Abstract
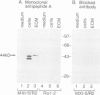
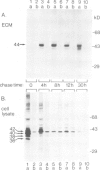
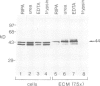
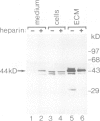
The proto-oncogene int-1 plays an important role in mammary tumorigenesis when activated by proviral insertions of the mouse mammary tumor virus. In normal mouse tissues the gene is expressed in the embryonic neural tube, suggesting a developmental function, while in Drosophila the homolog of int-1 is the segment polarity gene wingless. In order to study the protein products of int-1 we have derived fibroblast cell lines infected with multiple copies of a retroviral vector expressing int-1 cDNA. By Western blot analysis and immunoprecipitation we have identified a 44 kd form of int-1 protein which is secreted from these cells. The 44 kd species is distinct from the major intracellular forms of int-1 protein as judged by its slower mobility in SDS-polyacrylamide gels and by its longer half-life in pulse-chase experiments. Under normal growth conditions, little or none of the 44 kd protein is detectable in the cell culture medium but instead the majority is found associated with the extracellular matrix (ECM). The protein appears to bind heparin in vitro, suggesting that it might bind glycosaminoglycans in the ECM. These data support the view that int-1 protein may play a role in cell-cell communication over short distances.
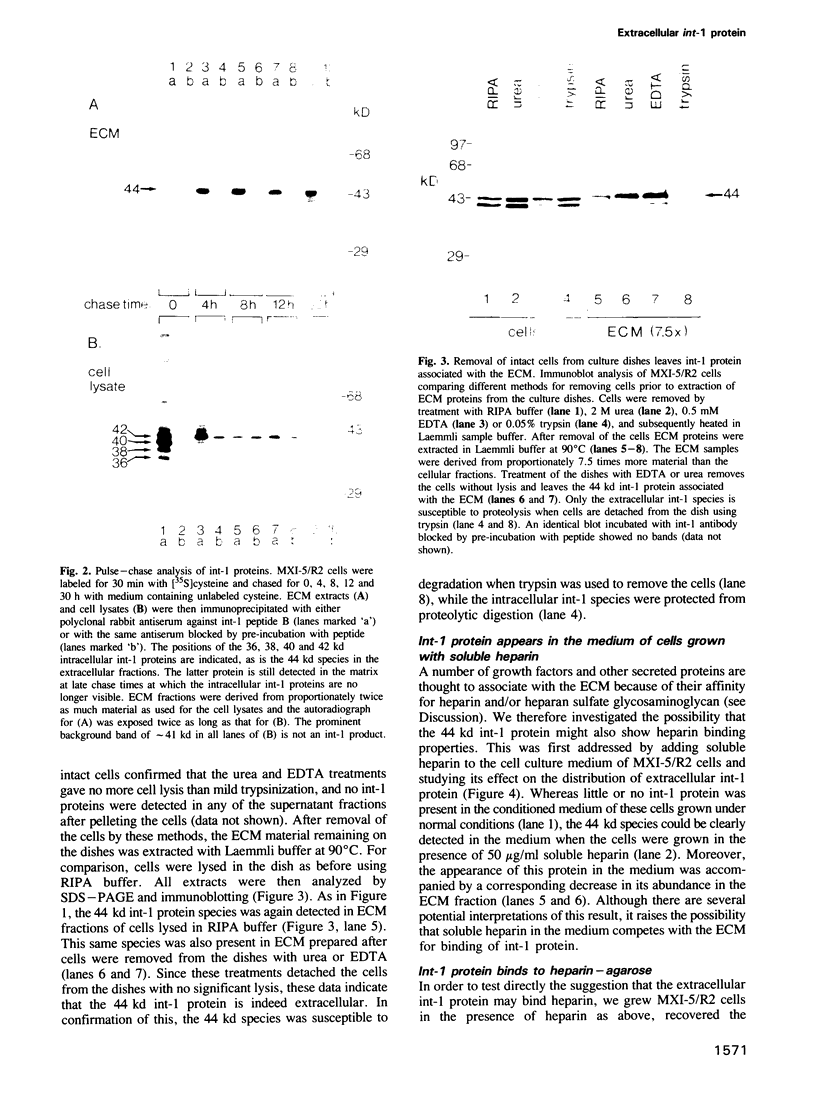
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird A., Ling N. Fibroblast growth factors are present in the extracellular matrix produced by endothelial cells in vitro: implications for a role of heparinase-like enzymes in the neovascular response. Biochem Biophys Res Commun. 1987 Jan 30;142(2):428–435. doi: 10.1016/0006-291x(87)90292-0. [DOI] [PubMed] [Google Scholar]
- Baker N. E. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987 Jun;6(6):1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole D. G., Hendershot L. M., Kearney J. F. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. J Cell Biol. 1986 May;102(5):1558–1566. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Papkoff J., Fung Y. K., Shackleford G. M., Varmus H. E. Identification of protein products encoded by the proto-oncogene int-1. Mol Cell Biol. 1987 Nov;7(11):3971–3977. doi: 10.1128/mcb.7.11.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Wildin R. S., Prendergast T. J., Varmus H. E. A retrovirus vector expressing the putative mammary oncogene int-1 causes partial transformation of a mammary epithelial cell line. Cell. 1986 Sep 26;46(7):1001–1009. doi: 10.1016/0092-8674(86)90699-9. [DOI] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Delli-Bovi P., Curatola A. M., Newman K. M., Sato Y., Moscatelli D., Hewick R. M., Rifkin D. B., Basilico C. Processing, secretion, and biological properties of a novel growth factor of the fibroblast growth factor family with oncogenic potential. Mol Cell Biol. 1988 Jul;8(7):2933–2941. doi: 10.1128/mcb.8.7.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Dixon M., Deed R., Acland P., Moore R., Whyte A., Peters G., Dickson C. Detection and characterization of the fibroblast growth factor-related oncoprotein INT-2. Mol Cell Biol. 1989 Nov;9(11):4896–4902. doi: 10.1128/mcb.9.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung Y. K., Shackleford G. M., Brown A. M., Sanders G. S., Varmus H. E. Nucleotide sequence and expression in vitro of cDNA derived from mRNA of int-1, a provirally activated mouse mammary oncogene. Mol Cell Biol. 1985 Dec;5(12):3337–3344. doi: 10.1128/mcb.5.12.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething M. J., McCammon K., Sambrook J. Expression of wild-type and mutant forms of influenza hemagglutinin: the role of folding in intracellular transport. Cell. 1986 Sep 12;46(6):939–950. doi: 10.1016/0092-8674(86)90076-0. [DOI] [PubMed] [Google Scholar]
- Hök M., Kjellén L., Johansson S. Cell-surface glycosaminoglycans. Annu Rev Biochem. 1984;53:847–869. doi: 10.1146/annurev.bi.53.070184.004215. [DOI] [PubMed] [Google Scholar]
- Jakobovits A., Shackleford G. M., Varmus H. E., Martin G. R. Two proto-oncogenes implicated in mammary carcinogenesis, int-1 and int-2, are independently regulated during mouse development. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7806–7810. doi: 10.1073/pnas.83.20.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassenbrock C. K., Garcia P. D., Walter P., Kelly R. B. Heavy-chain binding protein recognizes aberrant polypeptides translocated in vitro. Nature. 1988 May 5;333(6168):90–93. doi: 10.1038/333090a0. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Lyons R. M., Moses H. L. Immunodetection and modulation of cellular growth with antibodies against native transforming growth factor-beta 1. Cancer Res. 1987 Dec 15;47(24 Pt 1):6451–6458. [PubMed] [Google Scholar]
- Maciag T., Mehlman T., Friesel R., Schreiber A. B. Heparin binds endothelial cell growth factor, the principal endothelial cell mitogen in bovine brain. Science. 1984 Aug 31;225(4665):932–935. doi: 10.1126/science.6382607. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Mester J., Wagenaar E., Sluyser M., Nusse R. Activation of int-1 and int-2 mammary oncogenes in hormone-dependent and -independent mammary tumors of GR mice. J Virol. 1987 Apr;61(4):1073–1078. doi: 10.1128/jvi.61.4.1073-1078.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. The int genes in mammary tumorigenesis and in normal development. Trends Genet. 1988 Oct;4(10):291–295. doi: 10.1016/0168-9525(88)90172-2. [DOI] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusse R., van Ooyen A., Cox D., Fung Y. K., Varmus H. Mode of proviral activation of a putative mammary oncogene (int-1) on mouse chromosome 15. Nature. 1984 Jan 12;307(5947):131–136. doi: 10.1038/307131a0. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Brown A. M., Varmus H. E. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol Cell Biol. 1987 Nov;7(11):3978–3984. doi: 10.1128/mcb.7.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J. Inducible overexpression and secretion of int-1 protein. Mol Cell Biol. 1989 Aug;9(8):3377–3384. doi: 10.1128/mcb.9.8.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. H., Schafer B., Goodman C. S., Holmgren R. The role of segment polarity genes during Drosophila neurogenesis. Genes Dev. 1989 Jun;3(6):890–904. doi: 10.1101/gad.3.6.890. [DOI] [PubMed] [Google Scholar]
- Peters G., Lee A. E., Dickson C. Concerted activation of two potential proto-oncogenes in carcinomas induced by mouse mammary tumour virus. Nature. 1986 Apr 17;320(6063):628–631. doi: 10.1038/320628a0. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987 Aug 14;50(4):649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F., van Deemter L., Wagenaar E., Sonnenberg A., Nusse R. Transfection of the int-1 mammary oncogene in cuboidal RAC mammary cell line results in morphological transformation and tumorigenicity. EMBO J. 1987 Jan;6(1):127–131. doi: 10.1002/j.1460-2075.1987.tb04729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. S., Osada H., Finch P. W., Taylor W. G., Rudikoff S., Aaronson S. A. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci U S A. 1989 Feb;86(3):802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Structure and biology of proteoglycans. Annu Rev Cell Biol. 1988;4:229–255. doi: 10.1146/annurev.cb.04.110188.001305. [DOI] [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Expression of the proto-oncogene int-1 is restricted to postmeiotic male germ cells and the neural tube of mid-gestational embryos. Cell. 1987 Jul 3;50(1):89–95. doi: 10.1016/0092-8674(87)90665-9. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Tsukamoto A. S., Grosschedl R., Guzman R. C., Parslow T., Varmus H. E. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell. 1988 Nov 18;55(4):619–625. doi: 10.1016/0092-8674(88)90220-6. [DOI] [PubMed] [Google Scholar]
- Uzvölgyi E., Kiss I., Pitt A., Arsenian S., Ingvarsson S., Udvardy A., Hamada M., Klein G., Sümegi J. Drosophila homolog of the murine Int-1 protooncogene. Proc Natl Acad Sci U S A. 1988 May;85(9):3034–3038. doi: 10.1073/pnas.85.9.3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlodavsky I., Folkman J., Sullivan R., Fridman R., Ishai-Michaeli R., Sasse J., Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2292–2296. doi: 10.1073/pnas.84.8.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- Zhan X., Bates B., Hu X. G., Goldfarb M. The human FGF-5 oncogene encodes a novel protein related to fibroblast growth factors. Mol Cell Biol. 1988 Aug;8(8):3487–3495. doi: 10.1128/mcb.8.8.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A., Nusse R. Structure and nucleotide sequence of the putative mammary oncogene int-1; proviral insertions leave the protein-encoding domain intact. Cell. 1984 Nov;39(1):233–240. doi: 10.1016/0092-8674(84)90209-5. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M., Nusse R., Johnston P., Lawrence P. A. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell. 1989 Nov 17;59(4):739–749. doi: 10.1016/0092-8674(89)90020-2. [DOI] [PubMed] [Google Scholar]