Abstract
BACKGROUND. In health, inflammation resolution is an active process governed by specialized proresolving mediators and receptors. ALX/FPR2 receptors (ALX) are targeted by both proresolving and proinflammatory ligands for opposing signaling events, suggesting pivotal roles for ALX in the fate of inflammatory responses. Here, we determined if ALX expression and ligands were linked to severe asthma (SA).
METHODS. ALX expression and levels of proresolving ligands (lipoxin A4 [LXA4], 15-epi-LXA4, and annexin A1 [ANXA1]), and a proinflammatory ligand (serum amyloid A [SAA]) were measured in bronchoscopy samples collected in Severe Asthma Research Program-3 (SA [n = 69], non-SA [NSA, n = 51] or healthy donors [HDs, n = 47]).
RESULTS. Bronchoalveolar lavage (BAL) fluid LXA4 and 15-epi-LXA4 were decreased and SAA was increased in SA relative to NSA. BAL macrophage ALX expression was increased in SA. Subjects with LXA4loSAAhi levels had increased BAL neutrophils, more asthma symptoms, lower lung function, increased relative risk for asthma exacerbation, sinusitis, and gastroesophageal reflux disease, and were assigned more frequently to SA clinical clusters. SAA and aliquots of LXA4loSAAhi BAL fluid induced IL-8 production by lung epithelial cells expressing ALX receptors, which was inhibited by coincubation with 15-epi-LXA4.
CONCLUSIONS. Together, these findings have established an association between select ALX receptor ligands and asthma severity that define a potentially new biochemical endotype for asthma and support a pivotal functional role for ALX signaling in the fate of lung inflammation.
TRIAL REGISTRATION. Severe Asthma Research Program-3 (SARP-3; ClinicalTrials.gov NCT01606826)
FUNDING Sources. National Heart, Lung and Blood Institute, the NIH, and the German Society of Pediatric Pneumology.
Keywords: Inflammation, Pulmonology
Levels of the ALX receptor ligands LXA4 and SAA serve as a new asthma biochemical endotype for non-Type 2, steroid resistant inflammation in severe asthma.
Introduction
Asthma is the most common disease of chronic lung inflammation, affecting nearly 1 in 13 Americans (1). The current clinical criteria for the diagnosis of asthma include a broad spectrum of patients with heterogeneous disease processes and distinct responses to medications (2). Approximately 10%–15% of asthmatic patients have severe asthma (SA) with daily symptoms and inadequate asthma control despite asthma-targeted controller medication use. These patients with SA have increased morbidity with significant adverse outcomes, including frequent outpatient visits, admissions to the hospital, and even life-threatening exacerbations (3). Cluster analyses utilizing patient clinical characteristics have identified at least 5 distinct clusters of asthmatic individuals (4, 5). Identifying disease mechanisms in asthma pathogenesis is critical to move the field from clinical phenotyping to molecular endotyping of patients to enable precision medicine approaches for improved asthma management (6). While type 2 “high” inflammation accounts for approximately 50% of asthma pathobiology (7), disease mechanisms for the remaining 50% of asthmatic subjects remain to be determined. A more detailed understanding of mechanisms underlying non–type 2 inflammation in asthma is needed.
In health, the resolution of inflammation is an active process governed by specific cellular events regulated by specialized proresolving mediators (SPMs) derived from essential fatty acids (8). Lipoxin A4 (LXA4) and 15-epi-LXA4 are endogenous arachidonic acid–derived SPMs that potently regulate acute inflammation, yet are underproduced in many inflammatory diseases, including SA (9). Lipoxins and their stable analogs are protective in murine models of allergic lung inflammation, and display cell type–specific actions for human leukocytes to inhibit proinflammatory IL-13 production by group 2 innate lymphoid cells, halt granulocyte trafficking and activation, decrease T cell cytokine production, enhance natural killer cell functions, and stimulate macrophage CD206 expression and efferocytosis to resolve tissue inflammation (reviewed in ref. 9). Lipoxins also inhibit leukotriene-mediated prophlogistic actions, including in vivo in asthma (10), and decrease cytokine-induced human airway contractile responses (11). In peripheral blood, exhaled breath condensates, sputum, and bronchoalveolar lavage (BAL) fluid (BALF), LXA4 levels are decreased in SA relative to non-SA (NSA) (12–15), suggesting a link between defective resolution mechanisms and persistent airway inflammation in some asthma patients.
LXA4 and 15-epi-LXA4 interact with specific receptors to exert their proresolving actions. Their high-affinity cognate receptors are ALX/FPR2 receptors (ALX) with a KD of approximately 1 nM (16). Of interest, ALX was the first receptor described to engage both lipid and peptide ligands (16), and subsequently several lipid and peptide ligands for ALX have been identified. Ligand recognition sites differ in the extracellular domains of ALX receptors and trigger distinct downstream events that dramatically change the signaling properties of the receptor depending on the engaging ligand (17). In sharp contrast to LXA4’s counter-regulatory signaling, SAA engages the same ALX receptors to promote inflammation (18, 19). SAA is generated as an acute-phase protein in COPD exacerbations in amounts that are 2–3 log orders higher than LXA4 that overwhelms SPM signaling via ALX (18). Another ALX ligand of potential interest in SA is annexin A1 (ANXA1), a corticosteroid-inducible protein that can interact with ALX receptors to transduce proresolving actions similar to LXA4 (20). Of interest, when apparently healthy individuals are challenged with a skin irritant, they segregate into fast and slow resolvers of the dermal wound based on lipoxin production and expression of ALX receptors (21). Thus, relative levels of these lipid and peptide ALX ligands could serve as a rheostat for inflammatory host responses in airway disease, as lipoxins can allosterically inhibit SAA interactions with ALX (18). Together, the relative abundance and actions of these proinflammatory versus proresolving ALX ligands may biochemically regulate airway phlogistic tone and contribute to unresolved inflammation in SA.
Here, we analyzed BALF samples collected from subjects participating in the National Heart, Lung and Blood Institute’s Severe Asthma Research Program-3 (SARP-3) and have identified a potentially new asthma biochemical endotype related to levels of the ALX receptor ligands LXA4 and SAA that was associated with neutrophilic inflammation, increased asthma symptoms, and decreased lung function in SA.
Results
Subject characteristics.
Subjects with SA and NSA, and nonasthmatic healthy donors (HD) were recruited to participate in SARP-3 at 7 research centers across the United States. Relative to NSA, subjects with SA had increased symptoms as manifested by lower Asthma Control Test (ACT) and higher Asthma Control Questionnaire (ACQ) scores. Spirometric measures of lung function were lower in SA than NSA and HD despite the SA cohort’s use of more asthma-targeted medications (Table 1). A subset of subjects agreed to bronchoscopy with BAL as part of their baseline phenotyping. SA subjects had more lung inflammation with increased BAL neutrophils (Table 1).
Table 1. Clinical characteristics and bronchoalveolar lavage leukocytes for subjects undergoing bronchoscopyA.
SA subjects have decreased lipoxins and increased SAA and macrophage ALX receptor expression.
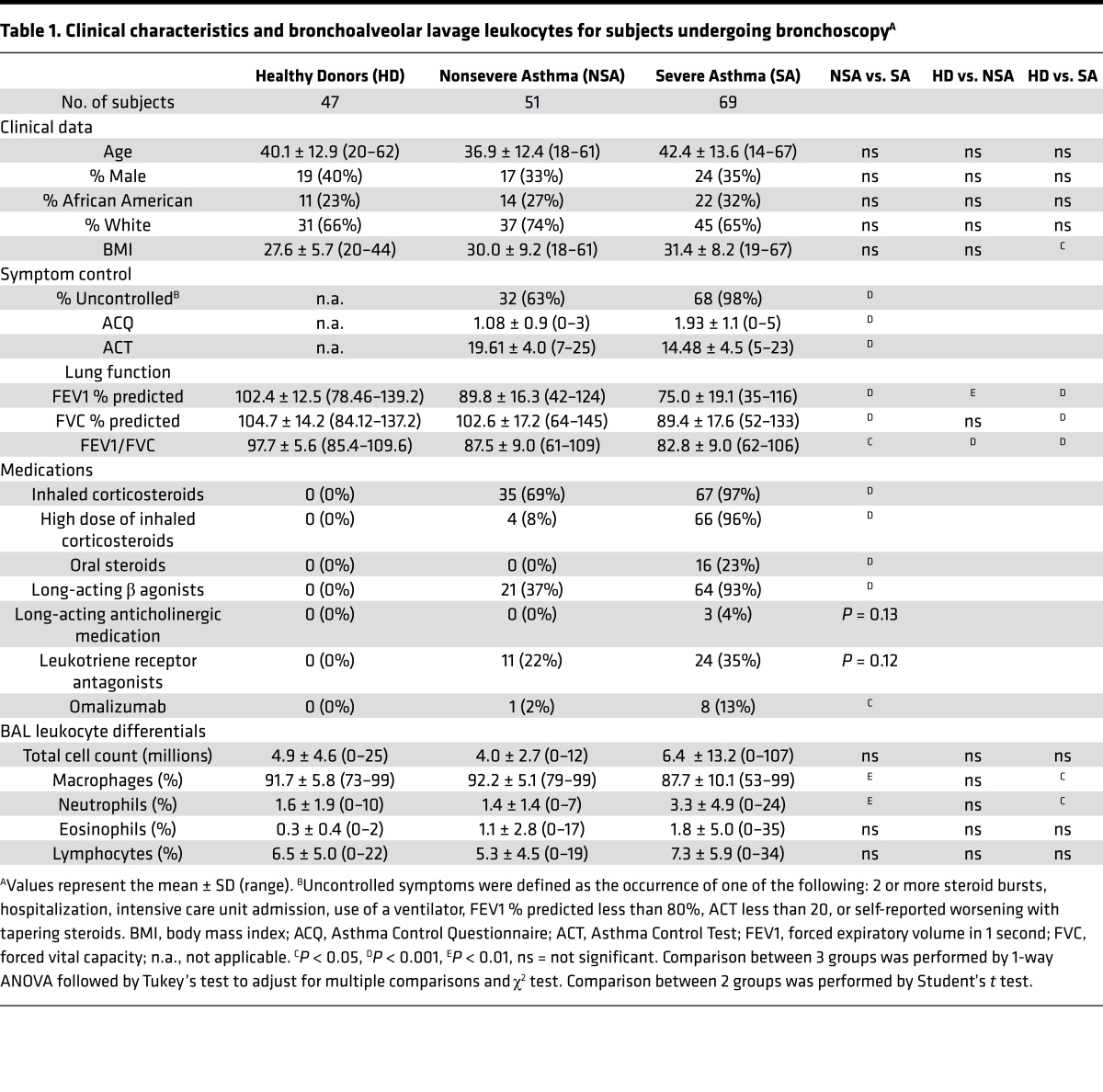
The fate of innate inflammatory responses is dictated in part by ALX receptor signaling (16, 21), so the presence of BALF ALX ligands with proinflammatory (i.e., SAA) or proresolving properties (i.e., LXA4, 15-epi-LXA4, and ANXA1) and BAL cell surface ALX receptor expression were determined. SA subjects had significantly less BALF LXA4 (median 0.23 pg/μg protein, mean 0.28 pg/μg protein) and 15-epi-LXA4 (median 1.02 pg/μg protein, mean 1.24 pg/μg protein) than NSA subjects (LXA4: median 0.32 pg/μg protein, mean 0.40 pg/μg protein; 15-epi-LXA4: median 1.47 pg/μg protein, mean 1.88 pg/μg protein) (Figure 1, A and B). BALF lipoxins were significantly increased in NSA relative to HD, without significant differences between SA and HD cohorts (Figure 1, A and B), consistent with the findings in an earlier SARP cohort (14). No significant differences in immunoreactive ANXA1 levels were identified between the cohorts (Figure 1C). In contrast, SAA levels were increased in SA (median 3.03 pg/μg protein, mean 11.35 pg/μg protein) relative to NSA (median 0 pg/μg protein, mean 11.21 pg/μg protein) (Figure 1D). Of note, BALF SAA levels were below the limit of detection in 51 of the 120 asthma subjects and these samples were arbitrarily assigned a value of 0 pg/μg protein for analysis. Differences in BALF ALX ligand levels were also present when BALF was not corrected for protein (Supplemental Figure 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.93534DS1). BAL macrophage surface ALX receptor expression was determined by flow cytometry, with data expressed as a normalized index for ALX (median fluorescence intensity [MFI] of ALX divided by MFI of isotype control) (see Methods). A stepwise increase in the BAL macrophage ALX index from HD to NSA to SA was detected (Figure 1E).
Figure 1. Relative abundance of BALF ALX ligands and ALX receptor expression differs in asthma.
BALF was obtained from subjects with asthma (n = 120) and healthy donors (n = 47, gray circles). Asthmatic subjects were assigned to NSA (n = 51, blue squares) and SA (n = 69, red triangles) cohorts by SARP criteria. (A) LXA4 and (B) 15-epi-LXA4 were extracted from BALF and quantified by ELISA (see Methods). (C) ANXA1 and (D) SAA levels were determined by ELISA. Scatter plots show individual data points for each subject normalized to protein levels with the median value noted by the horizontal line. (E) Flow cytometry was performed on viable BAL macrophages in n = 32 HD, n = 33 NSA, and n = 38 SA subjects to measure surface ALX expression. Data are expressed as the ALX index (MFI ALX divided by MFI isotype control). *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001 by Kruskal-Wallis test, followed by Dunn’s test for multiple comparisons. BALF, bronchoalveolar lavage fluid; ALX, airway lipoxin A4 receptor; HD, healthy donors; NSA, nonsevere asthma; SA, severe asthma; SARP, Severe Asthma Research Program; LXA4, lipoxin A4; 15-epi-LXA4, 15-epimer lipoxin A4; ELISA, enzyme-linked immunosorbent assay; ANXA1, annexin A1; SAA, serum amyloid A; MFI, median fluorescence intensity.
ALX ligands differentially correlate with asthma inflammation, symptoms, and lung function.
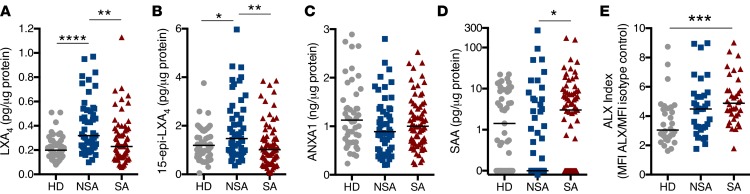
To screen for relationships between the BALF ALX ligand levels and asthma clinical parameters, a Pearson correlation matrix was constructed using measures of disease activity from all asthma subjects (NSA and SA; n = 120) (Figure 2). LXA4 levels correlated positively with 15-epi-LXA4 and inversely with SAA. The ALX ligands were also compared to clinical parameters of lung inflammation, asthma symptoms, and measures of lung function. Notably, the percentage of BALF neutrophils was inversely correlated with LXA4 and 15-epi-LXA4 and positively correlated with SAA. As expected, the ACQ and ACT scores were inversely correlated and significantly related to lung function. BALF levels of LXA4 were inversely correlated with asthma symptoms (i.e., high ACQ, low ACT scores). LXA4 and 15-epi-LXA4 were also positively associated with lung function. SAA was not significantly related to asthma symptoms or lung function parameters. There were no significant correlations between BALF ALX ligand levels and fractional exhaled nitric oxide (FeNO), methacholine PC 20 (provocation challenge causing a 20% fall in forced expiratory volume in 1 second [FEV1]), or lung function reversibility with albuterol. Because of limited sample size, this analysis was not corrected for multiple comparison testing, yet it strongly suggested relationships between select ALX ligands and major characteristics of clinical asthma, namely lung inflammation, asthma symptoms, and lung function.
Figure 2. Relationship between ALX ligands, lung inflammation, asthma symptoms, and lung function in asthma.
The relationships between BALF ALX ligand levels, BAL leukocytes, asthma symptom score, and measures of lung function, were determined by Pearson correlation matrix (see Methods) for n = 51 NSA and n = 69 SA subjects. Positive correlations are noted in blue and negative correlations in red. The color intensity is proportional to Pearson’s correlation coefficient, with deeper colors denoting stronger associations. *P < 0.05, **P < 0.01 by Pearson correlation analysis. BALF, bronchoalveolar lavage fluid; ALX, airway lipoxin A4 receptor; NSA, nonsevere asthma; SA, severe asthma; LXA4, lipoxin A4; 15-epi-LXA4, 15-epimer lipoxin A4; ANXA1, annexin A1; SAA, serum amyloid A; ACQ, Asthma Control Questionnaire; ACT, Asthma Control Test; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; FeNO, fractional exhaled nitric oxide; PC 20, provocation challenge causing a 20% fall in FEV1.
BAL neutrophilia in SA is associated with decreased LXA4 and increased SAA levels.
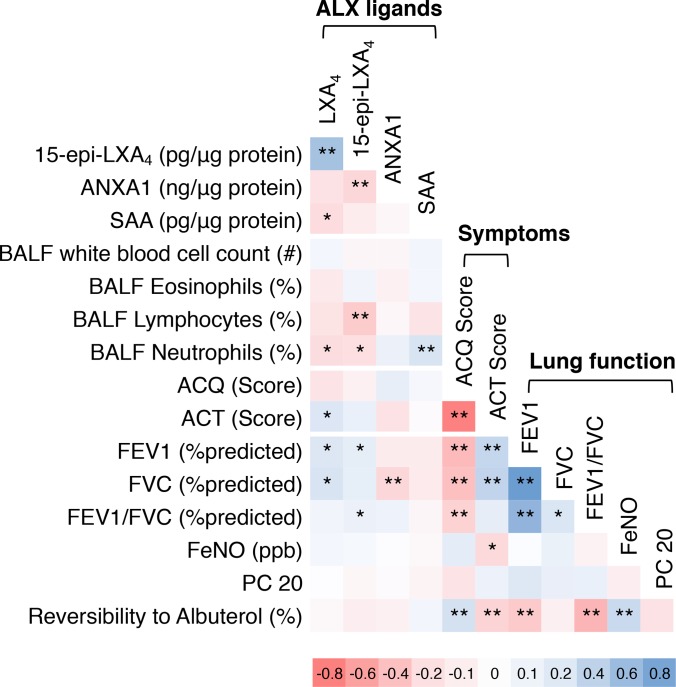
BAL leukocyte subsets were determined in NSA and SA subjects and HD. As expected, macrophages were the most numerous BAL cell type accounting for 88%–92% of BAL leukocytes (Figure 3A). SA subjects had significantly higher percentages of BAL neutrophils than NSA subjects (mean ± SEM 3.32% ± 0.59% SA versus 1.38% ± 0.19% NSA; Figure 3B). There were also trends for increased BAL lymphocytes and eosinophils in SA relative to NSA and HD (Figure 3, C and D). BALF LXA4 levels inversely correlated with BAL neutrophil percentage in the total asthma cohort or when the SA cohort was analyzed independently (Figure 3, E and F). In contrast, SAA levels were positively correlated with BAL neutrophil percentage in the complete asthma cohort as well as when SA was analyzed separately (Figure 3, G and H). LXA4 levels were also positively correlated with lung function (i.e., FEV1 and forced vital capacity [FVC] [percentage predicted values]), but there was no correlation between SAA levels and lung function in this cohort (Supplemental Figure 2).
Figure 3. BAL neutrophils are increased in severe asthma and differentially related to BALF LXA4 and SAA levels.
BAL samples were obtained from NSA and SA subjects and leukocyte subsets were enumerated. (A) Pie charts express the mean percentage of BAL neutrophils, lymphocytes, eosinophils, and macrophages in n = 47 HD, n = 51 NSA, and n = 69 SA subjects. (B–D) Scatter plots show individual subject data points with mean ± SEM for BAL (B) neutrophils, (C) lymphocytes, and (D) eosinophils in the HD (gray circles), NSA (blue squares), and SA (red triangles) cohorts. *P < 0.05, **P < 0.01 by 1-way ANOVA. (E and F) The relationship between BALF neutrophils and LXA4 was determined for (E) all asthma subjects and (F) for the SA cohort only. (G and H) The relationship between BALF neutrophils and SAA was determined for (G) all asthma subjects and (H) for the SA cohort only. SAA levels that were undetectable were assigned a value of 0 pg/μg protein and were included in the correlation analysis. Pearson correlation r value and significance are noted and regression lines are shown. BALF, bronchoalveolar lavage fluid; HD, healthy donors; NSA, nonsevere asthma; SA, severe asthma; LXA4, lipoxin A4; SAA, serum amyloid A.
ALX receptor ligands and expression are associated with asthma symptoms.
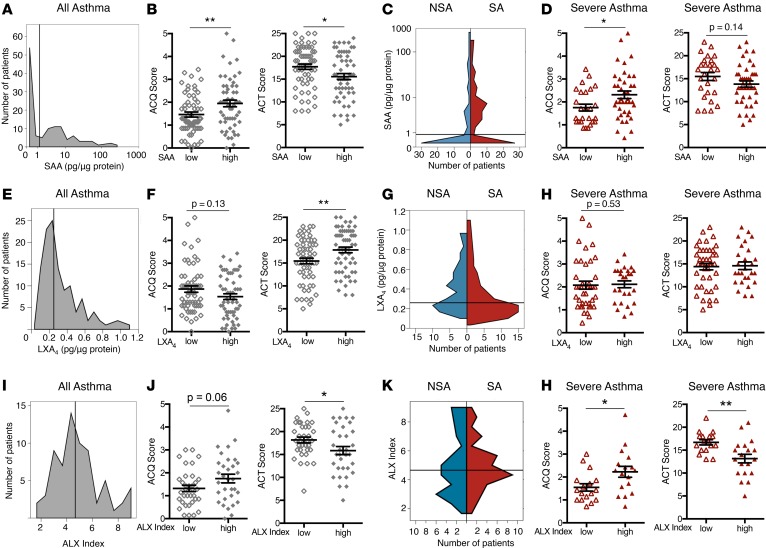
To further investigate relationships between the ALX signaling pathway and measures of asthma symptoms, the continuous variables for BAL ALX ligands were converted to categorical high and low subgroups using the median value to define a cutoff between the subgroups. Using the median BALF SAA value (1.22 pg/μg protein; Figure 4A), the relationships between SAAhi and SAAlo subjects and measures of asthma symptoms were determined. Subjects defined as SAAhi had significantly increased ACQ and decreased ACT scores relative to SAAlo subjects (Figure 4B), consistent with increased asthma symptoms in the SAAhi group. When stratified by asthma severity, there were more NSA subjects that were SAAlo and more SA subjects that were SAAhi (Figure 4C). ACQ scores were significantly different between SAAlo and SAAhi subjects when considering only those subjects with SA (Figure 4D). Of note, significant differences between the SAAhi and SAAlo cohorts for ACQ and ACT scores were not apparent in the NSA cohort (Supplemental Figure 3A).
Figure 4. SAA and macrophage ALX expression are associated with increased symptoms in severe asthma.
Asthma subjects were categorized into subgroups based on low or high BALF levels of SAA (A–D), LXA4 (E–H), and macrophage ALX expression (I–L). The median value for each variable was used to define the cutoff between the low and high subgroups (SAA cutoff = 1.22 pg/μg protein, LXA4 cutoff = 0.25 pg/μg protein, ALX index cutoff = 4.6 pg/μg protein). Cutoff values are delineated by the gray vertical line. A histogram shows the distribution of subjects based on BALF (A) SAA level, (E) LXA4 level, and (I) BAL macrophage ALX index. (B, F, and J) Validated measures of asthma symptoms (ACQ and ACT scores) were compared between low (open diamonds) and high (closed diamonds) subgroups for SAA, LXA4, and ALX index. (C, G, and K) The distributions of SAA, LXA4, and ALX index among NSA (blue) and SA (red) subjects are shown in violin plots. (D, H, and L) ACQ and ACT scores were compared in SA subjects for low (open triangles) and high (closed triangles) subgroups for SAA, LXA4, and ALX index. Scatter plots show individual subject data with mean ± SEM. n = 51 NSA and n = 69 SA subjects.*P < 0.05, **P < 0.01 by Mann-Whitney test or 2-tailed Student’s t test. BALF, bronchoalveolar lavage fluid; SAA, serum amyloid A; ACQ, asthma control questionnaire; ACT, asthma control test; NSA, nonsevere asthma; SA, severe asthma; LXA4, lipoxin A4; ALX, airway lipoxin A4 receptor.
Because BALF LXA4 levels were inversely related to SAA levels and asthma symptoms by Pearson correlation (Figure 2), the difference between LXA4hi and LXA4lo groups of subjects and asthma symptoms was determined. The median LXA4 level was used as a cutoff between groups (0.23 pg/μg protein; Figure 4E). LXA4lo subjects had higher ACQ scores and significantly lower ACT scores compared with LXA4hi subjects, consistent with more symptoms in the LXA4lo group (Figure 4F). When stratified by severity, NSA subjects were more numerous in the LXA4hi group and approximately 70% of the LXA4lo cohort were subjects with SA (Figure 4G). In contrast to our findings with SAA, the low LXA4 levels were not significantly related to symptom scores in the SA cohort (Figure 4H); however, among NSA subjects, the LXA4hi group did have significantly fewer symptoms as evidenced by higher ACT scores (Supplemental Figure 3B). Of note, administration of a single dose of intramuscular triamcinolone did not result in discernible changes in either LXA4 or SAA levels after 3 to 6 weeks in either SA or NSA subjects (Supplemental Figure 4).
With opposing relationships for asthma symptoms for the ALX ligands SAA and LXA4, we next performed similar analyses for the BAL macrophage ALX index. Asthma subjects were categorized into ALXhi and ALXlo cohorts using the median ALX index to define the cutoff between subgroups (median 4.61; Figure 4I). ALXhi asthma subjects had higher ACQ scores and significantly lower ACT scores, consistent with increased asthma symptoms (Figure 4J). After stratification by asthma severity (Figure 4K), ALXhi subjects had significantly increased symptoms by both measures (i.e., ACQ and ACT) in the SA cohort (Figure 4L). The ALX index was not significantly linked to symptom score in the NSA cohort (Supplemental Figure 3C). Of added interest, the BAL macrophage ALX index correlated with macrophage indices of MHC class 2 and CD206 expression in asthma subjects (Supplemental Figure 5).
SAA and LXA4 levels together represent a biochemical endotype that distinguishes SA from NSA.
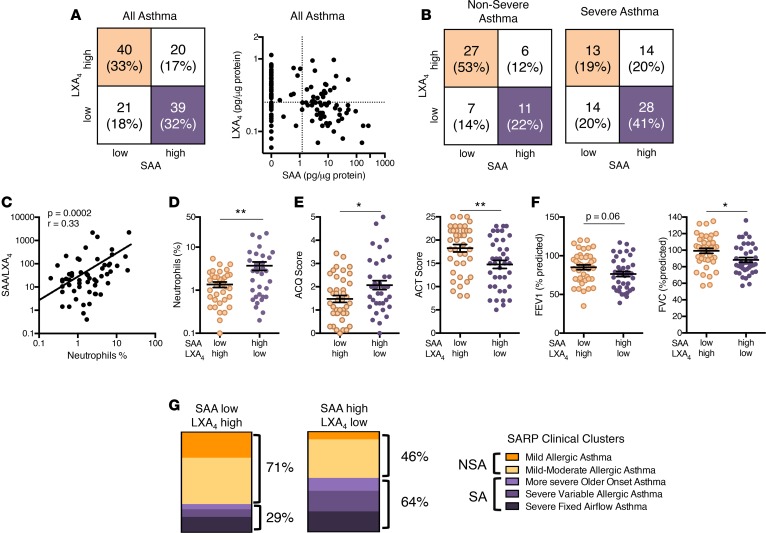
Individually, SAA and LXA4 levels were both associated with asthma severity. More SA subjects had SAAhi and LXA4lo levels and more NSA subjects had SAAlo and LXA4hi levels (Figure 4). In SA, macrophage ALX expression was increased (Figure 1) and associated with increased asthma symptoms (Figure 4), so we next determined if BALF levels for the combination of the ALX ligands was linked to SA. Asthma subjects were divided into 4 groups based on BALF LXA4 and SAA levels (Figure 5A). The approach of categorical grouping of high and low (based on median values) was chosen because the relationship between an individual’s BALF LXA4 and SAA levels suggested that these ALX ligands were independently regulated (Figure 5A). When subjects were stratified by clinical severity, it was striking that more than half of NSA but fewer than a quarter of SA were LXA4hiSAAlo (beige, Figure 5B). In contrast, 41% of SA subjects were LXA4loSAAhi compared with 22% of NSA subjects (purple, Figure 5B). Of note, for asthmatic subjects, the relative ratio of BALF SAA to LXA4 levels was strongly correlated with BAL neutrophilia (Figure 5C), more so than the level of either ALX ligand independently (Figure 3, E and G). When comparing asthmatic subjects in these 2 distinct groups, the LXA4loSAAhi group had significantly higher BAL neutrophils and asthma symptoms (i.e., higher ACQ, lower ACT) and lower lung function (i.e., percentage predicted FEV1 and FVC) than the LXA4hiSAAlo group (Figure 5, D–F). The total BAL white blood cell count and percentage of lymphocytes and eosinophils did not differ between the 2 groups (Supplemental Figure 6, A–C). The percentage of subjects with an ACQ score greater than 1.5 was higher in the LXA4loSAAhi cohort (Supplemental Figure 6D), representing another measure of the increased symptoms in this group.
Figure 5. BALF SAA and LXA4 levels are distinct in clinically severe and nonsevere asthma.
(A) The number and percentages of asthma subjects with BALF levels of LXA4 and SAA that were below (low) or above (high) the median value were identified and subjects were grouped into 4 phenotypes based on LXA4 and SAA levels. Noted are subjects in the LXA4hiSAAlo group (beige quadrant) and LXA4loSAAhi group (purple quadrant). The relationship between individual subject levels of LXA4 and SAA was determined for all asthma subjects. (B) The 4 groups of subjects based on BALF SAA and LXA4 low and high cohorts were determined for subjects and stratified by asthma severity; NSA (left), SA (right). (C) The relationship between the SAA/LXA4 ratio and BAL neutrophils (%) was determined in n = 120 asthma subjects. Pearson correlation r value and significance are noted and regression line is shown. (D–F) Scatter plots show comparisons of subjects in the LXA4hiSAAlo group (beige) to subjects in the LXA4loSAAhi group (purple) for measures of (D) inflammation (BALF neutrophils [%]), (E) asthma symptoms (ACQ and ACT scores), and (F) lung function (percentage predicted FEV1 and FVC). (G) Subjects in the LXA4hiSAAlo and LXA4loSAAhi groups were assigned to clinical clusters as defined in SARP-1 (5) and the percentage of subjects assigned to NSA and SA clusters is indicated. n = 51 NSA and n = 69 SA subjects.*P < 0.05, **P < 0.01 by 2-tailed Student’s t test. BALF, bronchoalveolar lavage fluid; LXA4, lipoxin A4; SAA, serum amyloid A; NSA, nonsevere asthma; SA, severe asthma; ACQ, Asthma Control Questionnaire; ACT, Asthma Control Test; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; SARP, Severe Asthma Research Program.
Cluster analyses from SARP-1 used clinical parameters to identify 5 asthma subtypes (mild allergic asthma, mild-moderate allergic asthma, more severe older-onset asthma, severe variable allergic asthma, and severe fixed-airflow asthma) (5). Using this classification here with SAA and LXA4 as biochemical markers of ALX receptor signaling, it was notable that 71% of the LXA4hiSAAlo group were assigned to one of the NSA clusters and 64% of the LXA4loSAAhi subjects were assigned to one of the SA clusters (Figure 5G and Supplemental Figure 6E).
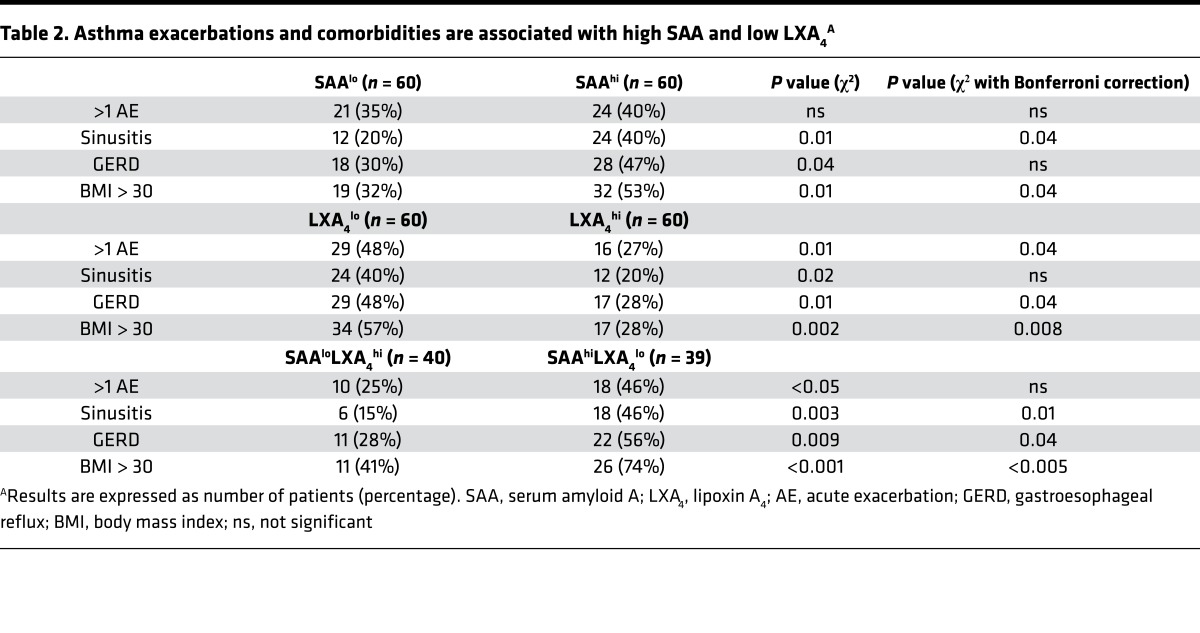
SAA is an acute-phase protein, and with the relationship for the LXA4loSAAhi endotype to asthma severity and neutrophilic lung inflammation, we next determined if there was a relationship for LXA4 and SAA to exacerbations and common asthma comorbidities. Both high SAA and low LXA4 levels were significantly related to sinusitis, gastroesophageal reflux disease (GERD), and obesity (BMI > 30), and low LXA4 was also related to history of more frequent acute exacerbation over the prior year (Table 2). Together, the combination of low LXA4 levels and high SAA levels (i.e., the LXA4loSAAhi endotype) was even more closely associated with these asthma comorbidities (Table 2).
Table 2. Asthma exacerbations and comorbidities are associated with high SAA and low LXA4A.
SAA and 15-epi-LXA4 signaling via ALX receptors regulates production of the neutrophil chemoattractant IL-8.
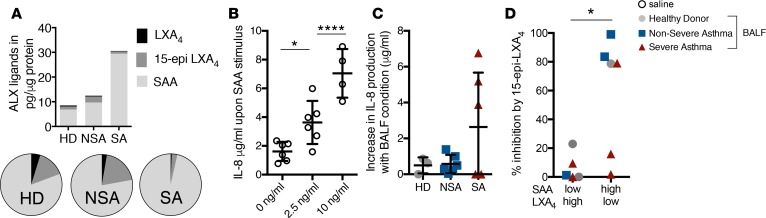
To determine the functional relationship for these ALX receptor ligands, we next turned to an ALX-dependent in vitro reporter assay that we have previously qualified in chronic obstructive pulmonary disease (18). A549 lung epithelial cells were stably transfected to express the human ALX/FPR2 receptor (hALX). BALF samples were selected from HD, NSA, and SA subjects with representative levels of ALX ligands (Figure 6A). SAA gave a concentration-dependent (0–10 ng/ml) increase in IL-8 production by A549hALX cells (Figure 6B). When the A549hALX cells were exposed to BALF (see Methods), several of the representative SA BALFs substantially increased IL-8 production (Figure 6C), reflective of the relative amounts of BALF SAA and LXA4. The addition of exogenous 15-epi-LXA4 inhibited A549hALX cell IL-8 production by cells conditioned with BALF (Figure 6D). Of note, maximal inhibition of IL-8 production by 15-epi-LXA4 was for A549hALX cells that had been conditioned with BALF from LXA4loSAAhi subjects (Figure 6D).
Figure 6. SAA and 15-epi-LXA4 signaling via ALX receptors regulates production of the neutrophil chemoattractant IL-8.
A549 human epithelial cells expressing human ALX receptors (A549hALX) were exposed to BALF from HD, NSA, or SA subjects (24 hours, 37°C, 5% CO2) and IL-8 levels were measured in the cell-free supernatant by ELISA (see Methods). (A) Mean levels of LXA4, 15-epi-LXA4, and SAA in BALF from n = 15 subjects used for A549hALX cell incubations are shown in stacked bar graphs and the relative proportions are noted in pie charts. (B) IL-8 production by A549hALX cells was measured after incubation with saline control or SAA (2.5 ng/ml or 10 ng/ml) for 24 hours. (C) IL-8 production by A549hALX cells was measured after 24 hours of exposure to BALF from HD (n = 3), NSA (n = 6), or SA (n = 5) and is expressed as an increase relative to saline controls. Incubations with BALF without an increase in IL-8 production relative to saline control were assigned a value of zero. (D) A549hALX epithelial cells were exposed to BALF from subjects with endogenous levels that were LXA4hiSAAlo (n = 5) or LXA4loSAAhi (n = 6) followed by exposure to exogenous 15-epi-LXA4 (100 nM). Percentage inhibition of IL-8 production after 15-epi-LXA4 exposure was calculated. *P < 0.05, ****P < 0.001 by (B) 1-way ANOVA, (C) Kruskal-Wallis test, and (D) Mann-Whitney test. ALX, airway lipoxin A4 receptor; LXA4, lipoxin A4; 15-epi-LXA4, 15-epimer lipoxin A4; SAA, serum amyloid A; HD, healthy donors; NSA, nonsevere asthma; SA, severe asthma; IL-8, interleukin-8; BALF, bronchoalveolar lavage fluid.
Discussion
Here, in SARP-3, we measured the abundance of 4 ligands for ALX receptors, namely LXA4, 15-epi-LXA4, ANXA1, and SAA, with the potential for opposing effects on asthmatic airway responses. In SA, BAL macrophage ALX receptor expression was increased and BALF ligands for ALX were selectively regulated. BALF levels of proresolving lipoxins were decreased and levels of proinflammatory SAA were increased in SA. Levels of lipoxins inversely correlated to SAA, BAL neutrophils, and asthma symptoms, and lipoxins were positively correlated to measures of lung function. SAAhi and ALXhi subjects more commonly had SA with increased ACQ and decreased ACT scores. When LXA4 and SAA levels were considered together in a combined endotype, a stronger association with asthma symptoms, lung function, and airway neutrophilia was noted than when either mediator was considered individually. LXA4loSAAhi subjects had more lung inflammation and asthma symptoms and lower lung function relative to LXA4hiSAAlo subjects. LXA4loSAAhi and LXA4hiSAAlo subjects segregated to SA and NSA clinical clusters, respectively. Importantly, LXA4loSAAhi subjects had significantly increased likelihood for asthma exacerbation in the past year and for the asthma comorbidities of sinusitis, GERD, and obesity. Exposure to SAA or BALF from SA subjects increased production of the neutrophil chemoattractant IL-8 by ALX-expressing human lung epithelial cells in vitro. This SAA-driven IL-8 production by epithelial cells was mitigated by exposure to 15-epi-LXA4 at pharmacological doses, supporting a functional interaction between the ALX ligands relevant to the neutrophilic inflammation in SA. Together, these findings support a mechanistic role for ALX receptor signaling by SAA and LXA4 in lung inflammation in SA that defines a potentially new biochemical endotype for patient stratification in asthma.
ALX receptors are intriguing targets for regulating the fate of inflammatory responses. LXA4 and SAA interact with ALX receptors to exert opposing effects on inflammation (18). ALX receptors are 7-membrane-spanning, G protein–coupled receptors that are present on several lung cell types relevant to asthma pathogenesis, including neutrophils (22), eosinophils (23), group 2 innate lymphoid cells (24), natural killer cells (24), lymphocytes (25), monocytes (26), dendritic cells (27, 28), macrophages (29), and airway structural cells (30). LXA4 is a high-affinity ligand for ALX that signals for antiinflammatory and proresolving cellular responses (16). SAA binds with lower affinity, yet this acute-phase reactant is substantially more abundant than LXA4 during infection and the upstroke of acute inflammation (18). Also relevant in SA patients with comorbidities, corticosteroids can enhance SAA production, especially in conjunction with LPS (18). Distinct from LXA4’s interaction with the seventh transmembrane domain and adjacent regions (31, 32), SAA interacts with the first and second extracellular loop domains (33), resulting in a marked shift in receptor conformation and dimerization that changes the receptor’s proresolving signaling to proinflammatory signaling (17). Here in SA, ALX expression was increased on BAL macrophages and associated with increased asthma symptoms. Macrophage ALX expression correlated with surface CD206 expression, which marks M2 macrophages that participate in endogenous pathways of inflammation resolution (34, 35). Together with the increased SAA and decreased lipoxins in SA BALF, the increase in ALX expression on BAL macrophages likely reflects SAA-driven outcomes, including the increased lung inflammation (i.e., BAL neutrophilia) despite higher doses of corticosteroids. With the presence of SAA and LXA4 in proximity to ALX receptors in the lung and their divergent influences on inflammatory responses, ALX receptors are poised to serve as a pivotal signaling nexus for acute inflammation or its resolution.
Lipoxins are products of arachidonic acid metabolism that are structurally and functionally distinct from leukotrienes and prostaglandins. LXA4 was first detected in humans in BALF from patients with lung disease, including asthma (36). Lipoxins are the lead members of a new genus of endogenous chemical signals, SPMs, which are partially defined by their ability to inhibit granulocyte recruitment and activation in inflamed tissues as well as to promote macrophage-mediated clearance of dead cells, microbes, and debris in catabasis (8). Distinct from increased leukotriene production by some asthmatic patients, lipoxin levels are decreased in uncontrolled asthma and SA (13). Current anti-leukotriene drugs would not be expected to increase lipoxins in asthma. There are likely multiple factors responsible for the defective lipoxin production in SA; however, the increased oxidative stress in SA airways was recently determined to be a major cause of reduced lipoxin generation (11). In human studies, inhaled LXA4 dampens bronchoprovocation in asthma (10) and lipoxins regulate cytokine-mediated increases in bronchial constriction induced by methacholine, histamine, and thromboxane (11). Recently, a stable LXA4 analog was shown to markedly decrease allergic inflammation and symptoms in patients with juvenile eczema (37). Preclinical studies have established that LXA4 analogs that resist metabolic inactivation can prevent and potently reduce allergen-driven airway hyperresponsiveness to methacholine, airway mucus metaplasia, and type 2 inflammation (38, 39). Transgenic expression of hALX receptors also leads to decreased inflammatory responses to allergens, supporting a role for endogenous ALX ligands in antiinflammation and proresolution (23). In addition to ALX, lipoxins can interact with additional receptors, including cysLT1 — the pharmacological target of one of the classes of anti-leukotriene drugs (40). Together, these findings point to pivotal proresolving roles for lipoxins in health to control airway phlogistic responses and promote their resolution. Here, the diminished BALF levels of lipoxins in SA would be predicted from prior publications to disable a major endogenous regulatory pathway for airway inflammation, mucus, and hyperreactivity, and our results show a strong and consistent correlation between low LXA4 and increased lung inflammation, asthma symptoms, and comorbidities, and lower lung function in SA.
In contrast to lipoxins, there are several peptide ligands for ALX receptors that engage these receptors to promote inflammatory responses. The acute-phase reactant SAA is one of the ALX peptide ligands and can induce neutrophil chemotaxis and activation via ALX (41, 42). SAA is increased in severe allergic asthma (43) and can prevent dendritic cell apoptosis to induce glucocorticoid resistance in CD4+ T cells (44). Here, BALF SAA levels were increased in SA and strongly associated with BAL neutrophils. SAAhi subjects had increased asthma symptoms and a higher relative risk of sinusitis, GERD, and obesity. If subjects had both low LXA4 levels and high SAA levels (i.e., LXA4loSAAhi) then their relative risk for BAL neutrophils, asthma symptoms, and lower lung function were all increased. Recently, some subjects with SA with non–type 2 inflammation were identified as IL-6hi (45). IL-6 induces SAA expression (46) and may conspire with this acute-phase protein to activate neutrophils and non–type 2 lung inflammation in SA, in particular in those with systemic metabolic alterations associated with obesity. In A549hALX epithelial cells, BALF from LXA4loSAAhi subjects increased production of the neutrophil chemoattractant IL-8, which was inhibitable by 15-epi-LXA4. At ALX receptors, 15-epi-LXA4 inhibition of SAA is allosteric in nature (18), and when given at pharmacological doses, 15-epi-LXA4 can decrease SAA-driven IL-8 production by human airway epithelial cells in vitro and SAA-mediated acute inflammation in vivo in mice (18). Of interest for SA, SAA production is increased by corticosteroids and its expression is synergistically increased by the combination of steroids and LPS (18). Additional soluble mediators acting via distinct or synergistic pathways with SAA also are likely to contribute to epithelial cell IL-8 production and neutrophil chemoattraction in SA. Subjects enrolled in SARP-3 were clinically characterized before and after intramuscular triamcinolone and adult subjects with SA continued to manifest lower FEV1 and worse asthma control as compared with NSA after the systemic corticosteroids (47). Of note, BALF levels of LXA4, 15-epi LXA4, and SAA were not significantly altered by a single dose of intramuscular triamcinolone when measured 3 to 6 weeks after steroid administration. Unlike 15-epi-LXA4, corticosteroids do not inhibit SAA-mediated lung inflammation (18), suggesting that for some subjects with SA their chronic neutrophilic lung inflammation could be perpetuated by corticosteroids and that SAA levels could inform more precise asthma management by helping to identify subjects at risk for this unintended consequence of corticosteroids.
Biochemical analyses here have linked ALX receptor signaling to the pathophysiology of SA. Using clinical and statistical approaches, 5 phenotypes of adult asthma have been defined (5), but there remains a need to connect these phenotypes to distinct molecular mechanisms for SA pathogenesis (6). We chose a candidate pathway approach based on preclinical evidence that linked ALX signaling to SA, and BAL LXA4 and SAA levels segregated subjects into discrete clinical clusters, suggesting that this biochemical pathway could convey additional value for patient stratification as an asthma endotype. Findings here suggest that these ALX ligands should be included in future studies designed to comprehensively model genetic, metabolic, and environmental influences and clinical characteristics for patient endotyping in SA.
Here, we have identified significant associations for BALF LXA4loSAAhi levels with neutrophilic lung inflammation and poorly controlled asthma; however, there are several potential limitations to consider. While it was advantageous for biochemical analyses to have a relatively large number of BAL samples from this carefully phenotyped group of asthma subjects, it would not be practical to routinely perform bronchoscopy in a clinical (or clinical trial) setting. It will be important in future studies to obtain and analyze respiratory samples collected by less invasive means (i.e., sputum, exhaled breath condensate) to determine the influence of anatomic compartment on the relationships uncovered here with bronchoscopy specimens. The cross-sectional nature of the analyses here does not address the stability of this endotype, a question best addressed with samples obtained by less invasive means. Regarding additional ALX ligands, the ELISA used here for ANXA1 does not distinguish between intact and cleaved protein, so the absence of a relationship here does not preclude its potential existence when more specific experimental tools become available. There are also several additional peptide and lipid ligands for ALX receptors that might further enhance the discriminatory power of ALX signaling for identification of asthma endotypes.
In summary, ALX receptor expression was increased in asthmatic BAL macrophages and we have identified a cassette of ALX receptor ligands that are selectively regulated in BALF in asthma. Levels of lipoxins and SAA correlated with lung inflammation and clinical parameters of asthma control. In particular, subjects with LXA4loSAAhi BALF were more likely to have SA with increased BAL neutrophils, asthma symptoms, and asthma comorbidities, and decreased lung function. At pharmacological levels, 15-epi-LXA4 functionally opposed SAA signaling at ALX receptors to inhibit production of the neutrophil chemoattractant IL-8. BALF LXA4loSAAhi subjects were assigned to discrete clinical clusters from LXA4hiSAAlo subjects, suggesting that these biochemical mediators could identify subgroups of asthma subjects and serve as a new asthma biochemical endotype for non–type 2, steroid resistant inflammation in SA.
Methods
Study design.
SARP-3 is an NHLBI-funded study designed to characterize molecular, cellular, and physiological phenotypes in subjects with SA and NSA (ClinicalTrials.gov NCT01606826). Asthmatic and healthy subjects were recruited and completed baseline characterization with some subjects agreeing to bronchoscopy. Details regarding SARP methods, subject enrollment, and study procedures can be found in Peters et al. (45).
Participants and sample collection.
Subjects 13 years of age and older with asthma and healthy control subjects were recruited between November 2012 and February 2015 by 7 geographically dispersed research centers in the USA. European Respiratory Society/American Thoracic Society guidelines were used to categorize subjects as SA or NSA (48). Control subjects were individuals who reported general health and were nonsmokers with no history of lung disease, atopic disease, or allergic rhinitis. BAL was performed with three 50-ml aliquots of warm saline, and BALF was recovered by hand suction. Subjects received intramuscular triamcinolone (1 mg/kg up to a maximum dose of 40 mg) and some subjects agreed to undergo a second bronchoscopy 3 to 6 weeks later and BAL samples were collected in the same manner. BAL cells were enumerated and differential leukocyte counts determined. Cell-free BALF supernatant was divided into several aliquots. One aliquot of BALF (1 ml) was directly mixed with iced methanol (2 ml, for 1:2, vol/vol) before storing at –80°C. The other aliquots were directly stored at –80°C. The stored aliquots were later shipped to Brigham and Women’s Hospital for analyses.
Lipid extraction.
Aliquots of BALF with methanol (1:2, vol/vol) were brought to dryness in vacuo using a BÜCHI Rotavapor R-200/205. The samples were resuspended with methanol (500 μl) and distilled/deionized water (10 ml) followed by extraction using C18 SepPak cartidges (Waters) as previously described (13). The methyl formate fraction was brought to dryness under a gentle stream of nitrogen and each sample was resuspended in 1 ml of methanol and stored at –80°C until LXA4 and 15-epi-LXA4 ELISAs were performed.
Protein assay.
The Pierce BCA Protein Assay Kit (Thermo Fisher) was used for BALF protein determination. Samples with less than 25 μg of protein were excluded from further analysis (n = 3; 1 NSA, 2 SA).
ELISA.
LXA4 and 15-epi-LXA4 levels in the BALF were measured by ELISA (Neogen). Extracted BALF samples stored in methanol were brought to dryness under a gentle stream of nitrogen and resuspended in ELISA buffer. SAA and ANXA1 levels were measured by ELISA (commercial kits from Abazyme and Cloud-Clone, respectively) in aliquots of BALF stored in the absence of methanol. LXA4, 15-epi-LXA4, SAA, and ANXA1 levels were normalized to the total protein content of the BALF. Some subjects had an SAA level below the limit of detection and these samples were assigned a value of 0 pg/μg protein for analysis. The median values for LXA4 and SAA were used to segregate subjects into high and low subgroups.
Flow cytometry.
BAL cell pellets were available from a subgroup of subjects. For ex vivo staining, BALF cells were blocked with mouse serum (Sigma-Aldrich) in PBS for 30 minutes at 4°C. The cells were then incubated with Viability Dye eFluorR660 (eBioscience) as per the manufacturer’s instructions followed by 30 minutes of incubation with the following antibodies against human proteins: anti-ALX-PerCP (clone 304405, R&D Systems); anti–HLA-DR (MHC class II)-allophycocyanin-Cy7 (APC-Cy7) (clone L243, BD Biosciences); and anti-CD206 (macrophage mannose receptor)-phycoerythrin (PE) (clone 19.2, eBioscience) or with directly conjugated unrelated antibodies of the same isotype (BD Biosciences) at 4°C. Data were acquired on a Canto II flow cytometer (Becton Dickinson) and analyzed using FlowJo software version 10.1 (Tree Star). Macrophages were identified as single cells (by doublet exclusion), viable (Viability Dye eFluorR660 negative), CD206+ cells. The MFI of ALX, MHC class II, and CD206 was assessed on macrophages and normalized with the MFI of the isotype control antibody (MFI cell surface marker/MFI isotype control = MFI index).
In vitro A549 cell culture.
A549 cells transfected to stably express the human ALX receptor were used (as in ref. 18). A549hALX cells were seeded into a 48-well plate (5 × 104 cells/well) and cultured in RPMI 1640 (Lonza) supplemented with 2 mM L-glutamine, 10% heat-inactivated fetal calf serum (Gibco), penicillin (100 IU/ml), and streptomycin (100 μg/ml) at 37°C in 5% CO2 until confluent. When confluent, A549hALX cells were cultured with serum-free media for 16 hours and then exposed to BALF (100 μl) and serum-free RPMI media (100 μl, 1:1 vol/vol) for 24 hours (37°C, 5% CO2). In select experiments, recombinant human SAA (0–10 ng/ml, Peprotech), 15-epi-LXA4 (100 nM, Cayman Chemical), or vehicle control was added. At the end of the incubations, supernatants were collected on ice and stored at –80°C. IL-8 levels in the supernatants were measured by ELISA (R&D Systems). If there was no increase in IL-8 production after exposure to BALF, the samples were assigned a value of zero (Figure 6C).
Statistics.
In figures, data are expressed either individually with indication of the median value or as mean ± SEM; in tables, data are expressed as mean ± SD. For violin plots in Figure 4, bin sizes and widths were determined for each variable automatically in SPSS based on the underlying data distribution. Statistical significance of differences was assessed by 2-tailed Student’s t test, 1-way ANOVA, Kruskal-Wallis test (when normality assumptions were not met), or χ2 test as noted using SPSS version 23.0 (IBM). Post hoc Tukey’s test (for ANOVA analyses) and Dunn’s test (for Kruskal-Wallis analyses) were used to correct for multiple comparisons. Correlations were evaluated by Pearson’s correlation coefficient (r) and linear or nonlinear (for graphs with log axes) regression lines are shown. Correlation analyses included samples with a value of 0 for statistical analysis, but data points with a value of 0 were excluded for regression line analyses of detectable ALX ligands. A P value less than 0.05 was considered significant and the reported P values were adjusted for multiple comparisons.
Study approval.
Written informed consent was obtained after institutional review board approval at each of the seven sites.
Author contributions
IR, IB, M. Cernadas, and MGD designed and performed experiments, analyzed data, and wrote the manuscript. NLG, EI, ERB, M. Castro, SCE, JVF, BMG, LCD, DTM, SEW, SAC, AMC, MLF, ATH, MWJ, MCP, and BRP collected specimens, analyzed data, and wrote the manuscript. BDL conceived of the study, designed experiments, analyzed data, and wrote the manuscript. All authors contributed to the editing of the final manuscript. All authors agreed to all of the content of the submitted manuscript.
Supplementary Material
Acknowledgments
The authors thank the study participants, the SARP-3 investigators and clinical research coordinators, and the data-coordinating center. See supplemental Acknowledgments for SARP-3 consortium details. We also thank Guangli Zhu for her expert technical assistance. This study was conducted with the support of grants that were awarded by the NHLBI as follows: Elliot Israel and Bruce Levy, Harvard Medical School U10 HL109172; Eugene Bleecker, Wake Forest University U10 HL109164; Mario Castro, Washington University U10 HL109257; Serpil Erzurum, Cleveland Clinic (Co-PI, Virginia-Cleveland Consortium) U10 HL109250; John Fahy, UCSF U10 HL109146; Benjamin Gaston, Case Western Reserve University U10 HL109250; Nizar Jarjour, University of Wisconsin U10 HL109168; Sally Wenzel, University of Pittsburgh U10 HL109152; David Mauger, Penn State University U10 HL109086-04; the work was also supported in part by R01-HL122531 (BDL), a fellowship from the German Society for Pediatric Pneumology (IR), and K12-HD047349 (MGD). In addition, this program is supported through the following NIH National Center for Advancing Translational Sciences (NCATS) awards: UL1 TR001420 to Wake Forest University, UL1 TR000427 to the University of Wisconsin, UL1 TR001102 to Harvard University, and UL1 TR000454 to Emory University. See supplemental acknowledgments for the NHLBI SARP-3 investigators’ details. The interests of Bruce Levy were reviewed and are managed by the Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict-of-interest policies.
Footnotes
Conflict of interest: B.D. Levy is a coinventor on patent 8,119,691 assigned to Brigham and Women’s Hospital.
Reference information: JCI Insight. 2017;2(14):e93534. https://doi.org/10.1172/jci.insight.93534.
Contributor Information
Isabell Ricklefs, Email: isabell_baumann@yahoo.de.
Ioanna Barkas, Email: joannabarkas@gmail.com.
Manuela Cernadas, Email: MCERNADAS@PARTNERS.ORG.
Elliot Israel, Email: eisrael@partners.org.
Eugene R. Bleecker, Email: erbleecker@email.arizona.edu.
Mario Castro, Email: castrom@dom.wustl.edu.
References
- 1. Most Recent Asthma Data. Center for Disease Control and Prevention. https://www.cdc.gov/asthma/most_recent_data.htm Updated February 27, 2017. Accessed June 6, 2017.
- 2.Levy BD, et al. Future research directions in asthma. An NHLBI working group report. Am J Respir Crit Care Med. 2015;192(11):1366–1372. doi: 10.1164/rccm.201505-0963WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray A, Raundhal M, Oriss TB, Ray P, Wenzel SE. Current concepts of severe asthma. J Clin Invest. 2016;126(7):2394–2403. doi: 10.1172/JCI84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modena BD, et al. Gene expression correlated with severe asthma characteristics reveals heterogeneous mechanisms of severe disease. Am J Respir Crit Care Med. 2017;195(11):1449–1463. doi: 10.1164/rccm.201607-1407OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore WC, et al. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181(4):315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18(5):716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 7.Fahy JV. Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi: 10.1038/nri3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy BD, Serhan CN. Resolution of acute inflammation in the lung. Annu Rev Physiol. 2014;76:467–492. doi: 10.1146/annurev-physiol-021113-170408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christie PE, Spur BW, Lee TH. The effects of lipoxin A4 on airway responses in asthmatic subjects. Am Rev Respir Dis. 1992;145(6):1281–1284. doi: 10.1164/ajrccm/145.6.1281. [DOI] [PubMed] [Google Scholar]
- 11.Ono E, et al. Lipoxin generation is related to soluble epoxide hydrolase activity in severe asthma. Am J Respir Crit Care Med. 2014;190(8):886–897. doi: 10.1164/rccm.201403-0544OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kazani S, et al. Exhaled breath condensate eicosanoid levels associate with asthma and its severity. J Allergy Clin Immunol. 2013;132(3):547–553. doi: 10.1016/j.jaci.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy BD, et al. Diminished lipoxin biosynthesis in severe asthma. Am J Respir Crit Care Med. 2005;172(7):824–830. doi: 10.1164/rccm.200410-1413OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Planagumà A, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178(6):574–582. doi: 10.1164/rccm.200801-061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vachier I, et al. Severe asthma is associated with a loss of LX4, an endogenous anti-inflammatory compound. J Allergy Clin Immunol. 2005;115(1):55–60. doi: 10.1016/j.jaci.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 16.Chiang N, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58(3):463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 17.Cooray SN, et al. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A. 2013;110(45):18232–18237. doi: 10.1073/pnas.1308253110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bozinovski S, et al. Serum amyloid A opposes lipoxin A4 to mediate glucocorticoid refractory lung inflammation in chronic obstructive pulmonary disease. Proc Natl Acad Sci U S A. 2012;109(3):935–940. doi: 10.1073/pnas.1109382109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He R, Sang H, Ye RD. Serum amyloid A induces IL-8 secretion through a G protein-coupled receptor, FPRL1/LXA4R. Blood. 2003;101(4):1572–1581. doi: 10.1182/blood-2002-05-1431. [DOI] [PubMed] [Google Scholar]
- 20.Perretti M, et al. Endogenous lipid- and peptide-derived anti-inflammatory pathways generated with glucocorticoid and aspirin treatment activate the lipoxin A4 receptor. Nat Med. 2002;8(11):1296–1302. doi: 10.1038/nm786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris T, et al. Dichotomy in duration and severity of acute inflammatory responses in humans arising from differentially expressed proresolution pathways. Proc Natl Acad Sci U S A. 2010;107(19):8842–8847. doi: 10.1073/pnas.1000373107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fiore S, Maddox JF, Perez HD, Serhan CN. Identification of a human cDNA encoding a functional high affinity lipoxin A4 receptor. J Exp Med. 1994;180(1):253–260. doi: 10.1084/jem.180.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levy BD, et al. Multi-pronged inhibition of airway hyper-responsiveness and inflammation by lipoxin A(4) Nat Med. 2002;8(9):1018–1023. doi: 10.1038/nm748. [DOI] [PubMed] [Google Scholar]
- 24.Barnig C, et al. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5(174):174ra26. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN. Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol. 2003;170(12):6266–6272. doi: 10.4049/jimmunol.170.12.6266. [DOI] [PubMed] [Google Scholar]
- 26.Romano M, Maddox JF, Serhan CN. Activation of human monocytes and the acute monocytic leukemia cell line (THP-1) by lipoxins involves unique signaling pathways for lipoxin A4 versus lipoxin B4: evidence for differential Ca2+ mobilization. J Immunol. 1996;157(5):2149–2154. [PubMed] [Google Scholar]
- 27.Aliberti J, Hieny S, Reis e Sousa C, Serhan CN, Sher A. Lipoxin-mediated inhibition of IL-12 production by DCs: a mechanism for regulation of microbial immunity. Nat Immunol. 2002;3(1):76–82. doi: 10.1038/ni745. [DOI] [PubMed] [Google Scholar]
- 28.Yang D, Chen Q, Le Y, Wang JM, Oppenheim JJ. Differential regulation of formyl peptide receptor-like 1 expression during the differentiation of monocytes to dendritic cells and macrophages. J Immunol. 2001;166(6):4092–4098. doi: 10.4049/jimmunol.166.6.4092. [DOI] [PubMed] [Google Scholar]
- 29.Maderna P, et al. FPR2/ALX receptor expression and internalization are critical for lipoxin A4 and annexin-derived peptide-stimulated phagocytosis. FASEB J. 2010;24(11):4240–4249. doi: 10.1096/fj.10-159913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonnans C, Fukunaga K, Levy MA, Levy BD. Lipoxin A(4) regulates bronchial epithelial cell responses to acid injury. Am J Pathol. 2006;168(4):1064–1072. doi: 10.2353/ajpath.2006.051056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang N, Fierro IM, Gronert K, Serhan CN. Activation of lipoxin A(4) receptors by aspirin-triggered lipoxins and select peptides evokes ligand-specific responses in inflammation. J Exp Med. 2000;191(7):1197–1208. doi: 10.1084/jem.191.7.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chiang N, Gronert K, Clish CB, O’Brien JA, Freeman MW, Serhan CN. Leukotriene B4 receptor transgenic mice reveal novel protective roles for lipoxins and aspirin-triggered lipoxins in reperfusion. J Clin Invest. 1999;104(3):309–316. doi: 10.1172/JCI7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bena S, Brancaleone V, Wang JM, Perretti M, Flower RJ. Annexin A1 interaction with the FPR2/ALX receptor: identification of distinct domains and downstream associated signaling. J Biol Chem. 2012;287(29):24690–24697. doi: 10.1074/jbc.M112.377101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chiang N, Dalli J, Colas RA, Serhan CN. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J Exp Med. 2015;212(8):1203–1217. doi: 10.1084/jem.20150225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dakin SG, et al. Inflammation activation and resolution in human tendon disease. Sci Transl Med. 2015;7(311):311ra173. doi: 10.1126/scitranslmed.aac4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee TH, et al. Identification of lipoxin A4 and its relationship to the sulfidopeptide leukotrienes C4, D4, and E4 in the bronchoalveolar lavage fluids obtained from patients with selected pulmonary diseases. Am Rev Respir Dis. 1990;141(6):1453–1458. doi: 10.1164/ajrccm/141.6.1453. [DOI] [PubMed] [Google Scholar]
- 37.Wu SH, Chen XQ, Liu B, Wu HJ, Dong L. Efficacy and safety of 15(R/S)-methyl-lipoxin A(4) in topical treatment of infantile eczema. Br J Dermatol. 2013;168(1):172–178. doi: 10.1111/j.1365-2133.2012.11177.x. [DOI] [PubMed] [Google Scholar]
- 38.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9(8):873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levy BD, et al. Lipoxin A4 stable analogs reduce allergic airway responses via mechanisms distinct from CysLT1 receptor antagonism. FASEB J. 2007;21(14):3877–3884. doi: 10.1096/fj.07-8653com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gronert K, Martinsson-Niskanen T, Ravasi S, Chiang N, Serhan CN. Selectivity of recombinant human leukotriene D(4), leukotriene B(4), and lipoxin A(4) receptors with aspirin-triggered 15-epi-LXA(4) and regulation of vascular and inflammatory responses. Am J Pathol. 2001;158(1):3–9. doi: 10.1016/S0002-9440(10)63937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su SB, et al. A seven-transmembrane, G protein-coupled receptor, FPRL1, mediates the chemotactic activity of serum amyloid A for human phagocytic cells. J Exp Med. 1999;189(2):395–402. doi: 10.1084/jem.189.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Kebir D, et al. Aspirin-triggered lipoxins override the apoptosis-delaying action of serum amyloid A in human neutrophils: a novel mechanism for resolution of inflammation. J Immunol. 2007;179(1):616–622. doi: 10.4049/jimmunol.179.1.616. [DOI] [PubMed] [Google Scholar]
- 43.Büyüköztürk S, et al. Acute phase reactants in allergic airway disease. Tohoku J Exp Med. 2004;204(3):209–213. doi: 10.1620/tjem.204.209. [DOI] [PubMed] [Google Scholar]
- 44.Ather JL, Fortner KA, Budd RC, Anathy V, Poynter ME. Serum amyloid A inhibits dendritic cell apoptosis to induce glucocorticoid resistance in CD4(+) T cells. Cell Death Dis. 2013;4:e786. doi: 10.1038/cddis.2013.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peters MC, et al. Plasma interleukin-6 concentrations, metabolic dysfunction, and asthma severity: a cross-sectional analysis of two cohorts. Lancet Respir Med. 2016;4(7):574–584. doi: 10.1016/S2213-2600(16)30048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hagihara K, et al. Essential role of STAT3 in cytokine-driven NF-kappaB-mediated serum amyloid A gene expression. Genes Cells. 2005;10(11):1051–1063. doi: 10.1111/j.1365-2443.2005.00900.x. [DOI] [PubMed] [Google Scholar]
- 47.Phipatanakul W, et al. Effects of age and disease severity on systemic corticosteroid responses in asthma. Am J Respir Crit Care Med. 2017;195(11):1439–1448. doi: 10.1164/rccm.201607-1453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung KF, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi: 10.1183/09031936.00202013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.