JA, Russel FG, Thien T, Smits P. Blockade of vascular ATP-sensitive potassium channels reduces the vasodilator response to ischaemia in humans. Diabetologia. 1996;39(12):1562–1568. doi: 10.1007/s001250050615. [DOI] [PubMed] [Google Scholar]
- Blanco-Rivero J, Gamallo C, Aras-Lopez R, Cobeno L, Cogolludo A, Perez-Vizcaino F, Balfagon G. Decreased expression of aortic KIR6.1 and SUR2B in hypertension does not correlate with changes in the functional role of K(ATP) channels. Eur J Pharmacol. 2008;587(1–3):204–208. doi: 10.1016/j.ejphar.2008.03.039. [DOI] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368(6474):850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Bonev AD, Nelson MT. Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. Journal of General Physiology. 1996;108(4):315–323. doi: 10.1085/jgp.108.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbouse L, Dick GM, Asano S, Bender SB, Dincer UD, Payne GA, Tune JD. Impaired function of coronary BK(Ca) channels in metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297(5):H1629–1637. doi: 10.1152/ajpheart.00466.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbouse L, Dick GM, Payne GA, Payne BD, Svendsen MC, Neeb ZP, Tune JD. Contribution of BK(Ca) channels to local metabolic coronary vasodilation: Effects of metabolic syndrome. Am J Physiol Heart Circ Physiol. 2010;298(3):H966–973. doi: 10.1152/ajpheart.00876.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard JF, Dumont E, Lamontagne D. Evidence that prostaglandins I2, E2, and D2 may activate ATP sensitive potassium channels in the isolated rat heart. Cardiovasc Res. 1994;28(6):901–905. doi: 10.1093/cvr/28.6.901. [DOI] [PubMed] [Google Scholar]
- Bouchard JF, Dumont EC, Lamontagne D. Modification of vasodilator response in streptozotocin-induced diabetic rat. Can J Physiol Pharmacol. 1999;77(12):980–985. [PubMed] [Google Scholar]
- Bratz IN, Dick GM, Partridge LD, Kanagy NL. Reduced molecular expression of K(+) channel proteins in vascular smooth muscle from rats made hypertensive with N{omega}-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289(3):H1277–1283. doi: 10.1152/ajpheart.01052.2004. [DOI] [PubMed] [Google Scholar]
- Bratz IN, Swafford AN, Jr, Kanagy NL, Dick GM. Reduced functional expression of K(+) channels in vascular smooth muscle cells from rats made hypertensive with N{omega}-nitro-L-arginine. Am J Physiol Heart Circ Physiol. 2005;289(3):H1284–1290. doi: 10.1152/ajpheart.01053.2004. [DOI] [PubMed] [Google Scholar]
- Brayden JE. Membrane Hyperpolarization Is a Mechanism of Endothelium-Dependent Cerebral Vasodilation. American Journal of Physiology. 1990;259(3):H668–H673. doi: 10.1152/ajpheart.1990.259.3.H668. [DOI] [PubMed] [Google Scholar]
- Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256(5056):532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- Brenner R, Perez GJ, Bonev AD, Eckman DM, Kosek JC, Wiler SW, Aldrich RW. Vasoregulation by the beta1 subunit of the calcium-activated potassium channel. Nature. 2000;407(6806):870–876. doi: 10.1038/35038011. [DOI] [PubMed] [Google Scholar]
- Bubolz AH, Li H, Wu Q, Liu Y. Enhanced oxidative stress impairs cAMP-mediated dilation by reducing Kv channel function in small coronary arteries of diabetic rats. Am J Physiol Heart Circ Physiol. 2005;289(5):H1873–1880. doi: 10.1152/ajpheart.00357.2005. [DOI] [PubMed] [Google Scholar]
- Burns WR, Cohen KD, Jackson WF. K+-induced dilation of hamster cremasteric arterioles involves both the Na+/K+-ATPase and inward-rectifier K+ channels. Microcirculation. 2004;11(3):279–293. doi: 10.1080/10739680490425985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23(8):374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261(5118):221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Kaiser RA, Malmquist NA, Tichenor S. NO-induced relaxation of labouring and non-labouring human myometrium is not mediated by cyclic GMP. Br J Pharmacol. 2001;134(1):206–214. doi: 10.1038/sj.bjp.0704226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bychkov R, Gollasch M, Ried C, Luft FC, Haller H. Regulation of spontaneous transient outward potassium currents in human coronary arteries. Circulation. 1997;95(2):503–510. doi: 10.1161/01.cir.95.2.503. [DOI] [PubMed] [Google Scholar]
- Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol Rev. 2009;231(1):59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callera GE, Yogi A, Tostes RC, Rossoni LV, Bendhack LM. Ca2+-activated K+ channels underlying the impaired acetylcholine-induced vasodilation in 2K-1C hypertensive rats. J Pharmacol Exp Ther. 2004;309(3):1036–1042. doi: 10.1124/jpet.103.062810. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78(3):415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Cankar K, Strucl M. The effect of glibenclamide on cutaneous laser-Doppler flux. Microvasc Res. 2008;75(1):97–103. doi: 10.1016/j.mvr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Chadha PS, Jepps TA, Carr G, Stott JB, Zhu HL, Cole WC, Greenwood IA. Contribution of kv7.4/kv7.5 heteromers to intrinsic and calcitonin gene-related peptide-induced cerebral reactivity. Arterioscler Thromb Vasc Biol. 2014;34(4):887–893. doi: 10.1161/ATVBAHA.114.303405. [DOI] [PubMed] [Google Scholar]
- Chadha PS, Zunke F, Zhu HL, Davis AJ, Jepps TA, Olesen SP, Greenwood IA. Reduced KCNQ4-encoded voltage-dependent potassium channel activity underlies impaired beta-adrenoceptor-mediated relaxation of renal arteries in hypertension. Hypertension. 2012;59(4):877–884. doi: 10.1161/HYPERTENSIONAHA.111.187427. [DOI] [PubMed] [Google Scholar]
- Chai Q, Liu Z, Chen L. Effects of streptozotocin-induced diabetes on Kv channels in rat small coronary smooth muscle cells. Chin J Physiol. 2005;48(1):57–63. [PubMed] [Google Scholar]
- Chai Q, Xu X, Jia Q, Dong Q, Liu Z, Zhang W, Chen L. Molecular basis of dysfunctional Kv channels in small coronary artery smooth muscle cells of streptozotocin-induced diabetic rats. Chin J Physiol. 2007;50(4):171–177. [PubMed] [Google Scholar]
- Cheang WS, Wong WT, Shen B, Lau CW, Tian XY, Tsang SY, Huang Y. 4-aminopyridine-sensitive K+ channels contributes to NaHS-induced membrane hyperpolarization and relaxation in the rat coronary artery. Vascul Pharmacol. 2010;53(3–04):94–98. doi: 10.1016/j.vph.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Lam J, Gregory CR, Schrepfer S, Wulff H. The Ca(2)(+)-activated K(+) channel KCa3.1 as a potential new target for the prevention of allograft vasculopathy. PLoS One. 2013;8(11):e81006. doi: 10.1371/journal.pone.0081006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Ndisang JF, Tang G, Cao K, Wang R. Hydrogen sulfide-induced relaxation of resistance mesenteric artery beds of rats. Am J Physiol Heart Circ Physiol. 2004;287(5):H2316–2323. doi: 10.1152/ajpheart.00331.2004. [DOI] [PubMed] [Google Scholar]
- Cheong A, Bingham AJ, Li J, Kumar B, Sukumar P, Munsch C, Wood IC. Downregulated REST transcription factor is a switch enabling critical potassium channel expression and cell proliferation. Mol Cell. 2005;20(1):45–52. doi: 10.1016/j.molcel.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Cheong A, Dedman AM, Beech DJ. Expression and function of native potassium channel [K(V)alpha1] subunits in terminal arterioles of rabbit. J Physiol. 2001;534(Pt 3):691–700. doi: 10.1111/j.1469-7793.2001.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong A, Dedman AM, Xu SZ, Beech DJ. K(V)alpha1 channels in murine arterioles: differential cellular expression and regulation of diameter. Am J Physiol Heart Circ Physiol. 2001;281(3):H1057–1065. doi: 10.1152/ajpheart.2001.281.3.H1057. [DOI] [PubMed] [Google Scholar]
- Cheong A, Li J, Sukumar P, Kumar B, Zeng F, Riches K, Beech DJ. Potent suppression of vascular smooth muscle cell migration and human neointimal hyperplasia by KV1.3 channel blockers. Cardiovasc Res. 2011;89(2):282–289. doi: 10.1093/cvr/cvq305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheranov SY, Jaggar JH. TNF-alpha dilates cerebral arteries via NAD(P)H oxidase-dependent Ca2+ spark activation. Am J Physiol Cell Physiol. 2006;290(4):C964–971. doi: 10.1152/ajpcell.00499.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton L, Loutzenhiser K, Morales E, Breaks J, Kargacin GJ, Loutzenhiser R. Inward rectifier K(+) currents and Kir2.1 expression in renal afferent and efferent arterioles. J Am Soc Nephrol. 2008;19(1):69–76. doi: 10.1681/ASN.2007010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilton L, Loutzenhiser R. Functional evidence for an inward rectifier potassium current in rat renal afferent arterioles. Circ Res. 2001;88(2):152–158. doi: 10.1161/01.res.88.2.152. [DOI] [PubMed] [Google Scholar]
- Chilton L, Smirnov SV, Loutzenhiser K, Wang X, Loutzenhiser R. Segment-specific differences in the inward rectifier K(+) current along the renal interlobular artery. Cardiovasc Res. 2011;92(1):169–177. doi: 10.1093/cvr/cvr179. [DOI] [PubMed] [Google Scholar]
- Chrissobolis S, Sobey CG. Inhibitory effects of protein kinase C on inwardly rectifying K+- and ATP-sensitive K+ channel-mediated responses of the basilar artery. Stroke. 2002;33(6):1692–1697. doi: 10.1161/01.str.0000016966.89226.67. [DOI] [PubMed] [Google Scholar]
- Cidad P, Jimenez-Perez L, Garcia-Arribas D, Miguel-Velado E, Tajada S, Ruiz-McDavitt C, Perez-Garcia MT. Kv1.3 channels can modulate cell proliferation during phenotypic switch by an ion-flux independent mechanism. Arterioscler Thromb Vasc Biol. 2012;32(5):1299–1307. doi: 10.1161/ATVBAHA.111.242727. [DOI] [PubMed] [Google Scholar]
- Cidad P, Miguel-Velado E, Ruiz-McDavitt C, Alonso E, Jimenez-Perez L, Asuaje A, Lopez-Lopez JR. Kv1.3 channels modulate human vascular smooth muscle cells proliferation independently of mTOR signaling pathway. Pflugers Arch. 2015;467(8):1711–1722. doi: 10.1007/s00424-014-1607-y. [DOI] [PubMed] [Google Scholar]
- Cidad P, Moreno-Dominguez A, Novensa L, Roque M, Barquin L, Heras M, Lopez-Lopez JR. Characterization of ion channels involved in the proliferative response of femoral artery smooth muscle cells. Arterioscler Thromb Vasc Biol. 2010;30(6):1203–1211. doi: 10.1161/ATVBAHA.110.205187. [DOI] [PubMed] [Google Scholar]
- Cidad P, Novensa L, Garabito M, Batlle M, Dantas AP, Heras M, Roque M. K+ channels expression in hypertension after arterial injury, and effect of selective Kv1.3 blockade with PAP-1 on intimal hyperplasia formation. Cardiovasc Drugs Ther. 2014;28(6):501–511. doi: 10.1007/s10557-014-6554-5. [DOI] [PubMed] [Google Scholar]
- Clement-Chomienne O, Walsh MP, Cole WC. Angiotensin II activation of protein kinase C decreases delayed rectifier K+ current in rabbit vascular myocytes. J Physiol. 1996;495(Pt 3):689–700. doi: 10.1113/jphysiol.1996.sp021626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole WC, Malcolm T, Walsh MP, Light PE. Inhibition by protein kinase C of the K(NDP) subtype of vascular smooth muscle ATP-sensitive potassium channel. Circ Res. 2000;87(2):112–117. doi: 10.1161/01.res.87.2.112. [DOI] [PubMed] [Google Scholar]
- Cook NS. Effect of Some Potassium Channel Blockers on Contractile Responses of the Rabbit Aorta. Journal of Cardiovascular Pharmacology. 1989;13(2):299–306. doi: 10.1097/00005344-198902000-00019. [DOI] [PubMed] [Google Scholar]
- Cooke JP, Rossitch E, Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest. 1991;88(5):1663–1671. doi: 10.1172/JCI115481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox RH. Comparison of K+ channel properties in freshly isolated myocytes from thoracic aorta of WKY and SHR. Am J Hypertens. 1996;9(9):884–894. doi: 10.1016/s0895-7061(96)00179-3. [DOI] [PubMed] [Google Scholar]
- Cox RH. Molecular determinants of voltage-gated potassium currents in vascular smooth muscle. Cell Biochem Biophys. 2005;42(2):167–195. doi: 10.1385/CBB:42:2:167. [DOI] [PubMed] [Google Scholar]
- Cox RH, Folander K, Swanson R. Differential expression of voltage-gated K(+) channel genes in arteries from spontaneously hypertensive and Wistar-Kyoto rats. Hypertension. 2001;37(5):1315–1322. doi: 10.1161/01.hyp.37.5.1315. [DOI] [PubMed] [Google Scholar]
- Cox RH, Fromme SJ, Folander KL, Swanson RJ. Voltage gated K+ channel expression in arteries of Wistar-Kyoto and spontaneously hypertensive rats. Am J Hypertens. 2008;21(2):213–218. doi: 10.1038/ajh.2007.44. [DOI] [PubMed] [Google Scholar]
- Cox RH, Lozinskaya I, Dietz NJ. Differences in K+ current components in mesenteric artery myocytes from WKY and SHR. Am J Hypertens. 2001;14(9 Pt 1):897–907. doi: 10.1016/s0895-7061(01)02145-8. [DOI] [PubMed] [Google Scholar]
- Cox RH, Petrou S. Ca(2+) influx inhibits voltage-dependent and augments Ca(2+)-dependent K(+) currents in arterial myocytes. Am J Physiol. 1999;277(1 Pt 1):C51–63. doi: 10.1152/ajpcell.1999.277.1.C51. [DOI] [PubMed] [Google Scholar]
- Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2013;305(1):H29–40. doi: 10.1152/ajpheart.00298.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crecelius AR, Luckasen GJ, Larson DG, Dinenno FA. KIR channel activation contributes to onset and steady-state exercise hyperemia in humans. Am J Physiol Heart Circ Physiol. 2014;307(5):H782–791. doi: 10.1152/ajpheart.00212.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankelman J, Van der Ploeg CP, Spaan JA. Glibenclamide decelerates the responses of coronary regulation in the goat. Am J Physiol. 1994;266(5 Pt 2):H1715–1721. doi: 10.1152/ajpheart.1994.266.5.H1715. [DOI] [PubMed] [Google Scholar]
- Dart C, Standen NB. Adenosine-activated potassium current in smooth muscle cells isolated from the pig coronary artery. J Physiol. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daut J, Maierrudolph W, Vonbeckerath N, Mehrke G, Gunther K, Goedelmeinen L. Hypoxic Dilation of Coronary-Arteries Is Mediated by Atp-Sensitive Potassium Channels. Science. 1990;247(4948):1341–1344. doi: 10.1126/science.2107575. [DOI] [PubMed] [Google Scholar]
- de Kreutzenberg SV, Puato M, Kiwanuka E, Del Prato S, Pauletto P, Pasini L, Avogaro A. Elevated non-esterified fatty acids impair nitric oxide independent vasodilation, in humans: evidence for a role of inwardly rectifying potassium channels. Atherosclerosis. 2003;169(1):147–153. doi: 10.1016/s0021-9150(03)00153-9. [DOI] [PubMed] [Google Scholar]
- del Valle-Rodriguez A, Lopez-Barneo J, Urena J. Ca2+ channel-sarcoplasmic reticulum coupling: a mechanism of arterial myocyte contraction without Ca2+ influx. EMBO J. 2003;22(17):4337–4345. doi: 10.1093/emboj/cdg432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GM, Bratz IN, Borbouse L, Payne GA, Dincer UD, Knudson JD, Tune JD. Voltage-dependent K+ channels regulate the duration of reactive hyperemia in the canine coronary circulation. Am J Physiol Heart Circ Physiol. 2008;294(5):H2371–2381. doi: 10.1152/ajpheart.01279.2007. [DOI] [PubMed] [Google Scholar]
- Dick GM, Tune JD. Role of potassium channels in coronary vasodilation. Exp Biol Med (Maywood) 2010;235(1):10–22. doi: 10.1258/ebm.2009.009201. [DOI] [PubMed] [Google Scholar]
- Dong H, Waldron GJ, Cole WC, Triggle CR. Roles of calcium-activated and voltage-gated delayed rectifier potassium channels in endothelium-dependent vasorelaxation of the rabbit middle cerebral artery. Br J Pharmacol. 1998;123(5):821–832. doi: 10.1038/sj.bjp.0701680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Waldron GJ, Galipeau D, Cole WC, Triggle CR. NO/PGI2-independent vasorelaxation and the cytochrome P450 pathway in rabbit carotid artery. Br J Pharmacol. 1997;120(4):695–701. doi: 10.1038/sj.bjp.0700945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Xie MJ, Zhang P, Ji LL, Liu WC, Dong MQ, Gao F. Rotenone partially reverses decreased BK Ca currents in cerebral artery smooth muscle cells from streptozotocin-induced diabetic mice. Clin Exp Pharmacol Physiol. 2009;36(10):e57–64. doi: 10.1111/j.1440-1681.2009.05222.x. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, Oei HH, Hu F, Stubenitsky R, Verdouw PD. Role of K(ATP)(+) channels in regulation of systemic, pulmonary, and coronary vasomotor tone in exercising swine. Am J Physiol Heart Circ Physiol. 2001;280(1):H22–33. doi: 10.1152/ajpheart.2001.280.1.H22. [DOI] [PubMed] [Google Scholar]
- Duncker DJ, van Zon NS, Pavek TJ, Herrlinger SK, Bache RJ. Endogenous adenosine mediates coronary vasodilation during exercise after K(ATP)+ channel blockade. J Clin Invest. 1995;95(1):285–295. doi: 10.1172/JCI117653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiol Rev. 2015;95(2):645–690. doi: 10.1152/physrev.00026.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Heppner TJ, Nelson MT, Brayden JE. TRPV4 forms a novel Ca2+ signaling complex with ryanodine receptors and BKCa channels. Circ Res. 2005;97(12):1270–1279. doi: 10.1161/01.RES.0000194321.60300.d6. [DOI] [PubMed] [Google Scholar]
- Eckman DM, Hopkins N, McBride C, Keef KD. Endothelium-dependent relaxation and hyperpolarization in guinea-pig coronary artery: role of epoxyeicosatrienoic acid. Br J Pharmacol. 1998;124(1):181–189. doi: 10.1038/sj.bjp.0701778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD. Inward rectification in submucosal arterioles of guinea-pig ileum. J Physiol. 1988;404:437–454. doi: 10.1113/jphysiol.1988.sp017298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Silverberg GD. Inward rectification in rat cerebral arterioles; involvement of potassium ions in autoregulation. J Physiol. 1988;404:455–466. doi: 10.1113/jphysiol.1988.sp017299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguchi S, Kawano T, Yinhua Tanaka K, Yasui S, Mawatari K, Nakajo N. Effects of prostaglandin E1 on vascular ATP-sensitive potassium channels. J Cardiovasc Pharmacol. 2007;50(6):686–691. doi: 10.1097/FJC.0b013e3181583d9b. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87(6):474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- Erdos B, Miller AW, Busija DW. Alterations in KATP and KCa channel function in cerebral arteries of insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2002;283(6):H2472–2477. doi: 10.1152/ajpheart.00516.2002. [DOI] [PubMed] [Google Scholar]
- Erdos B, Simandle SA, Snipes JA, Miller AW, Busija DW. Potassium channel dysfunction in cerebral arteries of insulin-resistant rats is mediated by reactive oxygen species. Stroke. 2004;35(4):964–969. doi: 10.1161/01.STR.0000119753.05670.F1. [DOI] [PubMed] [Google Scholar]
- Evanson KW, Bannister JP, Leo MD, Jaggar JH. LRRC26 is a functional BK channel auxiliary gamma subunit in arterial smooth muscle cells. Circ Res. 2014;115(4):423–431. doi: 10.1161/CIRCRESAHA.115.303407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Mohler ER, 3rd, Hsieh E, Osman H, Hashemi SM, Davies PF, Levitan I. Hypercholesterolemia suppresses inwardly rectifying K+ channels in aortic endothelium in vitro and in vivo. Circ Res. 2006;98(8):1064–1071. doi: 10.1161/01.RES.0000218776.87842.43. [DOI] [PubMed] [Google Scholar]
- Fanger CM, Ghanshani S, Logsdon NJ, Rauer H, Kalman K, Zhou J, Aiyar J. Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J Biol Chem. 1999;274(9):5746–5754. doi: 10.1074/jbc.274.9.5746. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Breese KR, Heistad DD. Cerebral vasodilation during hypercapnia. Role of glibenclamide-sensitive potassium channels and nitric oxide. Stroke. 1994;25(8):1679–1683. doi: 10.1161/01.str.25.8.1679. [DOI] [PubMed] [Google Scholar]
- Faraci FM, Heistad DD. Regulation of the cerebral circulation: role of endothelium and potassium channels. Physiol Rev. 1998;78(1):53–97. doi: 10.1152/physrev.1998.78.1.53. [DOI] [PubMed] [Google Scholar]
- Farouque HM, Meredith IT. Effects of inhibition of ATP-sensitive potassium channels on metabolic vasodilation in the human forearm. Clin Sci (Lond) 2003a;104(1):39–46. doi: 10.1042/. doi: 10.1042/ [DOI] [PubMed] [Google Scholar]
- Farouque HM, Meredith IT. Inhibition of vascular ATP-sensitive K+ channels does not affect reactive hyperemia in human forearm. Am J Physiol Heart Circ Physiol. 2003b;284(2):H711–718. doi: 10.1152/ajpheart.00315.2002. [DOI] [PubMed] [Google Scholar]
- Farouque HM, Meredith IT. Relative contribution of vasodilator prostanoids, NO, and KATP channels to human forearm metabolic vasodilation. Am J Physiol Heart Circ Physiol. 2003c;284(6):H2405–2411. doi: 10.1152/ajpheart.00879.2002. [DOI] [PubMed] [Google Scholar]
- Farouque HM, Meredith IT. Effect of adenosine triphosphate-sensitive potassium channel inhibitors on coronary metabolic vasodilation. Trends Cardiovasc Med. 2007;17(2):63–68. doi: 10.1016/j.tcm.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Farouque HM, Worthley SG, Meredith IT. Effect of ATP-sensitive potassium channel inhibition on coronary metabolic vasodilation in humans. Arterioscler Thromb Vasc Biol. 2004;24(5):905–910. doi: 10.1161/01.ATV.0000125701.18648.48. [DOI] [PubMed] [Google Scholar]
- Farouque HM, Worthley SG, Meredith IT, Skyrme-Jones RA, Zhang MJ. Effect of ATP-sensitive potassium channel inhibition on resting coronary vascular responses in humans. Circ Res. 2002;90(2):231–236. doi: 10.1161/hh0202.103713. [DOI] [PubMed] [Google Scholar]
- Feliciangeli S, Chatelain FC, Bichet D, Lesage F. The family of K2P channels: salient structural and functional properties. J Physiol. 2015;593(12):2587–2603. doi: 10.1113/jphysiol.2014.287268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Tenorio M, González-Rodríguez P, Porras C, Castellano A, Moosmang S, Hofmann F, López-Barneo J. Short Communication: Genetic Ablation of L-Type Ca2+ Channels Abolishes Depolarization-Induced Ca2+ Release in Arterial Smooth Muscle. Circ Res. 2010;106(7):1285–1289. doi: 10.1161/circresaha.109.213967. [DOI] [PubMed] [Google Scholar]
- Fernandez-Tenorio M, Porras-Gonzalez C, Castellano A, del Valle-Rodriguez A, Lopez-Barneo J, Urena J. Metabotropic Regulation of RhoA/Rho-Associated Kinase by L-type Ca2+ Channels. Circ Res. 2011 doi: 10.1161/circresaha.111.240127. CIRCRESAHA.111.240127. [DOI] [PubMed] [Google Scholar]
- Fernandez-Velasco M, Ruiz-Hurtado G, Gomez AM, Rueda A. Ca(2+) handling alterations and vascular dysfunction in diabetes. Cell Calcium. 2014;56(5):397–407. doi: 10.1016/j.ceca.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9(11):1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Foster MN, Coetzee WA. KATP Channels in the Cardiovascular System. Physiol Rev. 2016;96(1):177–252. doi: 10.1152/physrev.00003.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee JC, Maier KG, Stepp DW. Oxidant stress-induced increase in myogenic activation of skeletal muscle resistance arteries in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2002;283(6):H2160–2168. doi: 10.1152/ajpheart.00379.2002. [DOI] [PubMed] [Google Scholar]
- Fujino K, Nakaya S, Wakatsuki T, Miyoshi Y, Nakaya Y, Mori H, Inoue I. Effects of nitroglycerin on ATP-induced Ca(++)-mobilization, Ca(++)-activated K channels and contraction of cultured smooth muscle cells of porcine coronary artery. J Pharmacol Exp Ther. 1991;256(1):371–377. [PubMed] [Google Scholar]
- Fukami Y, Toki Y, Numaguchi Y, Nakashima Y, Mukawa H, Matsui H, Ito T. Nitroglycerin-induced aortic relaxation mediated by calcium-activated potassium channel is markedly diminished in hypertensive rats. Life Sci. 1998;63(12):1047–1055. doi: 10.1016/s0024-3205(98)00366-x. [DOI] [PubMed] [Google Scholar]
- Furspan PB, Webb RC. Decreased ATP sensitivity of a K+ channel and enhanced vascular smooth muscle relaxation in genetically hypertensive rats. J Hypertens. 1993;11(10):1067–1072. doi: 10.1097/00004872-199310000-00010. [DOI] [PubMed] [Google Scholar]
- Furstenau M, Lohn M, Ried C, Luft FC, Haller H, Gollasch M. Calcium sparks in human coronary artery smooth muscle cells resolved by confocal imaging. J Hypertens. 2000;18(9):1215–1222. doi: 10.1097/00004872-200018090-00007. [DOI] [PubMed] [Google Scholar]
- Ganitkevich V, Isenberg G. Isolated Guinea-Pig Coronary Smooth-Muscle Cells - Acetylcholine Induces Hyperpolarization Due to Sarcoplasmic-Reticulum Calcium Release Activating Potassium Channels. Circ Res. 1990;67(2):525–528. doi: 10.1161/01.res.67.2.525. [DOI] [PubMed] [Google Scholar]
- Ganitkevich VY, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. Journal of Physiology (London) 1993;470:35–44. doi: 10.1113/jphysiol.1993.sp019845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland CJ, McPherson GA. Evidence that nitric oxide does not mediate the hyperpolarization and relaxation to acetylcholine in the rat small mesenteric artery. Br J Pharmacol. 1992;105(2):429–435. doi: 10.1111/j.1476-5381.1992.tb14270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelband CH, Ishikawa T, Post JM, Keef KD, Hume JR. Intracellular divalent cations block smooth muscle K+ channels. Circ Res. 1993;73(1):24–34. doi: 10.1161/01.res.73.1.24. [DOI] [PubMed] [Google Scholar]
- Ghanshani S, Wulff H, Miller MJ, Rohm H, Neben A, Gutman GA, Chandy KG. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J Biol Chem. 2000;275(47):37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- Ghisdal P, Gomez JP, Morel N. Action of a NO donor on the excitation-contraction pathway activated by noradrenaline in rat superior mesenteric artery. J Physiol. 2000;522(Pt 1):83–96. doi: 10.1111/j.1469-7793.2000.t01-3-00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M, Hanna ST, Wang R, McNeill JR. Altered vascular reactivity and KATP channel currents in vascular smooth muscle cells from deoxycorticosterone acetate (DOCA)-salt hypertensive rats. J Cardiovasc Pharmacol. 2004;44(5):525–531. doi: 10.1097/00005344-200411000-00003. [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107(8):3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gole HK, Tharp DL, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channels (KCNN4) in porcine coronary smooth muscle requires NADPH oxidase 5 (NOX5) PLoS One. 2014;9(8):e105337. doi: 10.1371/journal.pone.0105337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollasch M, Lohn M, Furstenau M, Nelson MT, Luft FC, Haller H. Ca2+ channels, Ca2+ sparks, and regulation of arterial smooth muscle function. Z Kardiol. 2000;89(Suppl 2):15–19. doi: 10.1007/s003920070095. [DOI] [PubMed] [Google Scholar]
- Greenwood IA, Ohya S. New tricks for old dogs: KCNQ expression and role in smooth muscle. Br J Pharmacol. 2009;156(8):1196–1203. doi: 10.1111/j.1476-5381.2009.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guia A, Wan X, Courtemanche M, Leblanc N. Local Ca2+ entry through L-type Ca2+ channels activates Ca2+-dependent K+ channels in rabbit coronary myocytes. Circ Res. 1999;84(9):1032–1042. doi: 10.1161/01.res.84.9.1032. [DOI] [PubMed] [Google Scholar]
- Gurney A, Manoury B. Two-pore potassium channels in the cardiovascular system. Eur Biophys J. 2009;38(3):305–318. doi: 10.1007/s00249-008-0326-8. [DOI] [PubMed] [Google Scholar]
- Gutman GA, Chandy KG, Grissmer S, Lazdunski M, Mckinnon D, Pardo LA, Wang X. International Union of Pharmacology. LIII. Nomenclature and Molecular Relationships of Voltage-Gated Potassium Channels. Pharmacol Rev. 2005;57(4):473–508. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- Haddock RE, Grayson TH, Morris MJ, Howitt L, Chadha PS, Sandow SL. Diet-induced obesity impairs endothelium-derived hyperpolarization via altered potassium channel signaling mechanisms. PLoS One. 2011;6(1):e16423. doi: 10.1371/journal.pone.0016423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290(3):R546–552. doi: 10.1152/ajpregu.00491.2005. [DOI] [PubMed] [Google Scholar]
- Hald BO, Jacobsen JC, Braunstein TH, Inoue R, Ito Y, Sorensen PG, Jensen LJ. BKCa and KV channels limit conducted vasomotor responses in rat mesenteric terminal arterioles. Pflugers Arch. 2012;463(2):279–295. doi: 10.1007/s00424-011-1049-8. [DOI] [PubMed] [Google Scholar]
- Hammer LW, Ligon AL, Hester RL. Differential inhibition of functional dilation of small arterioles by indomethacin and glibenclamide. Hypertension. 2001;37(2 Pt 2):599–603. doi: 10.1161/01.hyp.37.2.599. [DOI] [PubMed] [Google Scholar]
- Hansen PR, Olesen SP. Relaxation of rat resistance arteries by acetylcholine involves a dual mechanism: activation of K+ channels and formation of nitric oxide. Pharmacol Toxicol. 1997;80(6):280–285. doi: 10.1111/j.1600-0773.1997.tb01974.x. [DOI] [PubMed] [Google Scholar]
- Harraz OF, Abd El-Rahman RR, Bigdely-Shamloo K, Wilson SM, Brett SE, Romero M, Welsh DG. Ca(V)3.2 channels and the induction of negative feedback in cerebral arteries. Circ Res. 2014;115(7):650–661. doi: 10.1161/CIRCRESAHA.114.304056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraz OF, Brett SE, Zechariah A, Romero M, Puglisi JL, Wilson SM, Welsh DG. Genetic ablation of CaV3.2 channels enhances the arterial myogenic response by modulating the RyR-BKCa axis. Arterioscler Thromb Vasc Biol. 2015;35(8):1843–1851. doi: 10.1161/ATVBAHA.115.305736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemzadeh-Gargari H, Rembold CM. Histamine activates whole cell K+ currents in swine carotid arterial smooth muscle cell. Comp Biochem Physiol C. 1992;102(1):33–37. doi: 10.1016/0742-8413(92)90039-a. [DOI] [PubMed] [Google Scholar]
- Hayabuchi Y, Davies NW, Standen NB. Angiotensin II inhibits rat arterial KATP channels by inhibiting steady-state protein kinase A activity and activating protein kinase Ce. J Physiol. 2001;530(Pt 2):193–205. doi: 10.1111/j.1469-7793.2001.0193l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayabuchi Y, Standen NB, Davies NW. Angiotensin II inhibits and alters kinetics of voltage-gated K(+) channels of rat arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2001;281(6):H2480–2489. doi: 10.1152/ajpheart.2001.281.6.H2480. [DOI] [PubMed] [Google Scholar]
- Heaps CL, Bowles DK. Gender-specific K(+)-channel contribution to adenosine-induced relaxation in coronary arterioles. J Appl Physiol (1985) 2002;92(2):550–558. doi: 10.1152/japplphysiol.00566.2001. [DOI] [PubMed] [Google Scholar]
- Heaps CL, Tharp DL, Bowles DK. Hypercholesterolemia abolishes voltage-dependent K+ channel contribution to adenosine-mediated relaxation in porcine coronary arterioles. Am J Physiol Heart Circ Physiol. 2005;288(2):H568–576. doi: 10.1152/ajpheart.00157.2004. [DOI] [PubMed] [Google Scholar]
- Hedegaard ER, Nielsen BD, Kun A, Hughes AD, Kroigaard C, Mogensen S, Simonsen U. KV 7 channels are involved in hypoxia-induced vasodilatation of porcine coronary arteries. Br J Pharmacol. 2014;171(1):69–82. doi: 10.1111/bph.12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heintz A, Damm M, Brand M, Koch T, Deussen A. Coronary flow regulation in mouse heart during hypercapnic acidosis: role of NO and its compensation during eNOS impairment. Cardiovasc Res. 2008;77(1):188–196. doi: 10.1093/cvr/cvm014. [DOI] [PubMed] [Google Scholar]
- Henry P, Pearson WL, Nichols CG. Protein kinase C inhibition of cloned inward rectifier (HRK1/KIR2.3) K+ channels expressed in Xenopus oocytes. J Physiol. 1996;495(Pt 3):681–688. doi: 10.1113/jphysiol.1996.sp021625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernanz R, Alonso MJ, Baena AB, Salaices M, Alvarez L, Castillo-Olivares JL, Marin J. Mechanisms involved in relaxation induced by exogenous nitric oxide in pig coronary arteries. Methods Find Exp Clin Pharmacol. 1999;21(3):155–160. doi: 10.1358/mf.1999.21.3.534823. [DOI] [PubMed] [Google Scholar]
- Hodnett BL, Xiang L, Dearman JA, Carter CB, Hester RL. K(ATP)-mediated vasodilation is impaired in obese Zucker rats. Microcirculation. 2008;15(6):485–494. doi: 10.1080/10739680801942240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hojs N, Strucl M, Cankar K. The effect of glibenclamide on acetylcholine and sodium nitroprusside induced vasodilatation in human cutaneous microcirculation. Clin Physiol Funct Imaging. 2009;29(1):38–44. doi: 10.1111/j.1475-097X.2008.00833.x. [DOI] [PubMed] [Google Scholar]
- Holdsworth CT, Copp SW, Ferguson SK, Sims GE, Poole DC, Musch TI. Acute inhibition of ATP-sensitive K+ channels impairs skeletal muscle vascular control in rats during treadmill exercise. Am J Physiol Heart Circ Physiol. 2015;308(11):H1434–1442. doi: 10.1152/ajpheart.00772.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinaka N, Kuang TY, Pak H, Wang R, Jehle J, Kennedy C, Sokoloff L. Blockade of cerebral blood flow response to insulin-induced hypoglycemia by caffeine and glibenclamide in conscious rats. J Cereb Blood Flow Metab. 1997;17(12):1309–1318. doi: 10.1097/00004647-199712000-00006. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Pantazis A, Olcese R. Transduction of voltage and Ca2+ signals by Slo1 BK channels. Physiology (Bethesda) 2013;28(3):172–189. doi: 10.1152/physiol.00055.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Tian Y, Xu R, Heinemann SH, Hou S. Mechanism of the modulation of BK potassium channel complexes with different auxiliary subunit compositions by the omega-3 fatty acid DHA. Proc Natl Acad Sci U S A. 2013;110(12):4822–4827. doi: 10.1073/pnas.1222003110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou S, Heinemann SH, Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda) 2009;24:26–35. doi: 10.1152/physiol.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House SJ, Potier M, Bisaillon J, Singer HA, Trebak M. The non-excitable smooth muscle: calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 2008;456(5):769–785. doi: 10.1007/s00424-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Pollock CA, Chen XM. KCa3.1: a new player in progressive kidney disease. Curr Opin Nephrol Hypertens. 2015;24(1):61–66. doi: 10.1097/MNH.0000000000000083. [DOI] [PubMed] [Google Scholar]
- Hutri-Kahonen N, Kahonen M, Wu X, Sand J, Nordback I, Taurio J, Porsti I. Control of vascular tone in isolated mesenteric arterial segments from hypertensive patients. Br J Pharmacol. 1999;127(7):1735–1743. doi: 10.1038/sj.bjp.0702716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura Y, Tomoike H, Narishige T, Takahashi T, Kasuya H, Takeshita A. Glibenclamide decreases basal coronary blood flow in anesthetized dogs. Am J Physiol. 1992;263(2 Pt 2):H399–404. doi: 10.1152/ajpheart.1992.263.2.H399. [DOI] [PubMed] [Google Scholar]
- Irat AM, Aslamaci S, Karasu C, Ari N. Alteration of vascular reactivity in diabetic human mammary artery and the effects of thiazolidinediones. J Pharm Pharmacol. 2006;58(12):1647–1653. doi: 10.1211/jpp.58.12.0012. [DOI] [PubMed] [Google Scholar]
- Ishii TM, Silvia C, Hirschberg B, Bond CT, Adelman JP, Maylie J. A human intermediate conductance calcium-activated potassium channel. Proc Natl Acad Sci U S A. 1997;94(21):11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Hume JR, Keef KD. Modulation of K+ and Ca2+ channels by histamine H1-receptor stimulation in rabbit coronary artery cells. J Physiol. 1993;468:379–400. doi: 10.1113/jphysiol.1993.sp019777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WF. Arteriolar Tone Is Determined by Activity of Atp-Sensitive Potassium Channels. American Journal of Physiology. 1993;265(5):H1797–H1803. doi: 10.1152/ajpheart.1993.265.5.H1797. [DOI] [PubMed] [Google Scholar]
- Jackson WF. Ion channels and vascular tone. Hypertension. 2000;35(1 Pt 2):173–178. doi: 10.1161/01.hyp.35.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12(1):113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson WF, Blair KL. Characterization and function of Ca(2+)-activated K+ channels in arteriolar muscle cells. Am J Physiol. 1998;274(1 Pt 2):H27–34. doi: 10.1152/ajpheart.1998.274.1.H27. [DOI] [PubMed] [Google Scholar]
- Jackson WF, Konig A, Dambacher T, Busse R. Prostacyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am J Physiol. 1993;264(1 Pt 2):H238–243. doi: 10.1152/ajpheart.1993.264.1.H238. [DOI] [PubMed] [Google Scholar]
- Jackson-Weaver O, Osmond JM, Riddle MA, Naik JS, Gonzalez Bosc LV, Walker BR, Kanagy NL. Hydrogen sulfide dilates rat mesenteric arteries by activating endothelial large-conductance Ca(2)(+)-activated K(+) channels and smooth muscle Ca(2)(+) sparks. Am J Physiol Heart Circ Physiol. 2013;304(11):H1446–1454. doi: 10.1152/ajpheart.00506.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, E S, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res. 2002;91(7):610–617. doi: 10.1161/01.res.0000036900.76780.95. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Li AL, Parfenova H, Liu JX, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97(8):805–812. doi: 10.1161/RES.000018618.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaggar JH, Porter VA, Lederer WJ, Nelson MT. Calcium sparks in smooth muscle. Am J Physiol Cell Physiol. 2000;278(2):C235–256. doi: 10.1152/ajpcell.2000.278.2.C235. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Stevenson AS, Nelson MT. Voltage dependence of Ca2+ sparks in intact cerebral arteries. Am J Physiol. 1998;274(6 Pt 1):C1755–1761. doi: 10.1152/ajpcell.1998.274.6.C1755. [DOI] [PubMed] [Google Scholar]
- Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Nelson MT. Ca2+ channels, ryanodine receptors and Ca(2+)-activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand. 1998;164(4):577–587. doi: 10.1046/j.1365-201X.1998.00462.x. [DOI] [PubMed] [Google Scholar]
- Jantzi MC, Brett SE, Jackson WF, Corteling R, Vigmond EJ, Welsh DG. Inward rectifying potassium channels facilitate cell-to-cell communication in hamster retractor muscle feed arteries. Am J Physiol Heart Circ Physiol. 2006;291(3):H1319–1328. doi: 10.1152/ajpheart.00217.2006. [DOI] [PubMed] [Google Scholar]
- Jepps TA, Olesen SP, Greenwood IA. One man’s side effect is another man’s therapeutic opportunity: targeting Kv7 channels in smooth muscle disorders. Br J Pharmacol. 2013;168(1):19–27. doi: 10.1111/j.1476-5381.2012.02133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell RP, Saundry CM, Bonev AD, Tranmer BI, Wellman GC. Inhibition of Ca++ sparks by oxyhemoglobin in rabbit cerebral arteries. J Neurosurg. 2004;100(2):295–302. doi: 10.3171/jns.2004.100.2.0295. [DOI] [PubMed] [Google Scholar]
- Jiang J, Thoren P, Caligiuri G, Hansson GK, Pernow J. Enhanced phenylephrine-induced rhythmic activity in the atherosclerotic mouse aorta via an increase in opening of KCa channels: relation to Kv channels and nitric oxide. Br J Pharmacol. 1999;128(3):637–646. doi: 10.1038/sj.bjp.0702855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang ZG, Si JQ, Lasarev MR, Nuttall AL. Two resting potential levels regulated by the inward-rectifier potassium channel in the guinea-pig spiral modiolar artery. J Physiol. 2001;537(Pt 3):829–842. doi: 10.1111/j.1469-7793.2001.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao J, Garg V, Yang B, Elton TS, Hu K. Protein kinase C-epsilon induces caveolin-dependent internalization of vascular adenosine 5′-triphosphate-sensitive K+ channels. Hypertension. 2008;52(3):499–506. doi: 10.1161/HYPERTENSIONAHA.108.110817. [DOI] [PubMed] [Google Scholar]
- Jimenez-Perez L, Cidad P, Alvarez-Miguel I, Santos-Hipolito A, Torres-Merino R, Alonso E, Perez-Garcia MT. Molecular Determinants of Kv1.3 Potassium Channels-induced Proliferation. J Biol Chem. 2016;291(7):3569–3580. doi: 10.1074/jbc.M115.678995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TD, Marrelli SP, Steenberg ML, Childres WF, Bryan RM., Jr Inward rectifier potassium channels in the rat middle cerebral artery. Am J Physiol. 1998;274(2 Pt 2):R541–547. doi: 10.1152/ajpregu.1998.274.2.R541. [DOI] [PubMed] [Google Scholar]
- Kalliovalkama J, Jolma P, Tolvanen JP, Kahonen M, Hutri-Kahonen N, Wu X, Porsti I. Arterial function in nitric oxide-deficient hypertension: influence of long-term angiotensin II receptor antagonism. Cardiovasc Res. 1999;42(3):773–782. doi: 10.1016/s0008-6363(98)00346-0. [DOI] [PubMed] [Google Scholar]
- Kam KL, Pfaffendorf M, van Zwieten PA. Drug-induced endothelium-dependent and -independent relaxations in isolated resistance vessels taken from simultaneously hypertensive and streptozotocin-diabetic rats. Blood Press. 1994;3(6):418–427. doi: 10.3109/08037059409102296. [DOI] [PubMed] [Google Scholar]
- Kamata K, Miyata N, Kasuya Y. Functional changes in potassium channels in aortas from rats with streptozotocin-induced diabetes. Eur J Pharmacol. 1989;166(2):319–323. doi: 10.1016/0014-2999(89)90076-9. doi: http://dx.doi.org/10.1016/0014-2999(89)90076-9. [DOI] [PubMed] [Google Scholar]
- Kawata T, Mimuro T, Onuki T, Tsuchiya K, Nihei H, Koike T. The K(ATP) channel opener nicorandil: effect on renal hemodynamics in spontaneously hypertensive and Wistar Kyoto rats. Kidney Int Suppl. 1998;67:S231–233. doi: 10.1046/j.1523-1755.1998.06758.x. [DOI] [PubMed] [Google Scholar]
- Khanamiri S, Soltysinska E, Jepps TA, Bentzen BH, Chadha PS, Schmitt N, Olesen SP. Contribution of Kv7 channels to basal coronary flow and active response to ischemia. Hypertension. 2013;62(6):1090–1097. doi: 10.1161/HYPERTENSIONAHA.113.01244. [DOI] [PubMed] [Google Scholar]
- Kilpatrick EV, Cocks TM. Evidence for Differential Roles of Nitric-Oxide (No) and Hyperpolarization in Endothelium-Dependent Relaxation of Pig Isolated Coronary-Artery. Br J Pharmacol. 1994;112(2):557–565. doi: 10.1111/j.1476-5381.1994.tb13110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Oh KH. Protein Network Interacting with BK Channels. Int Rev Neurobiol. 2016;128:127–161. doi: 10.1016/bs.irn.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Kinoshita H, Azma T, Iranami H, Nakahata K, Kimoto Y, Dojo M, Hatano Y. Synthetic peroxisome proliferator-activated receptor-gamma agonists restore impaired vasorelaxation via ATP-sensitive K+ channels by high glucose. J Pharmacol Exp Ther. 2006;318(1):312–318. doi: 10.1124/jpet.106.100958. [DOI] [PubMed] [Google Scholar]
- Kitazono T, Heistad DD, Faraci FM. ATP-sensitive potassium channels in the basilar artery during chronic hypertension. Hypertension. 1993a;22(5):677–681. doi: 10.1161/01.hyp.22.5.677. [DOI] [PubMed] [Google Scholar]
- Kitazono T, Heistad DD, Faraci FM. Role of ATP-sensitive K+ channels in CGRP-induced dilatation of basilar artery in vivo. Am J Physiol. 1993b;265(2 Pt 2):H581–585. doi: 10.1152/ajpheart.1993.265.2.H581. [DOI] [PubMed] [Google Scholar]
- Kleppisch T, Nelson MT. Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1995;92(26):12441–12445. doi: 10.1073/pnas.92.26.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Nelson MT. Regulation of arterial diameter and wall [Ca2+] in cerebral arteries of rat by membrane potential and intravascular pressure. J Physiol. 1998;508(Pt 1):199–209. doi: 10.1111/j.1469-7793.1998.199br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knot HJ, Standen NB, Nelson MT. Ryanodine receptors regulate arterial diameter and wall [Ca2+] in cerebral arteries of rat via Ca2+-dependent K+ channels. J Physiol. 1998;508(Pt 1):211–221. doi: 10.1111/j.1469-7793.1998.211br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EA, Park WS, Firth AL, Hong DH, Choi SW, Heo HJ, Han J. Increased sensitivity of serotonin on the voltage-dependent K+ channels in mesenteric arterial smooth muscle cells of OLETF rats. Prog Biophys Mol Biol. 2010;103(1):88–94. doi: 10.1016/j.pbiomolbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Ko EA, Park WS, Firth AL, Kim N, Yuan JX, Han J. Pathophysiology of voltage-gated K+ channels in vascular smooth muscle cells: modulation by protein kinases. Prog Biophys Mol Biol. 2010;103(1):95–101. doi: 10.1016/j.pbiomolbio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Kohler R, Wulff H, Eichler I, Kneifel M, Neumann D, Knorr A, Hoyer J. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108(9):1119–1125. doi: 10.1161/01.CIR.0000086464.04719.DD. [DOI] [PubMed] [Google Scholar]
- Kolias TJ, Chai S, Webb RC. Potassium channel antagonists and vascular reactivity in stroke-prone spontaneously hypertensive rats. Am J Hypertens. 1993;6(6 Pt 1):528–533. doi: 10.1093/ajh/6.6.528. [DOI] [PubMed] [Google Scholar]
- Kondratskyi A, Kondratska K, Skryma R, Prevarskaya N. Ion channels in the regulation of apoptosis. Biochim Biophys Acta. 2015;1848(10 Pt B):2532–2546. doi: 10.1016/j.bbamem.2014.10.030. [DOI] [PubMed] [Google Scholar]
- Kosmas EN, Levy RD, Hussain SN. Acute effects of glyburide on the regulation of peripheral blood flow in normal humans. Eur J Pharmacol. 1995;274(1–03):193–199. doi: 10.1016/0014-2999(94)00732-m. [DOI] [PubMed] [Google Scholar]
- Kuang Q, Purhonen P, Hebert H. Structure of potassium channels. Cell Mol Life Sci. 2015;72(19):3677–3693. doi: 10.1007/s00018-015-1948-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukuljan M, Rojas E, Catt KJ, Stojilkovic SS. Membrane potential regulates inositol 1,4,5-trisphosphate-controlled cytoplasmic Ca2+ oscillations in pituitary gonadotrophs. J Biol Chem. 1994;269(7):4860–4865. [PubMed] [Google Scholar]
- Kume H, Graziano MP, Kotlikoff MI. Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide-binding proteins. Proc Natl Acad Sci U S A. 1992;89(22):11051–11055. doi: 10.1073/pnas.89.22.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. Beta-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest. 1994;93(1):371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Takai A, Tokuno H, Tomita T. Regulation of Ca2+-dependent K+-channel activity in tracheal myocytes by phosphorylation. Nature. 1989;341(6238):152–154. doi: 10.1038/341152a0. [DOI] [PubMed] [Google Scholar]
- Lang RJ, Harvey JR, McPhee GJ, Klemm MF. Nitric oxide and thiol reagent modulation of Ca2+-activated K+ (BKCa) channels in myocytes of the guinea-pig taenia caeci. J Physiol. 2000;525(Pt 2):363–376. doi: 10.1111/j.1469-7793.2000.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RJ, Harvey JR, Mulholland EL. Sodium (2-sulfonatoethyl) methanethiosulfonate prevents S-nitroso-L-cysteine activation of Ca2+-activated K+ (BKCa) channels in myocytes of the guinea-pig taenia caeca. Br J Pharmacol. 2003;139(6):1153–1163. doi: 10.1038/sj.bjp.0705349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange A, Gebremedhin D, Narayanan J, Harder D. 20-hydroxyeicosatetraenoic acid-induced vasoconstriction and inhibition of potassium current in cerebral vascular smooth muscle is dependent on activation of protein kinase C. Journal of Biological Chemistry. 1997;272(43):27345–27352. doi: 10.1074/jbc.272.43.27345. [DOI] [PubMed] [Google Scholar]
- Leffler CW, Parfenova H, Basuroy S, Jaggar JH, Umstot ES, Fedinec AL. Hydrogen sulfide and cerebral microvascular tone in newborn pigs. Am J Physiol Heart Circ Physiol. 2011;300(2):H440–447. doi: 10.1152/ajpheart.00722.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo MD, Bannister JP, Narayanan D, Nair A, Grubbs JE, Gabrick KS, Jaggar JH. Dynamic regulation of beta1 subunit trafficking controls vascular contractility. Proc Natl Acad Sci U S A. 2014;111(6):2361–2366. doi: 10.1073/pnas.1317527111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo MD, Bulley S, Bannister JP, Kuruvilla KP, Narayanan D, Jaggar JH. Angiotensin II stimulates internalization and degradation of arterial myocyte plasma membrane BK channels to induce vasoconstriction. Am J Physiol Cell Physiol. 2015;309(6):C392–402. doi: 10.1152/ajpcell.00127.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan IB. Signaling protein complexes associated with neuronal ion channels. Nat Neurosci. 2006;9(3):305–310. doi: 10.1038/nn1647. [DOI] [PubMed] [Google Scholar]
- Li A, Knutsen RH, Zhang H, Osei-Owusu P, Moreno-Dominguez A, Harter TM, Nichols CG. Hypotension due to Kir6.1 gain-of-function in vascular smooth muscle. J Am Heart Assoc. 2013;2(4):e000365. doi: 10.1161/JAHA.113.000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, Leffler CW. Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle Cell KCa channels. Circ Res. 2008;102(2):234–241. doi: 10.1161/CIRCRESAHA.107.164145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP-mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003;285(3):H1213–1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- Li H, Gutterman DD, Rusch NJ, Bubolz A, Liu Y. Nitration and functional loss of voltage-gated K+ channels in rat coronary microvessels exposed to high glucose. Diabetes. 2004;53(9):2436–2442. doi: 10.2337/diabetes.53.9.2436. [DOI] [PubMed] [Google Scholar]
- Li PL, Jin MW, Campbell WB. Effect of selective inhibition of soluble guanylyl cyclase on the K(Ca) channel activity in coronary artery smooth muscle. Hypertension. 1998;31(1 Pt 2):303–308. doi: 10.1161/01.hyp.31.1.303. [DOI] [PubMed] [Google Scholar]
- Li PL, Zou AP, Campbell WB. Regulation of potassium channels in coronary arterial smooth muscle by endothelium-derived vasodilators. Hypertension. 1997;29(1 Pt 2):262–267. doi: 10.1161/01.hyp.29.1.262. [DOI] [PubMed] [Google Scholar]
- Li SS, Cui N, Yang Y, Trower TC, Wei YM, Wu Y, Jiang C. Impairment of the Vascular KATP Channel Imposes Fatal Susceptibility to Experimental Diabetes Due to Multi-Organ Injuries. J Cell Physiol. 2015;230(12):2915–2926. doi: 10.1002/jcp.25003. [DOI] [PubMed] [Google Scholar]
- Li Z, Lu N, Shi L. Exercise training reverses alterations in Kv and BKCa channel molecular expression in thoracic aorta smooth muscle cells from spontaneously hypertensive rats. J Vasc Res. 2014;51(6):447–457. doi: 10.1159/000369928. [DOI] [PubMed] [Google Scholar]
- Liang GH, Adebiyi A, Leo MD, McNally EM, Leffler CW, Jaggar JH. Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am J Physiol Heart Circ Physiol. 2011;300(6):H2088–2095. doi: 10.1152/ajpheart.01290.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang GH, Xi Q, Leffler CW, Jaggar JH. Hydrogen sulfide activates Ca(2)(+) sparks to induce cerebral arteriole dilatation. J Physiol. 2012;590(11):2709–2720. doi: 10.1113/jphysiol.2011.225128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindauer U, Vogt J, Schuh-Hofer S, Dreier JP, Dirnagl U. Cerebrovascular vasodilation to extraluminal acidosis occurs via combined activation of ATP-sensitive and Ca2+-activated potassium channels. J Cereb Blood Flow Metab. 2003;23(10):1227–1238. doi: 10.1097/01.WCB.0000088764.02615.B7. [DOI] [PubMed] [Google Scholar]
- Liu QH, Zheng YM, Korde AS, Yadav VR, Rathore R, Wess J, Wang YX. Membrane depolarization causes a direct activation of G protein-coupled receptors leading to local Ca2+ release in smooth muscle. Proc Natl Acad Sci U S A. 2009;106(27):11418–11423. doi: 10.1073/pnas.0813307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Gutterman DD. The coronary circulation in diabetes: influence of reactive oxygen species on K+ channel-mediated vasodilation. Vascul Pharmacol. 2002;38(1):43–49. doi: 10.1016/s1537-1891(02)00125-8. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hudetz AG, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats: evidence for their protection against cerebral vasospasm. Circ Res. 1998;82(6):729–737. doi: 10.1161/01.res.82.6.729. [DOI] [PubMed] [Google Scholar]
- Liu Y, Jones AW, Sturek M. Increased barium influx and potassium current in stroke-prone spontaneously hypertensive rats. Hypertension. 1994;23(6 Pt 2):1091–1095. doi: 10.1161/01.hyp.23.6.1091. [DOI] [PubMed] [Google Scholar]
- Liu Y, Pleyte K, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30(6):1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated K(+) channel current in rat small coronary arteries. Circ Res. 2001;89(2):146–152. doi: 10.1161/hh1401.093294. [DOI] [PubMed] [Google Scholar]
- Longden TA, Dabertrand F, Hill-Eubanks DC, Hammack SE, Nelson MT. Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc Natl Acad Sci U S A. 2014;111(20):7462–7467. doi: 10.1073/pnas.1401811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longden TA, Nelson MT. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation. 2015;22(3):183–196. doi: 10.1111/micc.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loutzenhiser RD, Parker MJ. Hypoxia inhibits myogenic reactivity of renal afferent arterioles by activating ATP-sensitive K+ channels. Circ Res. 1994;74(5):861–869. doi: 10.1161/01.res.74.5.861. [DOI] [PubMed] [Google Scholar]
- Lovren F, Triggle C. Nitric oxide and sodium nitroprusside-induced relaxation of the human umbilical artery. Br J Pharmacol. 2000;131(3):521–529. doi: 10.1038/sj.bjp.0703588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S, Xiang L, Clemmer JS, Gowdey AR, Mittwede PN, Hester RL. Impaired vascular KATP function attenuates exercise capacity in obese zucker rats. Microcirculation. 2013;20(7):662–669. doi: 10.1111/micc.12065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luykenaar KD, Brett SE, Wu BN, Wiehler WB, Welsh DG. Pyrimidine nucleotides suppress KDR currents and depolarize rat cerebral arteries by activating Rho kinase. Am J Physiol Heart Circ Physiol. 2004;286(3):H1088–1100. doi: 10.1152/ajpheart.00903.2003. [DOI] [PubMed] [Google Scholar]
- Luykenaar KD, El-Rahman RA, Walsh MP, Welsh DG. Rho-kinase-mediated suppression of KDR current in cerebral arteries requires an intact actin cytoskeleton. Am J Physiol Heart Circ Physiol. 2009;296(4):H917–926. doi: 10.1152/ajpheart.01206.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie AR, Byron KL. Cardiovascular KCNQ (Kv7) potassium channels: physiological regulators and new targets for therapeutic intervention. Mol Pharmacol. 2008;74(5):1171–1179. doi: 10.1124/mol.108.049825. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith MP, Martinez-Pinna J, Gurung IS. A role for membrane potential in regulating GPCRs? Trends Pharmacol Sci. 2008;29(8):421–429. doi: 10.1016/j.tips.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Mandala M, Heppner TJ, Bonev AD, Nelson MT. Effect of endogenous and exogenous nitric oxide on calcium sparks as targets for vasodilation in rat cerebral artery. Nitric Oxide. 2007;16(1):104–109. doi: 10.1016/j.niox.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Marijic J, Li Q, Song M, Nishimaru K, Stefani E, Toro L. Decreased expression of voltage- and Ca(2+)-activated K(+) channels in coronary smooth muscle during aging. Circ Res. 2001;88(2):210–216. doi: 10.1161/01.res.88.2.210. [DOI] [PubMed] [Google Scholar]
- Marrelli SP, Johnson TD, Khorovets A, Childres WF, Bryan RM., Jr Altered function of inward rectifier potassium channels in cerebrovascular smooth muscle after ischemia/reperfusion. Stroke. 1998;29(7):1469–1474. doi: 10.1161/01.str.29.7.1469. [DOI] [PubMed] [Google Scholar]
- Marshall JM, Thomas T, Turner L. A Link between Adenosine, Atp-Sensitive K+ Channels, Potassium and Muscle Vasodilatation in the Rat in Systemic Hypoxia. Journal of Physiology-London. 1993;472:1–9. doi: 10.1113/jphysiol.1993.sp019931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli A, Testai L, Breschi MC, Lawson K, McKay NG, Miceli F, Calderone V. Vasorelaxation by hydrogen sulphide involves activation of Kv7 potassium channels. Pharmacol Res. 2013;70(1):27–34. doi: 10.1016/j.phrs.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Martens JR, Gelband CH. Alterations in rat interlobar artery membrane potential and K+ channels in genetic and nongenetic hypertension. Circ Res. 1996;79(2):295–301. doi: 10.1161/01.res.79.2.295. [DOI] [PubMed] [Google Scholar]
- Martinez AC, Pagan RM, Prieto D, Recio P, Garcia-Sacristan A, Hernandez M, Benedito S. Modulation of noradrenergic neurotransmission in isolated rat radial artery. J Pharmacol Sci. 2009;111(3):299–311. doi: 10.1254/jphs.09135fp. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Szasz T, Tostes RC, Webb RC. Impaired beta-adrenoceptor-induced relaxation in small mesenteric arteries from DOCA-salt hypertensive rats is due to reduced K(Ca) channel activity. Pharmacol Res. 2012;65(5):537–545. doi: 10.1016/j.phrs.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Puro DG. Topographical heterogeneity of K(IR) currents in pericyte-containing microvessels of the rat retina: effect of diabetes. J Physiol. 2006;573(Pt 2):483–495. doi: 10.1113/jphysiol.2006.107102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhan WG. Effect of diabetes mellitus on response of the basilar artery to activation of ATP-sensitive potassium channels. Brain Res. 1994;636(1):35–39. doi: 10.1016/0006-8993(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Faraci FM. Responses of cerebral arterioles in diabetic rats to activation of ATP-sensitive potassium channels. Am J Physiol. 1993;265(1 Pt 2):H152–157. doi: 10.1152/ajpheart.1993.265.1.H152. [DOI] [PubMed] [Google Scholar]
- Mayhan WG, Mayhan JF, Sun H, Patel KP. In vivo properties of potassium channels in cerebral blood vessels during diabetes mellitus. Microcirculation. 2004;11(7):605–613. doi: 10.1080/10739680490503410. [DOI] [PubMed] [Google Scholar]
- McCarron JG, Halpern W. Potassium dilates rat cerebral arteries by two independent mechanisms. Am J Physiol. 1990;259(3 Pt 2):H902–908. doi: 10.1152/ajpheart.1990.259.3.H902. [DOI] [PubMed] [Google Scholar]
- McGahon MK, Dash DP, Arora A, Wall N, Dawicki J, Simpson DA, Curtis TM. Diabetes downregulates large-conductance Ca2+-activated potassium beta 1 channel subunit in retinal arteriolar smooth muscle. Circ Res. 2007;100(5):703–711. doi: 10.1161/01.RES.0000260182.36481.c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus OB, Helms LM, Pallanck L, Ganetzky B, Swanson R, Leonard RJ. Functional role of the beta subunit of high conductance calcium-activated potassium channels. Neuron. 1995;14(3):645–650. doi: 10.1016/0896-6273(95)90321-6. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between alpha (hslo) and beta subunits (KV,Ca beta) of maxi K channels. FEBS Lett. 1996;382:84–88. doi: 10.1016/0014-5793(96)00151-2. [DOI] [PubMed] [Google Scholar]
- Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0–S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. Proc Natl Acad Sci U S A. 1997;94(25):14066–14071. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkus D, Haitsma DB, Fung TY, Assen YJ, Verdouw PD, Duncker DJ. Coronary blood flow regulation in exercising swine involves parallel rather than redundant vasodilator pathways. Am J Physiol Heart Circ Physiol. 2003;285(1):H424–433. doi: 10.1152/ajpheart.00916.2002. [DOI] [PubMed] [Google Scholar]
- Merkus D, Sorop O, Houweling B, Hoogteijling BA, Duncker DJ. KCa+ channels contribute to exercise-induced coronary vasodilation in swine. Am J Physiol Heart Circ Physiol. 2006;291(5):H2090–2097. doi: 10.1152/ajpheart.00315.2006. [DOI] [PubMed] [Google Scholar]
- Miguel-Velado E, Moreno-Dominguez A, Colinas O, Cidad P, Heras M, Perez-Garcia MT, Lopez-Lopez JR. Contribution of Kv channels to phenotypic remodeling of human uterine artery smooth muscle cells. Circ Res. 2005;97(12):1280–1287. doi: 10.1161/01.RES.0000194322.91255.13. [DOI] [PubMed] [Google Scholar]
- Miguel-Velado E, Perez-Carretero FD, Colinas O, Cidad P, Heras M, Lopez-Lopez JR, Perez-Garcia MT. Cell cycle-dependent expression of Kv3.4 channels modulates proliferation of human uterine artery smooth muscle cells. Cardiovasc Res. 2010;86(3):383–391. doi: 10.1093/cvr/cvq011. [DOI] [PubMed] [Google Scholar]
- Miki T, Suzuki M, Shibasaki T, Uemura H, Sato T, Yamaguchi K, Seino S. Mouse model of Prinzmetal angina by disruption of the inward rectifier Kir6.1. Nat Med. 2002;8(5):466–472. doi: 10.1038/nm0502-466. [DOI] [PubMed] [Google Scholar]
- Miller AW, Tulbert C, Puskar M, Busija DW. Enhanced endothelin activity prevents vasodilation to insulin in insulin resistance. Hypertension. 2002;40(1):78–82. doi: 10.1161/01.hyp.0000022806.87281.62. [DOI] [PubMed] [Google Scholar]
- Minami K, Fukuzawa K, Nakaya Y. Protein kinase C inhibits the Ca(2+)-activated K+ channel of cultured porcine coronary artery smooth muscle cells. Biochem Biophys Res Commun. 1993;190(1):263–269. doi: 10.1006/bbrc.1993.1040. [DOI] [PubMed] [Google Scholar]
- Ming Z, Parent R, Lavallee M. Beta 2-adrenergic dilation of resistance coronary vessels involves KATP channels and nitric oxide in conscious dogs. Circulation. 1997;95(6):1568–1576. doi: 10.1161/01.cir.95.6.1568. [DOI] [PubMed] [Google Scholar]
- Mistry DK, Garland CJ. Nitric oxide (NO)-induced activation of large conductance Ca2+-dependent K+ channels (BK(Ca)) in smooth muscle cells isolated from the rat mesenteric artery. Br J Pharmacol. 1998;124(6):1131–1140. doi: 10.1038/sj.bjp.0701940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry DK, Garland CJ. The influence of phenylephrine outward potassium currents in single smooth muscle cells from the rabbit mesenteric artery. Gen Pharmacol. 1999;33(5):389–399. doi: 10.1016/s0306-3623(99)00031-2. [DOI] [PubMed] [Google Scholar]
- Miura H, Wachtel RE, Loberiza FR, Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. Circ Res. 2003;92(2):151–158. doi: 10.1161/01.res.0000052671.53256.49. [DOI] [PubMed] [Google Scholar]
- Miyata N, Tsuchida K, Otomo S. Functional changes in potassium channels in carotid arteries from stroke-prone spontaneously hypertensive rats. Eur J Pharmacol. 1990;182(1):209–210. doi: 10.1016/0014-2999(90)90517-a. [DOI] [PubMed] [Google Scholar]
- Mokelke EA, Dietz NJ, Eckman DM, Nelson MT, Sturek M. Diabetic dyslipidemia and exercise affect coronary tone and differential regulation of conduit and microvessel K+ current. Am J Physiol Heart Circ Physiol. 2005;288(3):H1233–1241. doi: 10.1152/ajpheart.00732.2004. [DOI] [PubMed] [Google Scholar]
- Moore CL, Nelson PL, Parelkar NK, Rusch NJ, Rhee SW. Protein kinase A-phosphorylated KV1 channels in PSD95 signaling complex contribute to the resting membrane potential and diameter of cerebral arteries. Circ Res. 2014;114(8):1258–1267. doi: 10.1161/CIRCRESAHA.114.303167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Dominguez A, Cidad P, Miguel-Velado E, Lopez-Lopez JR, Perez-Garcia MT. De novo expression of Kv6.3 contributes to changes in vascular smooth muscle cell excitability in a hypertensive mice strain. J Physiol. 2009;587(3):625–640. doi: 10.1113/jphysiol.2008.165217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Chujo M, Tanaka E, Yamakawa A, Shinozaki Y, Mohamed MU, Nakazawa H. Modulation of adrenergic coronary vasoconstriction via ATP-sensitive potassium channel. Am J Physiol. 1995;268(3 Pt 2):H1077–1085. doi: 10.1152/ajpheart.1995.268.3.H1077. [DOI] [PubMed] [Google Scholar]
- Munoz E, Hernandez-Morales M, Sobradillo D, Rocher A, Nunez L, Villalobos C. Intracellular Ca(2+) remodeling during the phenotypic journey of human coronary smooth muscle cells. Cell Calcium. 2013;54(5):375–385. doi: 10.1016/j.ceca.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Murrant CL, Sarelius IH. Multiple dilator pathways in skeletal muscle contraction-induced arteriolar dilations. Am J Physiol Regul Integr Comp Physiol. 2002;282(4):R969–978. doi: 10.1152/ajpregu.00405.2001. [DOI] [PubMed] [Google Scholar]
- Mustafa AK, Sikka G, Gazi SK, Steppan J, Jung SM, Bhunia AK, Snyder SH. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109(11):1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata K, Kinoshita H, Tokinaga Y, Ishida Y, Kimoto Y, Dojo M, Hatano Y. Vasodilation mediated by inward rectifier K+ channels in cerebral microvessels of hypertensive and normotensive rats. Anesth Analg. 2006;102(2):571–576. doi: 10.1213/01.ane.0000194303.00844.5e. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Vanhoutte PM. Isoproterenol Causes Hyperpolarization through Opening of Atp-Sensitive Potassium Channels in Vascular Smooth-Muscle of the Canine Saphenous-Vein. Journal of Pharmacology and Experimental Therapeutics. 1995;272(1):379–384. [PubMed] [Google Scholar]
- Nakhostine N, Lamontagne D. Adenosine Contributes to Hypoxia-Induced Vasodilation through Atp-Sensitive K+ Channel Activation. American Journal of Physiology. 1993;265(4):H1289–H1293. doi: 10.1152/ajpheart.1993.265.4.H1289. [DOI] [PubMed] [Google Scholar]
- Nakhostine N, Lamontagne D. Contribution of prostaglandins in hypoxia-induced vasodilation in isolated rabbit hearts. Relation to adenosine and KATP channels. Pflugers Arch. 1994;428(5–6):526–532. doi: 10.1007/BF00374574. [DOI] [PubMed] [Google Scholar]
- Nara M, Dhulipala PD, Wang YX, Kotlikoff MI. Reconstitution of beta-adrenergic modulation of large conductance, calcium-activated potassium (maxi-K) channels in Xenopus oocytes. Identification of the camp-dependent protein kinase phosphorylation site. J Biol Chem. 1998;273(24):14920–14924. doi: 10.1074/jbc.273.24.14920. [DOI] [PubMed] [Google Scholar]
- Nelson CP, Rainbow RD, Brignell JL, Perry MD, Willets JM, Davies NW, Challiss RA. Principal role of adenylyl cyclase 6 in K(+) channel regulation and vasodilator signalling in vascular smooth muscle cells. Cardiovasc Res. 2011;91(4):694–702. doi: 10.1093/cvr/cvr137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks [see comments] Science. 1995;270:633–637. doi: 10.1126/science.270.5236.633. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Huang Y, Brayden JE, Hescheler J, Standen NB. Arterial dilations in response to calcitonin gene-related peptide involve activation of K+ channels. Nature. 1990;344(6268):770–773. doi: 10.1038/344770a0. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259(1 Pt 1):C3–18. doi: 10.1152/ajpcell.1990.259.1.C3. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268(4 Pt 1):C799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- Neylon CB. Potassium channels and vascular proliferation. Vascul Pharmacol. 2002;38(1):35–41. doi: 10.1016/s1537-1891(02)00124-6. [DOI] [PubMed] [Google Scholar]
- Nieves-Cintron M, Amberg GC, Nichols CB, Molkentin JD, Santana LF. Activation of NFATc3 down-regulates the beta1 subunit of large conductance, calcium-activated K+ channels in arterial smooth muscle and contributes to hypertension. J Biol Chem. 2007;282(5):3231–3240. doi: 10.1074/jbc.M608822200. [DOI] [PubMed] [Google Scholar]
- Nieves-Cintron M, Nystoriak MA, Prada MP, Johnson K, Fayer W, Dell’Acqua ML, Navedo MF. Selective down-regulation of KV2.1 function contributes to enhanced arterial tone during diabetes. J Biol Chem. 2015;290(12):7918–7929. doi: 10.1074/jbc.M114.622811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nnorom CC, Davis C, Fedinec AL, Howell K, Jaggar JH, Parfenova H, Leffler CW. Contributions of KATP and KCa channels to cerebral arteriolar dilation to hypercapnia in neonatal brain. Physiol Rep. 2014;2(8) doi: 10.14814/phy2.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystoriak MA, Nieves-Cintron M, Nygren PJ, Hinke SA, Nichols CB, Chen CY, Navedo MF. AKAP150 contributes to enhanced vascular tone by facilitating large-conductance Ca2+-activated K+ channel remodeling in hyperglycemia and diabetes mellitus. Circ Res. 2014;114(4):607–615. doi: 10.1161/CIRCRESAHA.114.302168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell AD, Morton MJ, Hunter M. Two-pore domain K+ channels-molecular sensors. Biochim Biophys Acta. 2002;1566(1–2):152–161. doi: 10.1016/s0005-2736(02)00597-7. [DOI] [PubMed] [Google Scholar]
- Ohya Y, Setoguchi M, Fujii K, Nagao T, Abe I, Fujishima M. Impaired action of levcromakalim on ATP-sensitive K+ channels in mesenteric artery cells from spontaneously hypertensive rats. Hypertension. 1996;27(6):1234–1239. doi: 10.1161/01.hyp.27.6.1234. [DOI] [PubMed] [Google Scholar]
- Okada Y, Yanagisawa T, Taira N. BRL 38227 (levcromakalim)-induced hyperpolarization reduces the sensitivity to Ca2+ of contractile elements in canine coronary artery. Naunyn Schmiedebergs Arch Pharmacol. 1993;347:438–444. doi: 10.1007/BF00165396. [DOI] [PubMed] [Google Scholar]
- Ozkor MA, Murrow JR, Rahman AM, Kavtaradze N, Lin J, Manatunga A, Quyyumi AA. Endothelium-derived hyperpolarizing factor determines resting and stimulated forearm vasodilator tone in health and in disease. Circulation. 2011;123(20):2244–2253. doi: 10.1161/CIRCULATIONAHA.110.990317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan RM, Martinez AC, Martinez MP, Hernandez M, Garcia-Sacristan A, Correa C, Benedito S. Endothelial and potassium channel dependent modulation of noradrenergic vasoconstriction in the pig radial artery. Eur J Pharmacol. 2009;616(1–3):166–174. doi: 10.1016/j.ejphar.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Paisansathan C, Xu H, Vetri F, Hernandez M, Pelligrino DA. Interactions between adenosine and K+ channel-related pathways in the coupling of somatosensory activation and pial arteriolar dilation. Am J Physiol Heart Circ Physiol. 2010;299(6):H2009–2017. doi: 10.1152/ajpheart.00702.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallanck L, Ganetzky B. Cloning and characterization of human and mouse homologs of the Drosophila calcium-activated potassium channel gene, slowpoke. Hum Mol Genet. 1994;3(8):1239–1243. doi: 10.1093/hmg/3.8.1239. [DOI] [PubMed] [Google Scholar]
- Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 2004;19:285–292. doi: 10.1152/physiol.00011.2004. [DOI] [PubMed] [Google Scholar]
- Park WS, Han J, Kim N, Ko JH, Kim SJ, Earm YE. Activation of inward rectifier K+ channels by hypoxia in rabbit coronary arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2005;289(6):H2461–2467. doi: 10.1152/ajpheart.00331.2005. [DOI] [PubMed] [Google Scholar]
- Park WS, Han J, Kim N, Youm JB, Joo H, Kim HK, Earm YE. Endothelin-1 inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase C. J Cardiovasc Pharmacol. 2005;46(5):681–689. doi: 10.1097/01.fjc.0000182846.08357.ed. [DOI] [PubMed] [Google Scholar]
- Park WS, Kim N, Youm JB, Warda M, Ko JH, Kim SJ, Han J. Angiotensin II inhibits inward rectifier K+ channels in rabbit coronary arterial smooth muscle cells through protein kinase Calpha. Biochem Biophys Res Commun. 2006;341(3):728–735. doi: 10.1016/j.bbrc.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Paterno R, Heistad DD, Faraci FM. Functional activity of Ca2+-dependent K+ channels is increased in basilar artery during chronic hypertension. American Journal of Physiology-Heart and Circulatory Physiology. 1997;272(3):H1287–H1291. doi: 10.1152/ajpheart.1997.272.3.H1287. [DOI] [PubMed] [Google Scholar]
- Perez GJ, Bonev AD, Nelson MT. Micromolar Ca(2+) from sparks activates Ca(2+)-sensitive K(+) channels in rat cerebral artery smooth muscle. Am J Physiol Cell Physiol. 2001;281(6):C1769–1775. doi: 10.1152/ajpcell.2001.281.6.C1769. [DOI] [PubMed] [Google Scholar]
- Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113(2):229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane F, Garland CJ. Differential effects of acetylcholine, nitric oxide and levcromakalim on smooth muscle membrane potential and tone in the rabbit basilar artery. Br J Pharmacol. 1993;110(2):651–656. doi: 10.1111/j.1476-5381.1993.tb13861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane F, Hurrell A, Jeremy JY, Garland CJ. Evidence that potassium channels make a major contribution to SIN-1-evoked relaxation of rat isolated mesenteric artery. Br J Pharmacol. 1996;119(8):1557–1562. doi: 10.1111/j.1476-5381.1996.tb16072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane F, Sampson LJ, Smith JJ, Garland CJ. Relaxation to authentic nitric oxide and SIN-1 in rat isolated mesenteric arteries: variable role for smooth muscle hyperpolarization. Br J Pharmacol. 2001;133(5):665–672. doi: 10.1038/sj.bjp.0704127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plane F, Wiley KE, Jeremy JY, Cohen RA, Garland CJ. Evidence that different mechanisms underlie smooth muscle relaxation to nitric oxide and nitric oxide donors in the rabbit isolated carotid artery. Br J Pharmacol. 1998;123(7):1351–1358. doi: 10.1038/sj.bjp.0701746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter VA, Bonev AD, Knot HJ, Heppner TJ, Stevenson AS, Kleppisch T, Nelson MT. Frequency modulation of Ca2+ sparks is involved in regulation of arterial diameter by cyclic nucleotides. American Journal of Physiology-Cell Physiology. 1998;274(5):C1346–C1355. doi: 10.1152/ajpcell.1998.274.5.C1346. [DOI] [PubMed] [Google Scholar]
- Povlsen GK, Longden TA, Bonev AD, Hill-Eubanks DC, Nelson MT. Uncoupling of neurovascular communication after transient global cerebral ischemia is caused by impaired parenchymal smooth muscle Kir channel function. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16638350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle JM, Bonev AD, Brayden JE, Nelson MT. Calcitonin gene-related peptide activated ATP-sensitive K+ currents in rabbit arterial smooth muscle via protein kinase A. J Physiol. 1994;475(1):9–13. doi: 10.1113/jphysiol.1994.sp020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol Rev. 1997;77(4):1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- Quinn KV, Cui Y, Giblin JP, Clapp LH, Tinker A. Do anionic phospholipids serve as cofactors or second messengers for the regulation of activity of cloned ATP-sensitive K+ channels? Circ Res. 2003;93(7):646–655. doi: 10.1161/01.RES.0000095247.81449.8E. [DOI] [PubMed] [Google Scholar]
- Quinn KV, Giblin JP, Tinker A. Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ Res. 2004;94(10):1359–1366. doi: 10.1161/01.RES.0000128513.34817.c4. [DOI] [PubMed] [Google Scholar]
- Randall MD. The involvement of ATP-sensitive potassium channels and adenosine in the regulation of coronary flow in the isolated perfused rat heart. Br J Pharmacol. 1995;116(7):3068–3074. doi: 10.1111/j.1476-5381.1995.tb15965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renigunta V, Schlichthorl G, Daut J. Much more than a leak: structure and function of K(2)p-channels. Pflugers Arch. 2015;467(5):867–894. doi: 10.1007/s00424-015-1703-7. [DOI] [PubMed] [Google Scholar]
- Richmond KN, Tune JD, Gorman MW, Feigl EO. Role of K+ATP channels in local metabolic coronary vasodilation. Am J Physiol. 1999;277(6 Pt 2):H2115–2123. doi: 10.1152/ajpheart.1999.277.6.H2115. [DOI] [PubMed] [Google Scholar]
- Richmond KN, Tune JD, Gorman MW, Feigl EO. Role of K(ATP)(+) channels and adenosine in the control of coronary blood flow during exercise. J Appl Physiol (1985) 2000;89(2):529–536. doi: 10.1152/jappl.2000.89.2.529. [DOI] [PubMed] [Google Scholar]
- Roberts OL, Kamishima T, Barrett-Jolley R, Quayle JM, Dart C. Exchange protein activated by cAMP (Epac) induces vascular relaxation by activating Ca2+-sensitive K+ channels in rat mesenteric artery. J Physiol. 2013;591(20):5107–5123. doi: 10.1113/jphysiol.2013.262006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson BE, Schubert R, Hescheler J, Nelson MT. cGMP-dependent protein kinase activates Ca-activated K channels in cerebral artery smooth muscle cells. Am J Physiol. 1993;265(1 Pt 1):C299–303. doi: 10.1152/ajpcell.1993.265.1.C299. [DOI] [PubMed] [Google Scholar]
- Rogers PA, Chilian WM, Bratz IN, Bryan RM, Jr, Dick GM. H2O2 activates redox- and 4-aminopyridine-sensitive Kv channels in coronary vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2007;292(3):H1404–1411. doi: 10.1152/ajpheart.00696.2006. [DOI] [PubMed] [Google Scholar]
- Rusch NJ. BK channels in cardiovascular disease: a complex story of channel dysregulation. Am J Physiol Heart Circ Physiol. 2009;297(5):H1580–1582. doi: 10.1152/ajpheart.00852.2009. [DOI] [PubMed] [Google Scholar]
- Rusch NJ, Delucena RG, Wooldridge TA, England SK, Cowley AW. A Ca2+-Dependent K+ Current Is Enhanced in Arterial Membranes of Hypertensive Rats. Hypertension. 1992;19(4):301–307. doi: 10.1161/01.hyp.19.4.301. [DOI] [PubMed] [Google Scholar]
- Rusch NJ, Liu Y. Potassium channels in hypertension: homeostatic pathways to buffer arterial contraction. J Lab Clin Med. 1997;130(3):245–251. doi: 10.1016/s0022-2143(97)90018-4. [DOI] [PubMed] [Google Scholar]
- Sadoshima J, Akaike N, Kanaide H, Nakamura M. Cyclic AMP modulates Ca-activated K channel in culture smooth muscle cells of rat aortas. Am J Physiol. 1988;255(4 Pt 2):H754–759. doi: 10.1152/ajpheart.1988.255.4.H754. [DOI] [PubMed] [Google Scholar]
- Saito Y, McKay M, Eraslan A, Hester RL. Functional hyperemia in striated muscle is reduced following blockade of ATP-sensitive potassium channels. American Journal of Physiology-Heart and Circulatory Physiology. 1996;270(5):H1649–H1654. doi: 10.1152/ajpheart.1996.270.5.H1649. [DOI] [PubMed] [Google Scholar]
- Samaha FF, Heineman FW, Ince C, Fleming J, Balaban RS. ATP-sensitive potassium channel is essential to maintain basal coronary vascular tone in vivo. Am J Physiol. 1992;262(5 Pt 1):C1220–1227. doi: 10.1152/ajpcell.1992.262.5.C1220. [DOI] [PubMed] [Google Scholar]
- Sampson LJ, Davies LM, Barrett-Jolley R, Standen NB, Dart C. Angiotensin II-activated protein kinase C targets caveolae to inhibit aortic ATP-sensitive potassium channels. Cardiovasc Res. 2007;76(1):61–70. doi: 10.1016/j.cardiores.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Satake N, Shibata M, Shibata S. The inhibitory effects of iberiotoxin and 4-aminopyridine on the relaxation induced by beta 1- and beta 2-adrenoceptor activation in rat aortic rings. Br J Pharmacol. 1996;119(3):505–510. doi: 10.1111/j.1476-5381.1996.tb15700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawmiller DR, Ashtari M, Urueta H, Leschinsky M, Henning RJ. Mechanisms of vasoactive intestinal peptide-elicited coronary vasodilation in the isolated perfused rat heart. Neuropeptides. 2006;40(5):349–355. doi: 10.1016/j.npep.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Schleifenbaum J, Kohn C, Voblova N, Dubrovska G, Zavarirskaya O, Gloe T, Gollasch M. Systemic peripheral artery relaxation by KCNQ channel openers and hydrogen sulfide. J Hypertens. 2010;28(9):1875–1882. doi: 10.1097/HJH.0b013e32833c20d5. [DOI] [PubMed] [Google Scholar]
- Schwab A, Hanley P, Fabian A, Stock C. Potassium channels keep mobile cells on the go. Physiology (Bethesda) 2008;23:212–220. doi: 10.1152/physiol.00003.2008. [DOI] [PubMed] [Google Scholar]
- Scornik FS, Codina J, Birnbaumer L, Toro L. Modulation of coronary smooth muscle KCa channels by Gs alpha independent of phosphorylation by protein kinase A. Am J Physiol. 1993;265(4 Pt 2):H1460–1465. doi: 10.1152/ajpheart.1993.265.4.H1460. [DOI] [PubMed] [Google Scholar]
- Scornik FS, Toro L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am J Physiol. 1992;262(3 Pt 1):C708–713. doi: 10.1152/ajpcell.1992.262.3.C708. [DOI] [PubMed] [Google Scholar]
- Seo EY, Kim HJ, Zhao ZH, Jang JH, Jin CZ, Yoo HY, Kim SJ. Low K(+) current in arterial myocytes with impaired K(+)-vasodilation and its recovery by exercise in hypertensive rats. Pflugers Arch. 2014;466(11):2101–2111. doi: 10.1007/s00424-014-1473-7. [DOI] [PubMed] [Google Scholar]
- Sepulveda FV, Pablo Cid L, Teulon J, Niemeyer MI. Molecular aspects of structure, gating, and physiology of pH-sensitive background K2P and Kir K+-transport channels. Physiol Rev. 2015;95(1):179–217. doi: 10.1152/physrev.00016.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Sanjani M, Zhou X, Asano S, Tilley S, Ledent C, Teng B, Mustafa SJ. Interactions between A(2A) adenosine receptors, hydrogen peroxide, and KATP channels in coronary reactive hyperemia. Am J Physiol Heart Circ Physiol. 2013;304(10):H1294–1301. doi: 10.1152/ajpheart.00637.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Chen X, Wu Z, Shi W, Yang Y, Cui N, Harrison RW. cAMP-dependent protein kinase phosphorylation produces interdomain movement in SUR2B leading to activation of the vascular KATP channel. J Biol Chem. 2008;283(12):7523–7530. doi: 10.1074/jbc.M709941200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Wu Z, Cui N, Shi W, Yang Y, Zhang X, Jiang C. PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1205–1214. doi: 10.1152/ajpregu.00337.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Yokoshiki H, Sperelakis N, Paul RJ. Role of voltage-dependent and Ca(2+)-activated K(+) channels on the regulation of isometric force in porcine coronary artery. J Vasc Res. 2000;37(1):16–25. doi: 10.1159/000025709. doi:25709. [DOI] [PubMed] [Google Scholar]
- Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97(8):781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- Smith PD, Brett SE, Luykenaar KD, Sandow SL, Marrelli SP, Vigmond EJ, Welsh DG. KIR channels function as electrical amplifiers in rat vascular smooth muscle. J Physiol. 2008;586(4):1147–1160. doi: 10.1113/jphysiol.2007.145474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey CG, Faraci FM. Inhibitory effect of 4-aminopyridine on responses of the basilar artery to nitric oxide. Br J Pharmacol. 1999;126(6):1437–1443. doi: 10.1038/sj.bjp.0702439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YK, Park WS, Ko JH, Han J, Kim N, Earm YE. Protein kinase A-dependent activation of inward rectifier potassium channels by adenosine in rabbit coronary smooth muscle cells. Biochem Biophys Res Commun. 2005;337(4):1145–1152. doi: 10.1016/j.bbrc.2005.09.176. [DOI] [PubMed] [Google Scholar]
- Sonkusare SK, Dalsgaard T, Bonev AD, Nelson MT. Inward rectifier potassium (Kir2.1) channels as end-stage boosters of endothelium-dependent vasodilators. J Physiol. 2016 doi: 10.1113/JP271652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spallarossa P, Schiavo M, Rossettin P, Cordone S, Olivotti L, Cordera R, Brunelli C. Sulfonylurea treatment of type 2 diabetic patients does not reduce the vasodilator response to ischemia. Diabetes Care. 2001;24(4):738–742. doi: 10.2337/diacare.24.4.738. [DOI] [PubMed] [Google Scholar]
- Stepp DW, Kroll K, Feigl EO. K+ATP channels and adenosine are not necessary for coronary autoregulation. Am J Physiol. 1997;273(3 Pt 2):H1299–1308. doi: 10.1152/ajpheart.1997.273.3.H1299. [DOI] [PubMed] [Google Scholar]
- Stott JB, Barrese V, Jepps TA, Leighton EV, Greenwood IA. Contribution of Kv7 channels to natriuretic peptide mediated vasodilation in normal and hypertensive rats. Hypertension. 2015;65(3):676–682. doi: 10.1161/HYPERTENSIONAHA.114.04373. [DOI] [PubMed] [Google Scholar]
- Sung DJ, Noh HJ, Kim JG, Park SW, Kim B, Cho H, Bae YM. Serotonin contracts the rat mesenteric artery by inhibiting 4-aminopyridine-sensitive Kv channels via the 5-HT2A receptor and Src tyrosine kinase. Exp Mol Med. 2013;45:e67. doi: 10.1038/emm.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Li RA, Miki T, Uemura H, Sakamoto N, Ohmoto-Sekine Y, Nakaya H. Functional roles of cardiac and vascular ATP-sensitive potassium channels clarified by Kir6.2-knockout mice. Circ Res. 2001;88(6):570–577. doi: 10.1161/01.res.88.6.570. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Yamamura H, Ohya S, Imaizumi Y. Caveolin-1 facilitates the direct coupling between large conductance Ca2+-activated K+ (BKCa) and Cav1.2 Ca2+ channels and their clustering to regulate membrane excitability in vascular myocytes. J Biol Chem. 2013;288(51):36750–36761. doi: 10.1074/jbc.M113.511485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swayze RD, Braun AP. A catalytically inactive mutant of type I cGMP-dependent protein kinase prevents enhancement of large conductance, calcium-sensitive K+ channels by sodium nitroprusside and cGMP. J Biol Chem. 2001;276(23):19729–19737. doi: 10.1074/jbc.M005711200. [DOI] [PubMed] [Google Scholar]
- Taguchi H, Heistad DD, Kitazono T, Faraci FM. ATP-sensitive K+ channels mediate dilatation of cerebral arterioles during hypoxia. Circ Res. 1994;74(5):1005–1008. doi: 10.1161/01.res.74.5.1005. [DOI] [PubMed] [Google Scholar]
- Taguchi H, Heistad DD, Kitazono T, Faraci FM. Dilatation of Cerebral Arterioles in Response to Activation of Adenylate-Cyclase Is Dependent on Activation of Ca2+-Dependent K+ Channels. Circ Res. 1995;76(6):1057–1062. doi: 10.1161/01.res.76.6.1057. [DOI] [PubMed] [Google Scholar]
- Tajada S, Cidad P, Moreno-Dominguez A, Perez-Garcia MT, Lopez-Lopez JR. High blood pressure associates with the remodelling of inward rectifier K+ channels in mice mesenteric vascular smooth muscle cells. J Physiol. 2012;590(23):6075–6091. doi: 10.1113/jphysiol.2012.236190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaba H, Nagao T, Ibayashi S, Kitazono T, Fujii K, Fujishima M. Altered cerebrovascular response to a potassium channel opener in hypertensive rats. Hypertension. 1996;28(1):143–146. doi: 10.1161/01.hyp.28.1.143. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Tang G, Takizawa K, Otsuka K, Eghbali M, Song M, Toro L. Kv channels contribute to nitric oxide- and atrial natriuretic peptide-induced relaxation of a rat conduit artery. J Pharmacol Exp Ther. 2006;317(1):341–354. doi: 10.1124/jpet.105.096115. [DOI] [PubMed] [Google Scholar]
- Taniguchi J, Furukawa KI, Shigekawa M. Maxi K+ channels are stimulated by cyclic guanosine monophosphate-dependent protein kinase in canine coronary artery smooth muscle cells. Pflugers Arch. 1993;423(3–4):167–172. doi: 10.1007/BF00374390. [DOI] [PubMed] [Google Scholar]
- Tano JY, Schleifenbaum J, Gollasch M. Perivascular adipose tissue, potassium channels, and vascular dysfunction. Arterioscler Thromb Vasc Biol. 2014;34(9):1827–1830. doi: 10.1161/ATVBAHA.114.303032. [DOI] [PubMed] [Google Scholar]
- Tharp DL, Bowles DK. The intermediate-conductance Ca2+ -activated K+ channel (KCa3.1) in vascular disease. Cardiovasc Hematol Agents Med Chem. 2009;7(1):1–11. doi: 10.2174/187152509787047649. [DOI] [PubMed] [Google Scholar]
- Tharp DL, Wamhoff BR, Turk JR, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2006;291(5):H2493–2503. doi: 10.1152/ajpheart.01254.2005. [DOI] [PubMed] [Google Scholar]
- Tharp DL, Wamhoff BR, Wulff H, Raman G, Cheong A, Bowles DK. Local delivery of the KCa3.1 blocker, TRAM-34, prevents acute angioplasty-induced coronary smooth muscle phenotypic modulation and limits stenosis. Arterioscler Thromb Vasc Biol. 2008;28(6):1084–1089. doi: 10.1161/ATVBAHA.107.155796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thengchaisri N, Kuo L. Hydrogen peroxide induces endothelium-dependent and -independent coronary arteriolar dilation: role of cyclooxygenase and potassium channels. Am J Physiol Heart Circ Physiol. 2003;285(6):H2255–2263. doi: 10.1152/ajpheart.00487.2003. [DOI] [PubMed] [Google Scholar]
- Tian L, Coghill LS, McClafferty H, MacDonald SH, Antoni FA, Ruth P, Shipston MJ. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 2004;101(32):11897–11902. doi: 10.1073/pnas.0402590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276(11):7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- Tobin AA, Joseph BK, Al-Kindi HN, Albarwani S, Madden JA, Nemetz LT, Rhee SW. Loss of cerebrovascular Shaker-type K(+) channels: a shared vasodilator defect of genetic and renal hypertensive rats. Am J Physiol Heart Circ Physiol. 2009;297(1):H293–303. doi: 10.1152/ajpheart.00991.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama Y, Brian JE, Jr, Todd MM. Cerebral blood flow during hemodilution and hypoxia in rats : role of ATP-sensitive potassium channels. Stroke. 1999;30(9):1942–1947. doi: 10.1161/01.str.30.9.1942. discussion 1947-1948. [DOI] [PubMed] [Google Scholar]
- Toro L, Amador M, Stefani E. ANG II inhibits calcium-activated potassium channels from coronary smooth muscle in lipid bilayers. Am J Physiol. 1990;258(3 Pt 2):H912–915. doi: 10.1152/ajpheart.1990.258.3.H912. [DOI] [PubMed] [Google Scholar]
- Toyama K, Wulff H, Chandy KG, Azam P, Raman G, Saito T, Miura H. The intermediate-conductance calcium-activated potassium channel KCa3.1 contributes to atherogenesis in mice and humans. J Clin Invest. 2008;118(9):3025–3037. doi: 10.1172/JCI30836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoda K, Fujii K, Ibayashi S, Kitazono T, Nagao T, Fujishima M. Role of ATP-sensitive potassium channels in brain stem circulation during hypotension. Am J Physiol. 1997;273(3 Pt 2):H1342–1346. doi: 10.1152/ajpheart.1997.273.3.H1342. [DOI] [PubMed] [Google Scholar]
- Trebak M. STIM/Orai signalling complexes in vascular smooth muscle. J Physiol. 2012;590(17):4201–4208. doi: 10.1113/jphysiol.2012.233353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso Brindeiro CM, Fallet RW, Lane PH, Carmines PK. Potassium channel contributions to afferent arteriolar tone in normal and diabetic rat kidney. Am J Physiol Renal Physiol. 2008;295(1):F171–178. doi: 10.1152/ajprenal.00563.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troncoso Brindeiro CM, Lane PH, Carmines PK. Tempol prevents altered K(+) channel regulation of afferent arteriolar tone in diabetic rat kidney. Hypertension. 2012;59(3):657–664. doi: 10.1161/HYPERTENSIONAHA.111.184218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng-Crank J, Godinot N, Johansen TE, Ahring PK, Strobaek D, Mertz R, Reinhart PH. Cloning, expression, and distribution of a Ca(2+)-activated K+ channel beta-subunit from human brain. Proc Natl Acad Sci U S A. 1996;93(17):9200–9205. doi: 10.1073/pnas.93.17.9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzeng BH, Chen YH, Huang CH, Lin SS, Lee KR, Chen CC. The Ca(v)3.1 T-type calcium channel is required for neointimal formation in response to vascular injury in mice. Cardiovasc Res. 2012;96(3):533–542. doi: 10.1093/cvr/cvs257. [DOI] [PubMed] [Google Scholar]
- Urena J, del Valle-Rodriguez A, Lopez-Barneo J. Metabotropic Ca2+ channel-induced calcium release in vascular smooth muscle. Cell Calcium. 2007;42(4–5):513–520. doi: 10.1016/j.ceca.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Urrego D, Tomczak AP, Zahed F, Stuhmer W, Pardo LA. Potassium channels in cell cycle and cell proliferation. Philos Trans R Soc Lond B Biol Sci. 2014;369(1638):20130094. doi: 10.1098/rstb.2013.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Voorde J, Vanheel B, Leusen I. Endothelium-dependent relaxation and hyperpolarization in aorta from control and renal hypertensive rats. Circ Res. 1992;70(1):1–8. doi: 10.1161/01.res.70.1.1. [DOI] [PubMed] [Google Scholar]
- Vanelli G, Chang HY, Gatensby AG, Hussain SN. Contribution of potassium channels to active hyperemia of the canine diaphragm. J Appl Physiol (1985) 1994;76(3):1098–1105. doi: 10.1152/jappl.1994.76.3.1098. [DOI] [PubMed] [Google Scholar]
- Vanelli G, Hussain SN. Effects of potassium channel blockers on basal vascular tone and reactive hyperemia of canine diaphragm. Am J Physiol. 1994;266(1 Pt 2):H43–51. doi: 10.1152/ajpheart.1994.266.1.H43. [DOI] [PubMed] [Google Scholar]
- Vetri F, Xu H, Paisansathan C, Pelligrino DA. Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BK(Ca) and Kir channels. Am J Physiol Heart Circ Physiol. 2012;302(6):H1274–1284. doi: 10.1152/ajpheart.01067.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigili de Kreutzenberg S, Kiwanuka E, Tiengo A, Avogaro A. Visceral obesity is characterized by impaired nitric oxide-independent vasodilation. Eur Heart J. 2003;24(13):1210–1215. doi: 10.1016/s0195-668x(03)00206-9. [DOI] [PubMed] [Google Scholar]
- von Beckerath N, Cyrys S, Dischner A, Daut J. Hypoxic vasodilatation in isolated, perfused guinea-pig heart: an analysis of the underlying mechanisms. J Physiol. 1991;442:297–319. doi: 10.1113/jphysiol.1991.sp018794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakatsuki T, Nakaya Y, Inoue I. Vasopressin modulates K(+)-channel activities of cultured smooth muscle cells from porcine coronary artery. Am J Physiol. 1992;263(2 Pt 2):H491–496. doi: 10.1152/ajpheart.1992.263.2.H491. [DOI] [PubMed] [Google Scholar]
- Wang R, Wang Z, Wu L. Carbon monoxide-induced vasorelaxation and the underlying mechanisms. Br J Pharmacol. 1997;121(5):927–934. doi: 10.1038/sj.bjp.0701222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem. 1997;272(13):8222–8226. doi: 10.1074/jbc.272.13.8222. [DOI] [PubMed] [Google Scholar]
- Wang R, Wu L, Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 1997;434(3):285–291. doi: 10.1007/s004240050398. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang HT, Su XL, Deng XL, Yuan BX, Zhang W, Yang YB. Experimental diabetes mellitus down-regulates large-conductance Ca2+-activated K+ channels in cerebral artery smooth muscle and alters functional conductance. Curr Neurovasc Res. 2010;7(2):75–84. doi: 10.2174/156720210791184925. [DOI] [PubMed] [Google Scholar]
- Wei EP, Kontos HA. Blockade of ATP-sensitive potassium channels in cerebral arterioles inhibits vasoconstriction from hypocapnic alkalosis in cats. Stroke. 1999;30(4):851–853. doi: 10.1161/01.str.30.4.851. discussion 854. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Bevan JA. Barium inhibits the endothelium-dependent component of flow but not acetylcholine-induced relaxation in isolated rabbit cerebral arteries. J Pharmacol Exp Ther. 1995;274(1):47–53. [PubMed] [Google Scholar]
- Wellman GC, Nathan DJ, Saundry CM, Perez G, Bonev AD, Penar PL, Nelson MT. Ca2+ sparks and their function in human cerebral arteries. Stroke. 2002;33(3):802–808. doi: 10.1161/hs0302.104089. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Nelson MT. Signaling between SR and plasmalemma in smooth muscle: sparks and the activation of Ca2+-sensitive ion channels. Cell Calcium. 2003;34(3):211–229. doi: 10.1016/s0143-4160(03)00124-6. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Quayle JM, Standen NB. ATP-sensitive K+ channel activation by calcitonin gene-related peptide and protein kinase A in pig coronary arterial smooth muscle. J Physiol. 1998;507(Pt 1):117–129. doi: 10.1111/j.1469-7793.1998.117bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman GC, Santana LF, Bonev AD, Nelson MT. Role of phospholamban in the modulation of arterial Ca(2+) sparks and Ca(2+)-activated K(+) channels by cAMP. Am J Physiol Cell Physiol. 2001;281(3):C1029–1037. doi: 10.1152/ajpcell.2001.281.3.C1029. [DOI] [PubMed] [Google Scholar]
- Welsh DG, Jackson WF, Segal SS. Oxygen induces electromechanical coupling in arteriolar smooth muscle cells: a role for L-type Ca2+ channels. Am J Physiol. 1998;274(6 Pt 2):H2018–2024. doi: 10.1152/ajpheart.1998.274.6.H2018. [DOI] [PubMed] [Google Scholar]
- Wesselman JP, Schubert R, VanBavel ED, Nilsson H, Mulvany MJ. KCa-channel blockade prevents sustained pressure-induced depolarization in rat mesenteric small arteries. Am J Physiol. 1997;272(5 Pt 2):H2241–2249. doi: 10.1152/ajpheart.1997.272.5.H2241. [DOI] [PubMed] [Google Scholar]
- Westcott EB, Goodwin EL, Segal SS, Jackson WF. Function and expression of ryanodine receptors and inositol 1,4,5-trisphosphate receptors in smooth muscle cells of murine feed arteries and arterioles. J Physiol. 2012;590(8):1849–1869. doi: 10.1113/jphysiol.2011.222083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott EB, Jackson WF. Heterogeneous function of ryanodine receptors, but not IP3 receptors, in hamster cremaster muscle feed arteries and arterioles. Am J Physiol Heart Circ Physiol. 2011;300(5):H1616–1630. doi: 10.1152/ajpheart.00728.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Jr, Katz GM, Roy-Contancin L, Reuben JP. Guanosine 5′-monophosphate modulates gating of high-conductance Ca2+-activated K+ channels in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1988;85(23):9360–9364. doi: 10.1073/pnas.85.23.9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AJ, Jabr RI, Clapp LH. Calcium modulation of vascular smooth muscle ATP-sensitive K(+) channels: role of protein phosphatase-2B. Circ Res. 2000;87(11):1019–1025. doi: 10.1161/01.res.87.11.1019. [DOI] [PubMed] [Google Scholar]
- Winquist RJ, Faison EP, Napier M, Vandlen R, Waldman SA, Murad F. The Effects of Atrial Natriuretic Factor on Vascular Smooth Muscle. In: Bevan JA, Godfraind T, Maxwell RA, Stoclet JC, Worcel M, editors. Vascular Neuroeffector Mecahnisms. Amsetrdam: Elsevier; 1985. pp. 349–353. [Google Scholar]
- Winquist RJ, Faison EP, Nutt RF. Vasodilator Profile of Synthetic Atrial Natriuretic Factor. European Journal of Pharmacology. 1984;102(1):169–173. doi: 10.1016/0014-2999(84)90353-4. [DOI] [PubMed] [Google Scholar]
- Wischmeyer E, Doring F, Karschin A. Acute suppression of inwardly rectifying Kir2.1 channels by direct tyrosine kinase phosphorylation. J Biol Chem. 1998;273(51):34063–34068. doi: 10.1074/jbc.273.51.34063. [DOI] [PubMed] [Google Scholar]
- Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol. 1996;154(2):91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]
- Wu BN, Luykenaar KD, Brayden JE, Giles WR, Corteling RL, Wiehler WB, Welsh DG. Hyposmotic challenge inhibits inward rectifying K+ channels in cerebral arterial smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;292(2):H1085–1094. doi: 10.1152/ajpheart.00926.2006. [DOI] [PubMed] [Google Scholar]
- Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of alpha-subunits. Am J Physiol Heart Circ Physiol. 2004;286(2):H610–618. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- Xi Q, Umstot E, Zhao G, Narayanan D, Leffler CW, Jaggar JH. Glutamate regulates Ca2+ signals in smooth muscle cells of newborn piglet brain slice arterioles through astrocyte- and heme oxygenase-dependent mechanisms. Am J Physiol Heart Circ Physiol. 2010;298(2):H562–569. doi: 10.1152/ajpheart.00823.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Isomoto S, Matsumoto S, Kondo C, Shindo T, Horio Y, Kurachi Y. Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J Physiol. 1997;499(Pt 3):715–720. doi: 10.1113/jphysiol.1997.sp021963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T, Yanagisawa T, Taira N. K+ channel openers, cromakalim and Ki4032, inhibit agonist-induced Ca2+ release in canine coronary artery. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:691–700. doi: 10.1007/BF00168744. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Kajita J, Madison JM. Isoproterenol increases peripheral [Ca2+]i and decreases inner [Ca2+]i in single airway smooth muscle cells. Am J Physiol. 1995;268(3 Pt 1):C771–779. doi: 10.1152/ajpcell.1995.268.3.C771. [DOI] [PubMed] [Google Scholar]
- Yamamura H, Ohya S, Muraki K, Imaizumi Y. Involvement of inositol 1,4,5-trisphosphate formation in the voltage-dependent regulation of the Ca(2+) concentration in porcine coronary arterial smooth muscle cells. J Pharmacol Exp Ther. 2012;342(2):486–496. doi: 10.1124/jpet.112.194233. [DOI] [PubMed] [Google Scholar]
- Yanagisawa T, Yamagishi T, Okada Y. Hyperpolarization induced by K+ channel openers inhibits Ca2+ influx and Ca2+ release in coronary artery. Cardiovasc Drugs Ther. 1993;7(Suppl 3):565–574. doi: 10.1007/BF00877622. [DOI] [PubMed] [Google Scholar]
- Yang Y, Jones AW, Thomas TR, Rubin LJ. Influence of sex, high-fat diet, and exercise training on potassium currents of swine coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293(3):H1553–1563. doi: 10.1152/ajpheart.00151.2007. [DOI] [PubMed] [Google Scholar]
- Yang Y, Li PY, Cheng J, Mao L, Wen J, Tan XQ, Zeng XR. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension. 2013;61(2):519–525. doi: 10.1161/HYPERTENSIONAHA.111.00211. [DOI] [PubMed] [Google Scholar]
- Yang Y, Murphy TV, Ella SR, Grayson TH, Haddock R, Hwang YT, Hill MA. Heterogeneity in function of small artery smooth muscle BKCa: involvement of the beta1-subunit. J Physiol. 2009;587(Pt 12):3025–3044. doi: 10.1113/jphysiol.2009.169920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shi Y, Guo S, Zhang S, Cui N, Shi W, Jiang C. PKA-dependent activation of the vascular smooth muscle isoform of KATP channels by vasoactive intestinal polypeptide and its effect on relaxation of the mesenteric resistance artery. Biochim Biophys Acta. 2008;1778(1):88–96. doi: 10.1016/j.bbamem.2007.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sohma Y, Nourian Z, Ella SR, Li M, Stupica A, Hill MA. Mechanisms underlying regional differences in the Ca2+ sensitivity of BK(Ca) current in arteriolar smooth muscle. J Physiol. 2013;591(5):1277–1293. doi: 10.1113/jphysiol.2012.241562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi F, Wang H, Chai Q, Wang X, Shen WK, Willis MS, Lu T. Regulation of large conductance Ca2+-activated K+ (BK) channel beta1 subunit expression by muscle RING finger protein 1 in diabetic vessels. J Biol Chem. 2014;289(15):10853–10864. doi: 10.1074/jbc.M113.520940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Sun CW, Maier KG, Harder DR, Roman RJ. Mechanism of cGMP contribution to the vasodilator response to NO in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol. 2002;282(5):H1724–1731. doi: 10.1152/ajpheart.00699.2001. [DOI] [PubMed] [Google Scholar]
- Yuill KH, McNeish AJ, Kansui Y, Garland CJ, Dora KA. Nitric oxide suppresses cerebral vasomotion by sGC-independent effects on ryanodine receptors and voltage-gated calcium channels. J Vasc Res. 2010;47(2):93–107. doi: 10.1159/000235964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavaritskaya O, Zhuravleva N, Schleifenbaum J, Gloe T, Devermann L, Kluge R, Schubert R. Role of KCNQ channels in skeletal muscle arteries and periadventitial vascular dysfunction. Hypertension. 2013;61(1):151–159. doi: 10.1161/HYPERTENSIONAHA.112.197566. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gao YJ, Zuo J, Lee RM, Janssen LJ. Alteration of arterial smooth muscle potassium channel composition and BKCa current modulation in hypertension. Eur J Pharmacol. 2005;514(2–3):111–119. doi: 10.1016/j.ejphar.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tazzeo T, Chu V, Janssen LJ. Membrane potassium currents in human radial artery and their regulation by nitric oxide donor. Cardiovasc Res. 2006;71(2):383–392. doi: 10.1016/j.cardiores.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20(21):6008–6016. doi: 10.1093/emboj/20.21.6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XZ, Harhun MI, Olesen SP, Ohya S, Moffatt JD, Cole WC, Greenwood IA. Participation of KCNQ (Kv7) potassium channels in myogenic control of cerebral arterial diameter. J Physiol. 2010;588(Pt 17):3277–3293. doi: 10.1113/jphysiol.2010.192823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Wang XL, Lamping KG, Lee HC. Inhibition of protein kinase Cbeta protects against diabetes-induced impairment in arachidonic acid dilation of small coronary arteries. J Pharmacol Exp Ther. 2006;319(1):199–207. doi: 10.1124/jpet.106.106666. [DOI] [PubMed] [Google Scholar]
- Zhou X, Teng B, Tilley S, Ledent C, Mustafa SJ. Metabolic hyperemia requires ATP-sensitive K+ channels and H2O2 but not adenosine in isolated mouse hearts. Am J Physiol Heart Circ Physiol. 2014;307(7):H1046–1055. doi: 10.1152/ajpheart.00421.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XB, Feng YX, Sun Q, Lukowski R, Qiu Y, Spiger K, Wieland T. Nucleoside diphosphate kinase B-activated intermediate conductance potassium channels are critical for neointima formation in mouse carotid arteries. Arterioscler Thromb Vasc Biol. 2015;35(8):1852–1861. doi: 10.1161/ATVBAHA.115.305881. [DOI] [PubMed] [Google Scholar]
- Zhu P, Beny JL, Flammer J, Luscher TF, Haefliger IO. Relaxation by bradykinin in porcine ciliary artery. Role of nitric oxide and K(+)-channels. Invest Ophthalmol Vis Sci. 1997;38(9):1761–1767. [PubMed] [Google Scholar]
- Zitron E, Gunth M, Scherer D, Kiesecker C, Kulzer M, Bloehs R, Karle CA. Kir2.x inward rectifier potassium channels are differentially regulated by adrenergic alpha1A receptors. J Mol Cell Cardiol. 2008;44(1):84–94. doi: 10.1016/j.yjmcc.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Zitron E, Kiesecker C, Luck S, Kathofer S, Thomas D, Kreye VA, Karle CA. Human cardiac inwardly rectifying current IKir2.2 is upregulated by activation of protein kinase A. Cardiovasc Res. 2004;63(3):520–527. doi: 10.1016/j.cardiores.2004.02.015. [DOI] [PubMed] [Google Scholar]


