Abstract
Topical therapy as monotherapy is useful in psoriasis patients with mild disease. Topical agents are also used as adjuvant for moderate-to-severe disease who are being concurrently treated with either ultraviolet light or systemic medications. Emollients are useful adjuncts to the treatment of psoriasis. Use of older topical agents such as anthralin and coal tar has declined over the years. However, they are cheaper and can still be used for the treatment of difficult psoriasis refractory to conventional treatment. Salicylic acid can be used in combination with other topical therapies such as topical corticosteroids (TCS) and calcineurin inhibitors for the treatment of thick limited plaques to increase the absorption of the latter into the psoriatic plaques. Low- to mid-potent TCS are used in facial/flexural psoriasis and high potent over palmoplantar/thick psoriasis lesions. The addition of noncorticosteroid treatment can also facilitate the avoidance of long-term daily TCS. Tacrolimus and pimecrolimus can be used for the treatment of facial and intertriginous psoriasis. Tazarotene is indicated for stable plaque psoriasis usually in combination with other therapies such as TCS. Vitamin D analogs alone in combination with TCS are useful in stable plaques over limbs and palmoplantar psoriasis. Topical therapies for scalp psoriasis include TCS, Vitamin D analogs, salicylic acid, coal tar, and anthralin in various formulations such as solutions, foams, and shampoos. TCS, vitamin D analogs, and tazarotene can be used in the treatment of nail psoriasis.
Keywords: Psoriasis, therapeutic guidelines, topical therapy
Introduction
In India, the prevalence of psoriasis varies from 0.44%–2.8%.[1] The majority of these patients have mild-to-moderate disease and can be treated with topical agents which provide potential therapeutic efficacy and limit the adverse effects of the systemic treatment to the target tissue.
Aim of Therapy
The aim of the therapy is to minimize the extent and severity of psoriasis to the point at which it is no longer detrimental to a patient's quality of life.
Indications
Topical therapy is the treatment of choice in patients with psoriasis affecting < 10% body surface area (BSA) (mild psoriasis).[2]
It can also be used for psoriasis affecting sensitive areas such as the face, flexures, and genitals.
Topical agents are also used as adjuvant for:
Psoriasis affecting >10% BSA (moderate/severe psoriasis) on ultraviolet (UV) light or systemic medications
Refractory palmoplantar or scalp psoriasis.
Factors Which Influence Topical Therapy
Patient factors
Treatment regimens must be individualized according to the patient's age, sex, occupation, understanding, and the available resources.
Disease factors
Treatment also depends on the site of the lesions and their extent and severity. Assessment of severity should include the patient's own perception of disability, the need for treatment, and an objective assessment of extent and severity.
Vehicle
The choice of vehicle can significantly alter the use and penetration of medications, and hence the therapeutic effect. There is a vast array of vehicles including creams, gels, solutions, foams, sprays, shampoos, and lotions. Different vehicles are indicated for different body sites. Scalp is commonly involved in psoriasis and requires gel, solutions, or foams that are not as messy as ointments and creams. Elsewhere, patients may prefer a less greasy preparation such as a cream during the daytime, and an ointment which is more effective but less cosmetically appealing at night.
Occlusion
Occlusive therapy, in which the skin is covered, often with a plastic membrane, enhances the penetration of topical agents such as corticosteroids. The occlusive dressings trap heat and moisture, hydrating and macerating the skin and forcing the medication through the plaques.[3,4,5]
Combination therapy
Combination therapy may be indicated when monotherapy fails, for example, the combination of super potent steroids and calcipotriene.[6,7] However, when using multiple topical agents, it is important to be aware of possible compatibility issues, for example, salicylic acid inactivates calcipotriene.[8] On the other hand, anthralin requires salicylic acid for its chemical stability.[9] When it is desirable to use multiple topical agents, patients may be instructed to apply the various medications at separate times throughout the day.
Topical agents can be used intermittently or continuously. More potent agents must be used on a short-term basis to allow for response, and then patients should be instructed to use these agents intermittently for long-term management. This strategy may reduce the risk of side effects.
Alternatively, patients who require continuous topical therapy should be instructed to use the least potent agent that allows for disease control or be transitioned to a topical agent that is associated with the lowest long-term risk.
All patients on topical therapy should be examined regularly to look for the development of side effects at the earliest.
Adherence to Therapy
Adherence to topical treatment is a major issue, being generally poor in the majority of the patients. In compliance studies, 39% of the patients admitted to nonadherence with topical therapy.[10] Adherence has been seen to improve with simple regimens and once a day therapy. Moreover, realistic treatment outcomes should be discussed with patients, and they should be encouraged to participate in decision making.
Emollients and Moisturizers
Emollients form the backbone of therapy for psoriasis. They are a valuable first-line treatment because dry skin is common in psoriasis and adds to its irritability.
Mechanism of action
Moisturizers help in normalizing hyperproliferation, differentiation, and apoptosis. They have anti-inflammatory effects in addition improving barrier function. This helps in combating the stresses generated in the skin and Koebner's phenomenon.[11]
Hence, emollients moisturize dry skin, reduce scaling, ease itching, soften cracks, and improve penetration of other topical agents.[12] They may also slow down the rate of epidermal turnover.
Efficacy
Emollients do not work as a monotherapy but as an adjuvant to topical/systemic therapy.
There are two randomized, placebo-controlled trials of aloe vera gel for the treatment of mild-to-moderate psoriasis with one showing better efficacy compared to placebo[13] and the other showing no benefit over placebo[14]
It has been reported for chronic plaque psoriasis that water-in-oil emollients are useful as steroid-sparing agent.[15] The hydration of the stratum corneum leads to an enhanced delivery of topical corticosteroids (TCS).
Vehicles and preparations
Emollients come in many different forms such as creams, ointments, lotion, bath oils, and soap substitutes. Creams and ointments are preferable to lotions. They tend to be thicker, more occlusive, and therefore more effective.[15] Ointments are more suited for extra dry, thickened, or brittle skin and can be used at night. Lighter, less greasy creams or lotions are ideal for daytime use. Creams, ointments, or lotions should be used liberally and frequently such that the skin does not dry out (dehydrating). The commonly used emollients are petroleum jelly, liquid paraffin, and mineral oils.
Adverse effects
Emollients can cause a few side effects such as irritant dermatitis, allergic contact dermatitis, fragrance allergy, stinging, cosmetic acne, and pigmentary disorders.[16]
There are no contraindications to the use of emollients. These are considered to be safe during pregnancy and lactation as well as for pediatric use.
Anthralin (Dithranol)
Over the years, anthralin has been shown to be one of the most effective topical treatments of stable plaque psoriasis. It may be used as a Short contact anthralin therapy (SCAT) for the treatment of localized, scaly plaques of psoriasis on the body or the scalp that have not cleared with other treatments.
Mechanism of action
Anthralin reduces keratinocyte proliferation, prevents T-cell activation, and restores cell differentiation, probably through mitochondrial dysfunction.[17] In addition, production of free radicals may contribute to its effect.[18]
Modes of application of anthralin
Although anthralin has been used as an overnight therapy, we recommend that it be used only as SCAT in which high concentration of anthralin, 1% or greater, is applied for approximately 20 min to 1 h before removal.[19]
In another regimen, anthralin in a concentration of 1% or greater is applied for 5 min on the 1st day. Daily applications continue with increases up to 5 min every other day until patients develop mild irritation. The period of application is then maintained until clearing.
Efficacy
There is limited placebo-controlled trial data. In a systematic review of topical preparations for the treatment of psoriasis, it was found that dithranol reduced psoriasis severity scores at 4–8 weeks significantly more than placebo.[20] Short contact (30 min) and overnight therapy with 1%, 2%, and 3% dithranol ointment have shown similar efficacy, suggesting SCAT to be more convenient and practical.[21] Anthralin, similar to monotherapy, appears to have lower efficacy than more potent TCS or Vitamin D derivatives.[22] Other randomized controlled trials have found that Vitamin D analogs are equally effective as short contact dithranol regimen but with better tolerability and acceptability of Vitamin D analogs.[23,24]
Adverse effects
The most common side effect of anthralin is skin irritation, which is dose related. Anthralin also stains lesional and adjoining skin, hair, nails, clothing, and other objects, with which the patients come into contact.[25] Attempts to reduce these using low concentrations SCAT and concomitant steroid therapy have been only partially successful. Triethanolamine applied after the removal of anthralin, prevents staining and irritation by neutralizing any anthralin residue remaining on the skin.[26] Chlorine bleach can be used to remove stains from household items.[27] If the psoriatic plaques are well-defined, the surrounding normal skin can be protected by the use of an agent such as zinc oxide paste. It should be applied with caution to the face and intertriginous areas due to the risk of skin irritation.
No systemic toxicity has been reported even following long-term application of anthralin. Anthralin is pregnancy Category C.
For children: Use with caution.
Salicylic acid is frequently added to improve the stability of anthralin and to increase its penetration and efficacy.[28]
Preparations
Derobin ointment containing dithranol 1.15%, coal tar solution 5.3%, salicylic acid 1.15%, and petrolatum base
Micanol is a 1% anthralin formulation in a temperature sensitive vehicle (microcapsules).[29] It is removed by washing with cold water which leads to re-crystallization. It is effective in short and long contact regimens. It is particularly useful for scalp psoriasis
New formulations in emulsifying ointment base with 0.1% w/w ascorbyl palmitate as an anti-oxidant are stable for up to 52 weeks[30]
Dithranol, in an emulsifying oil base (bio-wash oil), is useful for scalp psoriasis[31]
Liposomal dithranol (lipogel) is an effective formulation with less irritation and staining.[32]
Coal Tar
Coal tar is frequently used as a part of an inpatient or daily dressing regimen. Its use in conjunction with UVB, the Goeckerman regimen, is well recognized.[33] Coal tar probably suppresses DNA synthesis, thereby reducing the hyperproliferation of keratinocytes.[34]
Efficacy
The success of coal tar has been demonstrated on chronic plaque psoriasis, palmoplantar psoriasis, and scalp psoriasis. Most chronic plaques improve after 1 month and patients remain in remission for longer than that with other psoriasis topical treatments.[35]
A recent Cochrane review of efficacy of coal tar preparations supports the use of coal tar products in the treatment of psoriasis, although the level of evidence is not strong. Coal tar is well tolerated with a few side effects[36]
Coal tar was as effective as calcipotriol after 12 weeks of treatment in a prospective study, however, Vitamin D analog was better tolerated and had a faster onset of action[37]
Similarly, Khandpur et al. compared the efficacy of coal tar–salicylic acid ointment versus calcipotriol/betamethasone dipropionate (BMD) ointment and concluded that both the treatment modalities were comparable in efficacy at the end of 12 weeks, although the calcipotriol/BMD ointment had quicker onset of action[38]
Other comparative studies have found calcipotriol superior to coal tar treatment.[39]
Side effects
The use of tar has waned due to its poor side effects profile and superior efficacy of alternative topical therapies.[40]
Adverse effects of coal tar include odor, staining, irritant contact dermatitis, erythema, stinging, folliculitis, and formation of keratoacanthomas.[41]
Occupational coal tar exposure is a recognized carcinogen. However, there is no evidence of carcinogenesis reported in patients with psoriasis who have had treatment with coal tar.[42,43]
Treatment with PUVA and coal tar is not recommended due to the risk of skin cancer.[43]
Coal tar (Category C) may be used during pregnancy.[44]
Coal tar should be used with caution in the pediatric population.
Newer formulations of coal tar
Recently new formulations of coal tar, ranging from 1% to 15% have been developed. They are claimed to be nonstaining, nonodorous, and easily spreadable. Their efficacy is superior to conventional coal tar preparations and equal to topical ultrapotent steroid preparations.[45,46,47]
Salicylic Acid
Salicylic acid is a topical keratolytic agent that has been used for many years in the topical treatment of psoriasis. It can be used in combination with TCS and calcineurin inhibitors to increase the absorption of the latter into the psoriatic plaques.
Mode of action
Salicylic acid leads to desquamation of corneocytes through two pathways. It reduces intercellular cohesiveness of the horny cells by dissolving the intercellular cement material. Moreover, it reduces the pH of the stratum corneum, thereby increasing hydration and softening.[48]
Efficacy
While there are no placebo-controlled studies verifying the efficacy and safety of salicylic acid used alone, salicylic acid is often combined with other topical therapies, including TCS[49] and immunomodulators[50] in the therapy of psoriasis. Salicylic acid significantly increases the penetration rate of TCS because of its keratolytic effects.[49]
Steroid–salicylic acid combination can be used as first line of treatment on thick, scaly plaques.
Salicylic acid can be applied to areas with thick stratum corneum including palms, soles, and scalp. It can also be used on the trunk. Its use should be avoided on genitals, the mucous membrane, and the eyes.
Tiplica et al. found that the combination of mometasone furoate (0.1%) and salicylic acid (5%) was more effective than monotherapy with mometasone furoate (0.1%).[49]
The addition of salicylic acid to dithranol formulations improves the clinical efficacy of dithranol because of the antioxidant properties of salicylic acid.[51]
It can be applied as a paste or in creams, ointments, and lotions in concentration of 2%–6%.
Adverse effects
The major problem in the topical treatment of psoriasis with salicylic acid is the potential chronic or acute systemic intoxication with the symptoms of oral mucosa burning, frontal headache, central nervous system symptoms, metabolic acidosis, tinnitus, nausea, and vomiting.[52,53]
These symptoms may occur in the topical treatment of large body surfaces, especially in children.[54,55] Even lethal cases are reported;[56] therefore, a concentration greater than 10% and an application on larger surfaces, especially in children, are not suitable. Salicylic acid should not be applied to more than 20% of the BSA.
If larger surfaces require a salicylic acid treatment for initial keratolysis, a sequential treatment is useful (e.g., affected areas in the upper part of the body at night and the lower part of the body in the morning). Careful clinical monitoring helps to avoid salicylic acid intoxication.
It should be noted calcipotriol is inactivated by salicylic acid.[57]
Salicylic acid blocks UV light, and therefore should be applied after phototherapy.[48]
Pregnancy and children
Salicylic acid can be safely used for localized psoriasis in pregnancy; however, because of a greater risk of systemic absorption and toxicity, salicylic acid should be avoided in children.
Topical Immunomodulators
Calcineurin inhibitors
Calcineurin inhibitors are approved for use in mild-to-moderate atopic dermatitis only; any use in psoriasis is off label.
Mechanism of action
Calcineurin inhibitors inhibit the action of calcineurin phosphatase and block the production of inflammatory substances that are thought to be important in causing skin lesions in psoriasis.[58]
There are two topical preparations of calcineurin inhibitors, namely, tacrolimus ointment (0.03% and 0.1%) and pimecrolimus cream (1%).
Tacrolimus and pimecrolimus
Neither tacrolimus ointment[59] nor pimecrolimus cream[60] appears to be effective for treating plaque-type psoriasis when simply applied as commercially available preparations. This is probably because of large molecular size and the inability to penetrate thick, psoriatic plaques. However, the penetration can be enhanced by occlusion[60] or by combining these agents with salicylic acid.[50]
Efficacy
A recent review of the efficacy of topical calcineurin inhibitors (TCIs) in psoriasis has concluded that topical tacrolimus (0.1%), and to a lesser extent, pimecrolimus (1%) have efficacy in the treatment of psoriasis. These are safe and effective in patients with facial, intertriginous, and genital psoriasis.[61]
However, they are less potent than 0.005% calcipotriol or 0.1% betamethasone valerate.[62]
Newer vehicle of topical tacrolimus, a 0.3% gel and 0.5% cream, have been developed to improve its penetration into thick psoriatic plaques.[63]
Adverse effects
The most common adverse event reported with the use of tacrolimus and pimecrolimus is a stinging sensation, which is usually transient.[61] Irritation is more significant with tacrolimus than pimecrolimus.
There is a theoretical risk of cancer with long-term use of TCI, and a black box warning has been added to the labels of these medications.[64] As a result, new Food and Drug Administration (FDA) recommendations state that topical immunomodulators should not be used as long-term treatment, over large surface areas, or in children under the age of 2 years.
Use of calcineurin inhibitors in pregnancy and pediatric population
Both topical tacrolimus and pimecrolimus have been labeled Category C, but are generally not considered teratogenic due to their low systemic absorption.[65] Both these medicines are found in human milk, and hence are not recommended for nursing mothers.
Topical tacrolimus (0.03%) ointment and topical pimecrolimus cream are approved for patients as young as 2 years with atopic dermatitis. There is minimal evidence regarding the safety and efficacy of these medications in young patients with psoriasis. A recent single-center, open label trial has demonstrated that tacrolimus ointment is an effective treatment for psoriasis on the face or intertriginous areas in children with minimal side effects.[66]
New calcineurin inhibitor
Sirolimus
It is a newly developed topical immunomodulator. It has recently been investigated as a possible treatment for chronic plaque psoriasis.[67]
Topical Retinoids
Tazarotene
Tazarotene, a Vitamin A derivative, is the first synthetically developed retinoid indicated for the topical treatment of psoriasis. It is available as a gel or cream at concentration of 0.1% or 0.05%. Tazarotene is indicated for stable plaque psoriasis usually in combination with TCS and calcipotriene. It has been demonstrated to be an effective maintenance therapy for psoriasis. Tazarotene under occlusion is also effective it the treatment of nail psoriasis.
Mechanism of action
Tazarotene selectively binds to β and γ retinoic acid on the cell membrane of keratinocytes and is then transported to the nucleus, altering transcription of genes in keratinocytes. This results in reduced epidermal hyperproliferation, normalizing keratinocyte differentiation, and decreasing inflammation.[68]
Efficacy
In a recent study, tazarotene 0.1% and 0.05% cream applied daily for 12 weeks was found to be more effective than vehicle in plaque psoriasis[69]
Tazarotene (0.1% and 0.05%) has been found to be as effective as flucinonide with greater remission rate and more irritation than the latter[70]
In a recent observer-blinded, randomized controlled trial conducted in India, 0.1% tazarotene cream was as effective as clobetasol propionate for palmoplantar psoriasis[71]
Its efficacy has been demonstrated to be comparable to calcipotriene 0.005% ointment[72] and 5% coal tar ointment[73]
The combination of tazarotene with mometasone demonstrated greater efficacy and more rapid and sustained improvement than corticosteroids monotherapy[74]
Once daily application of 0.1% tazarotene for 12–24 weeks has been shown to improve nail psoriasis including onycholysis, hyperkeratosis, pitting, and salmon patches.[75]
Adverse effects
The most common side effect of tazarotene is localized irritation.[69] The use of cream, low concentration, alternate day application and short contact (30–60 min) application may help alleviate such symptoms.[76] Concomitant use of TCS may also minimize symptoms.
Tazarotene must not be used in sensitive areas such as the face, flexures and genitals.[77]
The FDA has issued a caution regarding the use of tazarotene and exposure to sunlight, and patients are advised to use sunscreens when using tazarotene. When it is combined with phototherapy, the dosimetry might need to be lowered to prevent skin burning.
Use of tazarotene in pregnanacy and children
Tazarotene is contraindicated in pregnancy, Category X, because of the theoretical risk of tertogenicity.[65] Children tolerate topical retinoids very well. Topical retinoids are, therefore, considered safe and efficacious in the pediatric populations, although no specific trials have been conducted in children and it remains an off-label use of the medication.
New retinoids
Bexarotene
Bexarotene gel is being studied as a potential treatment for psoriasis. It selectively binds nuclear retinoid X receptor.
Bexarotene gel 1% has been found to be effective in treating mild-to-moderate plaque psoriasis as monotherapy[78] as well as in combination with narrowband UVB therapy.[79]
Topical Corticosteroids
TCS are universally used in the management of all grades of psoriasis. They are used as monotherapy when the disease is mild and as a complement to systemic therapy in patients with moderate-to-severe psoriasis.
Mechanism of action
Corticosteroids are vasoconstrictive, antiproliferative, anti-inflammatory, and immunosuppressive. They bind to intracellular corticosteroid receptor and regulate gene transcription of numerous genes, particularly those that code for proinflammatory cytokines.
Selecting potency of topical corticosteroids
While selecting the potency of corticosteroids and its vehicle, one should take into consideration the disease severity, the site being treated, patient preference, as well as the age of the patient. Penetration correlates inversely with the thickness of the stratum corneum[80] and is greatly increased on areas such as the scrotum and face [Table 1].[81]
Table 1.
Selecting potency of topical corticosteroids and vehicle according to the site involved
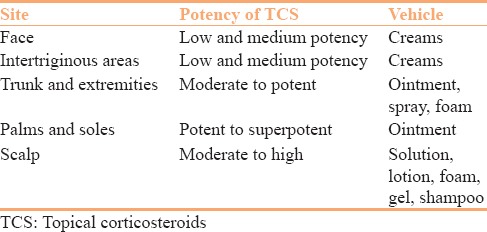
Potent and superpotent TCS may be used for stubborn, thick, and chronic plaques.
Efficacy
Clinical efficacy of Class I TCS such as clobetasol and halobetasol is well established in the treatment of plaque psoriasis.[82,83] Treatment is generally twice a day for approximately 2 weeks followed by an intermittent dosing regimen to preserve remission. Similarly, midpotent TCS such as fluticasone propionate has also been found to be effective in plaque psoriasis.[84]
Different regimens have been tried for maintenance therapy with TCS. Three consecutive applications (12 h apart) once a week therapy with BMD ointment has been used for maintenance of remission.[85] Another maintenance regimen of clobetasol propionate twice weekly achieved remission for an average of 4 months in 75% of treated patients.[86]
TCS in the form of lotions, solutions, and foams are effective in scalp psoriasis.[75,87,88]
Potent topical steroids such as clobetasol propionate can be used for the treatment of psoriasis involving nail bed and/or nail matrix.[75]
Limitations
TCS are often used for longer period. This can lead to tachyphylaxis.
The side effects of TCS[89] include skin atrophy striae, telangiectasia, or secondary infection. Therefore, potent TCS should not be used on the face or intertriginous sites. Systemic adverse events occur when TCS are used for prolonged periods of time or at doses higher than commonly prescribed. Prolonged use of potent TCS may result in its significant systemic absorption which can lead to HPA axis suppression, Cushing's syndrome, and hyperglycemia.
Another possible concern is the rebound phenomenon after abrupt discontinuation of TCS.
TCS usage in higher doses can lead to a pustular flare of psoriasis on their discontinuation.
Topical corticosteroids use in pregnancy and in pediatric population
TCS are labeled as pregnancy Category C.[90] Their safety in breastfeeding is unknown.
Infants and young children are at greater risk for side effects due to their higher skin surface to body mass ratio. Both growth retardation and suppression of the HPA axis have been documented in children more frequently than in older population.[91] As a result of systemic absorption, regular assessment of growth in children using TCS for long-term is recommended.
New vehicle formulations for topical corticosteroids
New formulations have been developed in the form of spray, shampoos, and foams in an effort to enhance the delivery of TCS. All of the newer topical clobetasol propionate formulations produce clearing of psoriasis for a large proportion of patients within 2–4 weeks with response, safety, and tolerability rates that are at least comparable to the conventional TCS creams and ointments. The spray and the foam may have an important role in the treatment of widespread plaque psoriasis while the shampoo and the foam would be useful in scalp psoriasis.[92,93] Eight percent clobetasol propionate in a nail lacquer vehicle has been formulated for treatment of nail psoriasis.[91]
Vitamin D Analogs
Vitamin D analogs provide a useful adjunct in the treatment of chronic plaque psoriasis. They are also useful in the treatment of nail psoriasis and chronic plaque psoriasis of the scalp. It should be used under occlusion for nail psoriasis. Synthetic Vitamin D analogs have been developed (by modification of the side chain) to enhance the antipsoriatic effect of Vitamin D3 and reduce their hypercalcemic action (because they are rapidly transformed into inactive metabolites).
Mechanism of action
Vitamin D analogs bind to the intracellular Vitamin D receptor which then binds to and regulates the genes involved in epidermal proliferation, inflammation, and keratinization.[94]
There are three Vitamin D analog preparations available to treat psoriasis, namely, calcipotriene (calcipotriol in Europe), calcitriol, and tacalcitol.
Calcipotriene
It is a synthetic Vitamin D analog available as 0.005% (5 mg/g) cream, ointment, and scalp lotion. Although it is as potent as 1, 25 dihydroxycholecalciferol in the regulation of cell proliferation and differentiation, it is at least 200 times less potent in its effects on calcium metabolism.[94]
Efficacy
It has similar efficacy to Class 2 and 3 TCS but has relatively fewer side effects. In comparison to superpotent TCS, calcipotriene has delayed onset of action but results in longer disease-free interval.[95] As monotherapy, the maximal response usually requires 2 months of therapy.[96]
The Cochrane systematic review provided evidence that twice daily calcipotriene is a safe and effective treatment for plaque psoriasis[97]
It is more effective than other Vitamin D analogs (tacalcitol or calcitriol), short contact dithranol therapy,[97] and 15% coal tar[39]
Calcipotriene solution has been found to be effective for the treatment of scalp psoriasis[98]
Combination of the calcipotriene and TCS in ointment and solution forms has proven superior to either agent used alone[99]
A new calcipotriene/BMD aerosol foam formulation has been found to be safe and effective in patients with extensive psoriasis vulgaris.[100]
Calcitriol
It is a synthetic form of the active metabolite of Vitamin D.[96] It is available as an ointment only (3 mg/g).
Efficacy
Multicenter and randomized clinical trials have demonstrated long-term safety and efficacy of caolcitriol.[101] It is less potent than BMD, 0.05% but induces longer remission than the latter.
Tacalcitol
It is a synthetic Vitamin D analog available as 4 mg/g ointment and lotion, applied once daily.
Efficacy
Clinical studies have shown tacalcitol ointment to be a safe and effective long-term treatment for chronic plaque psoriasis with no systemic side effects and good tolerability in sensitive areas.[102] It is, however, less potent than calcipotriene.[103] The combination with clobetasol propionate in lacquer for the treatment of nail psoriasis where tacalcitol ointment under occlusion was applied twice daily on weekdays and the steroid lacquer on weekends has been found to be effective.[75]
Adverse effects
Vitamin D analogs are relatively safe with few side effects. The most common adverse effect is skin irritation on or around the psoriasis plaques.[102] Face and intertriginous areas are especially prone to irritation. Calcitriol and tacalcitol have a better tolerability on sensitive areas as compared to calcipotriene, and therefore serve as better options in these areas.[102,104]
Systemic side effects such as hypercalcemia, hypercalciuria, and parathyroid hormone suppression are very rare if the maximum dose is not exceeded: 100 g/week for calcipotriene, 210 g/week for calcitriol, and 70 g/week for tacalcitol.[105]
Calcitriol may have greater effects on serum calcium in comparison to other analogs.[106]
Vitamin D analogs are contraindicated in patients already suffering from hypercalcemia. Patients with renal impairment need to be observed carefully.
Calcipotriene is a relatively unstable molecule that is inactivated by an acid pH.[107] It can be combined with halobetasol ointment or cream or with 5% tar gel, however, it is degraded when mixed with 6% salicylic acid, 12% ammonium lactate or hydrocortisone-17 valerate ointment.[108]
Topical Vitamin D analog use in pregnancy and pediatric population
Treatment with Vitamin D analogs during pregnancy is rated Category C.[65] Vitamin D analogs have not been found to be teratogenic in animals, although no clinical data on humans has been reported. The use of calcipotriene for the treatment of psoriasis in children is effective, and the dose should not exceed 50 g/week.[109]
Newer Vitamin D analogs
Newer Vitamin D analogs including maxacalcitol, paricalcitol, and becocalcidiol are being studied for the treatment of psoriasis. They appear to be promising drugs for the treatment of plaque psoriasis.[110,111,112]
Conclusion
Topical therapies are the backbone of management of psoriasis. They are safe and well tolerated by the patients. TCS and Vitamin D analogs are the treatment of choice among the various topical agents available for psoriasis. TCI can be used as steroid-sparing agents on the face and intertriginous areas. Tazarotene can be used as an effective maintenance therapy.
New vehicle formulations such as gels, lotions, solutions, shampoos, foams, etc., have been developed to improve the aesthetics, and thereby the patient compliance which is of utmost importance for optimum results.
New insights in the pathogenesis of psoriasis have enabled identification of new therapeutic targets. Target-based topical agents are being developed and tested. Moreover, advancement in nanotechnology has led to the possibility of improving the efficacy of topical agents and minimizing their side effects. The advent of newer molecules and newer drug delivery systems will significantly expand the therapeutic armamentarium for the treatment of psoriasis.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Dogra S, Yadav S. Psoriasis in India: Prevalence and pattern. Indian J Dermatol Venereol Leprol. 2010;76:595–601. doi: 10.4103/0378-6323.72443. [DOI] [PubMed] [Google Scholar]
- 2.van de Kerkhof PC, Barker J, Griffiths CE, Kragballe K, Mason J, Menter A, et al. Psoriasis: Consensus on topical therapies. J Eur Acad Dermatol Venereol. 2008;22:859–70. doi: 10.1111/j.1468-3083.2007.02534.x. [DOI] [PubMed] [Google Scholar]
- 3.Bourke JF, Berth-Jones J, Hutchinson PE. Occlusion enhances the efficacy of topical calcipotriol in the treatment of psoriasis vulgaris. Clin Exp Dermatol. 1993;18:504–6. doi: 10.1111/j.1365-2230.1993.tb01018.x. [DOI] [PubMed] [Google Scholar]
- 4.Patel T, Bhutani T, Busse KL, Koo J. Evaluating the efficacy and safety of calcipotriene/betamethasone ointment occluded with a hydrogel patch: A 6-week bilaterally controlled, investigator-blinded trial. Cutis. 2011;88:149–54. [PubMed] [Google Scholar]
- 5.Volden G, Kragballe K, van de Kerkhoffe PC, Aberg K, White RJ. Remission and relapse of chronic plaque psoriasis treated once a week with clobetasol propionate occluded with a hydrocolloid dressing v/s twice daily treatment with clobetasol propionate alone. J Dermatol Treat. 2001;12:141–4. doi: 10.1080/09546630152607862. [DOI] [PubMed] [Google Scholar]
- 6.Kaufmann R, Bibby AJ, Bissonnette R, Cambazard F, Chu AC, Decroix J, et al. A new calcipotriol/betamethasone dipropionate formulation (Daivobet) is an effective once-daily treatment for psoriasis vulgaris. Dermatology. 2002;205:389–93. doi: 10.1159/000066440. [DOI] [PubMed] [Google Scholar]
- 7.Vakirlis E, Kastanis A, Ioannides D. Calcipotriol/betamethasone dipropionate in the treatment of psoriasis vulgaris. Ther Clin Risk Manag. 2008;4:141–8. doi: 10.2147/tcrm.s1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Rosso J. Pharmacotherapy updates: Current therapies and research for common dermatologic conditions. The many roles of salicyclic acid. Skin Aging. 2005;13:38–42. [Google Scholar]
- 9.Green PG, Forbes DR, Kennedy CTC. The stability of dithranol in various bases. Br J Dermatol. 1985;113(s29):26. [Google Scholar]
- 10.van de Kerkhof PC, de Hoop D, de Korte J, Cobelens SA, Kuipers MV. Patient compliance and disease management in the treatment of psoriasis in the Netherlands. Dermatology. 2000;200:292–8. doi: 10.1159/000018390. [DOI] [PubMed] [Google Scholar]
- 11.Fluhr JW, Cavallotti C, Berardesca E. Emollients, moisturizers, and keratolytic agents in psoriasis. Clin Dermatol. 2008;26:380–6. doi: 10.1016/j.clindermatol.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Gelmetti C. Therapeutic moisturizers as adjuvant therapy for psoriasis patients. Am J Clin Dermatol. 2009;10(Suppl 1):7–12. doi: 10.2165/0128071-200910001-00002. [DOI] [PubMed] [Google Scholar]
- 13.Syed TA, Ahmad SA, Holt AH, Ahmad SA, Ahmad SH, Afzal M. Management of psoriasis with Aloe vera extract in a hydrophilic cream: A placebo-controlled, double-blind study. Trop Med Int Health. 1996;1:505–9. doi: 10.1046/j.1365-3156.1996.d01-91.x. [DOI] [PubMed] [Google Scholar]
- 14.Paulsen E, Korsholm L, Brandrup F. A double-blind, placebo-controlled study of a commercial Aloe vera gel in the treatment of slight to moderate psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2005;19:326–31. doi: 10.1111/j.1468-3083.2004.01186.x. [DOI] [PubMed] [Google Scholar]
- 15.Witman PM. Topical therapies for localized psoriasis. Mayo Clin Proc. 2001;76:943–9. doi: 10.4065/76.9.943. [DOI] [PubMed] [Google Scholar]
- 16.Nola I, Kostovic K, Kotrulja L, Lugovic L. The use of emollients as sophisticated therapy in dermatology. Acta Dermatovenerol Croat. 2003;11:80–7. [PubMed] [Google Scholar]
- 17.McGill A, Frank A, Emmett N, Turnbull DM, Birch-Machin MA, Reynolds NJ. The anti-psoriatic drug anthralin accumulates in keratinocyte mitochondria, dissipates mitochondrial membrane potential, and induces apoptosis through a pathway dependent on respiratory competent mitochondria. FASEB J. 2005;19:1012–4. doi: 10.1096/fj.04-2664fje. [DOI] [PubMed] [Google Scholar]
- 18.Mahrle G. Dithranol. Clin Dermatol. 1997;15:723–37. doi: 10.1016/s0738-081x(97)00019-9. [DOI] [PubMed] [Google Scholar]
- 19.Fleischmann HE. Short-contact anthralin therapy for psoriasis. West J Med. 1987;147:461. [PMC free article] [PubMed] [Google Scholar]
- 20.Mason J, Mason AR, Cork MJ. Topical preparations for the treatment of psoriasis: A systematic review. Br J Dermatol. 2002;146:351–64. doi: 10.1046/j.1365-2133.2000.04713.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones SK, Campbell WC, Mackie RM. Out-patient treatment of psoriasis: Short contact and overnight dithranol therapy compared. Br J Dermatol. 1985;113:331–7. doi: 10.1111/j.1365-2133.1985.tb02086.x. [DOI] [PubMed] [Google Scholar]
- 22.Berth-Jones J, Chu AC, Dodd WA, Ganpule M, Griffiths WA, Haydey RP, et al. A multicentre, parallel-group comparison of calcipotriol ointment and short-contact dithranol therapy in chronic plaque psoriasis. Br J Dermatol. 1992;127:266–71. doi: 10.1111/j.1365-2133.1992.tb00126.x. [DOI] [PubMed] [Google Scholar]
- 23.Hutchinson PE, Marks R, White J. The efficacy, safety and tolerance of calcitriol 3 microg/g ointment in the treatment of plaque psoriasis: A comparison with short-contact dithranol. Dermatology. 2000;201:139–45. doi: 10.1159/000018457. [DOI] [PubMed] [Google Scholar]
- 24.de Korte J, van der Valk PG, Sprangers MA, Damstra RJ, Kunkeler AC, Lijnen RL, et al. A comparison of twice-daily calcipotriol ointment with once-daily short-contact dithranol cream therapy: Quality-of-life outcomes of a randomized controlled trial of supervised treatment of psoriasis in a day-care setting. Br J Dermatol. 2008;158:375–81. doi: 10.1111/j.1365-2133.2007.08337.x. [DOI] [PubMed] [Google Scholar]
- 25.Loffler H, Effendy I, Happle R. Skin susceptibility to dithranol: Contact allergy or irritation? Eur J Dermatol. 1999;9:32–4. [PubMed] [Google Scholar]
- 26.Ramsay B, Lawrence CM, Bruce JM, Shuster S. The effect of triethanolamine application on anthralin-induced inflammation and therapeutic effect in psoriasis. J Am Acad Dermatol. 1990;23:73–6. doi: 10.1016/0190-9622(90)70189-o. [DOI] [PubMed] [Google Scholar]
- 27.Wang JC, Krazmien RJ, Dahlheim CE, Patel B. Anthralin stain removal. J Am Acad Dermatol. 1986;15(5 Pt 1):951–5. doi: 10.1016/s0190-9622(86)70255-7. [DOI] [PubMed] [Google Scholar]
- 28.Kneczke M, Rahm C, Landersjö L. The influence of salicylic acid on the in vitro release of anthralin from an oil in water cream. Acta Pharm Nord. 1990;2:313–8. [PubMed] [Google Scholar]
- 29.Volden G, Björnberg A, Tegner E, Pedersen NB, Arlés UB, Agren S, et al. Short-contact treatment at home with micanol. Acta Derm Venereol. 1992;172:20–2. [PubMed] [Google Scholar]
- 30.Weller PJ, Newman CM, Middleton KR, Wicker SM. Stability of a novel dithranol ointment formulation, containing ascorbyl palmitate as an anti-oxidant. J Clin Pharm Ther. 1990;15:419–23. doi: 10.1111/j.1365-2710.1990.tb00406.x. [DOI] [PubMed] [Google Scholar]
- 31.Wulff-Woesten A, Ohlendorf D, Henz BM, Haas N. Dithranol in an emulsifying oil base (bio-wash-oil) for the treatment of psoriasis of the scalp. Skin Pharmacol Physiol. 2004;17:91–7. doi: 10.1159/000076019. [DOI] [PubMed] [Google Scholar]
- 32.Saraswat A, Agarwal R, Katare OP, Kaur I, Kumar B. A randomized, double-blind, vehicle-controlled study of a novel liposomal dithranol formulation in psoriasis. J Dermatolog Treat. 2007;18:40–5. doi: 10.1080/09546630601028729. [DOI] [PubMed] [Google Scholar]
- 33.Goeckerman W. Treatment of psoriasis. Northwest Med. 1925;24:229–31. [Google Scholar]
- 34.Arbiser JL, Govindarajan B, Battle TE, Lynch R, Frank DA, Ushio-Fukai M, et al. Carbazole is a naturally occurring inhibitor of angiogenesis and inflammation isolated from antipsoriatic coal tar. J Invest Dermatol. 2006;126:1396–402. doi: 10.1038/sj.jid.5700276. [DOI] [PubMed] [Google Scholar]
- 35.Koo J, Lebwohl M. Duration of remission of psoriasis therapies. J Am Acad Dermatol. 1999;41:51–9. doi: 10.1016/s0190-9622(99)70406-8. [DOI] [PubMed] [Google Scholar]
- 36.Slutsky JB, Clark RA, Remedios AA, Klein PA. An evidence-based review of the efficacy of coal tar preparations in the treatment of psoriasis and atopic dermatitis. J Drugs Dermatol. 2010;9:1258–64. [PubMed] [Google Scholar]
- 37.Sharma V, Kaur I, Kumar B. Calcipotriol versus coal tar: A prospective randomized study in stable plaque psoriasis. Int J Dermatol. 2003;42:834–8. doi: 10.1046/j.1365-4362.2003.01974.x. [DOI] [PubMed] [Google Scholar]
- 38.Khandpur S, Sahni K. An open label prospective randomized trial to compare the efficacy of coal tar-salicylic acid ointment versus calcipotriol/betamethasone dipropionate ointment in the treatment of limited chronic plaque psoriasis. Indian J Dermatol. 2014;59:579–83. doi: 10.4103/0019-5154.143523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tham SN, Lun KC, Cheong WK. A comparative study of calcipotriol ointment and tar in chronic plaque psoriasis. Br J Dermatol. 1994;131:673–7. doi: 10.1111/j.1365-2133.1994.tb04981.x. [DOI] [PubMed] [Google Scholar]
- 40.Lebwohl M, Ali S. Treatment of psoriasis. Part 1. Topical therapy and phototherapy. J Am Acad Dermatol. 2001;45:487–98. doi: 10.1067/mjd.2001.117046. [DOI] [PubMed] [Google Scholar]
- 41.Lin AN, Moses K. Tar revisited. Int J Dermatol. 1985;24:216–8. doi: 10.1111/j.1365-4362.1985.tb05762.x. [DOI] [PubMed] [Google Scholar]
- 42.Maughan WZ, Muller SA, Perry HO, Pittelkow MR, O'Brien PC. Incidence of skin cancers in patients with atopic dermatitis treated with coal tar. A 25-year follow-up study. J Am Acad Dermatol. 1980;3:612–5. doi: 10.1016/s0190-9622(80)80075-2. [DOI] [PubMed] [Google Scholar]
- 43.Stern RS, Zierler S, Parrish JA. Skin carcinoma in patients with psoriasis treated with topical tar and artificial ultraviolet radiation. Lancet. 1980;1:732–5. doi: 10.1016/s0140-6736(80)91231-3. [DOI] [PubMed] [Google Scholar]
- 44.Lam J, Polifka JE, Dohil MA. Safety of dermatologic drugs used in pregnant patients with psoriasis and other inflammatory skin diseases. J Am Acad Dermatol. 2008;59:295–315. doi: 10.1016/j.jaad.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Frankel AJ, Zeichner JA, Del Rosso JQ. Coal tar 2% foam in combination with a superpotent corticosteroid foam for plaque psoriasis: Case report and clinical implications. J Clin Aesthet Dermatol. 2010;3:42–5. [PMC free article] [PubMed] [Google Scholar]
- 46.Alora-Palli MB, Perkins AC, Van Cott A, Kimball AB. Efficacy and tolerability of a cosmetically acceptable coal tar solution in the treatment of moderate plaque psoriasis: A controlled comparison with calcipotriene (calcipotriol) cream. J Clin Dermatol. 2010;11:275–83. doi: 10.2165/11530380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 47.Katare OP, Raza K, Singh B, Dogra S. Novel drug delivery systems in topical treatment of psoriasis: Rigors and vigors. Indian J Dermatol Venereol Leprol. 2010;76:612–21. doi: 10.4103/0378-6323.72451. [DOI] [PubMed] [Google Scholar]
- 48.Lebwohl M. The role of salicylic acid in the treatment of psoriasis. Int J Dermatol. 1999;38:16–24. doi: 10.1046/j.1365-4362.1999.00500.x. [DOI] [PubMed] [Google Scholar]
- 49.Tiplica GS, Salavastru CM. Mometasone furoate 0.1% and salicylic acid 5% vs. mometasone furoate 0.1% as sequential local therapy in psoriasis vulgaris. J Eur Acad Dermatol Venereol. 2009;23:905–12. doi: 10.1111/j.1468-3083.2009.03214.x. [DOI] [PubMed] [Google Scholar]
- 50.Carroll CL, Clarke J, Camacho F, Balkrishnan R, Feldman SR. Topical tacrolimus ointment combined with 6% salicylic acid gel for plaque psoriasis treatment. Arch Dermatol. 2005;141:43–6. doi: 10.1001/archderm.141.1.43. [DOI] [PubMed] [Google Scholar]
- 51.Runne U. Anthralin-salicylic acid therapy of psoriasis. Cignolin-salicylic acid-vaseline treatment and Lasan paste in a right-left comparison. Hautarzt. 1974;25:199–200. [PubMed] [Google Scholar]
- 52.Zesch A. Short and long-term risks of topical drugs. Br J Dermatol. 1986;115(Suppl 31):63–70. doi: 10.1111/j.1365-2133.1986.tb02111.x. [DOI] [PubMed] [Google Scholar]
- 53.Chapman BJ, Proudfoot AT. Adult salicylate poisoning: Deaths and outcome in patients with high plasma salicylate concentrations. Q J Med. 1989;72:699–707. [PubMed] [Google Scholar]
- 54.Péc J, Strmenová M, Palencárová E, Pullmann R, Funiaková S, Visnovský P, et al. Salicylate intoxication after use of topical salicylic acid ointment by a patient with psoriasis. Cutis. 1992;50:307–9. [PubMed] [Google Scholar]
- 55.Germann R, Schindera I, Kuch M, Seitz U, Altmeyer S, Schindera F. Life threatening salicylate poisoning caused by percutaneous absorption in severe ichthyosis vulgaris. Hautarzt. 1996;47:624–7. doi: 10.1007/s001050050479. [DOI] [PubMed] [Google Scholar]
- 56.Taylor JR, Halprin KM. Percutaneous absorption of salicylic acid. Arch Dermatol. 1975;111:740–3. [PubMed] [Google Scholar]
- 57.van de Kerkhof PC, Vissers WH. The topical treatment of psoriasis. Skin Pharmacol Appl Skin Physiol. 2003;16:69–83. doi: 10.1159/000069029. [DOI] [PubMed] [Google Scholar]
- 58.Marsland AM, Griffiths CE. The macrolide immunosuppressants in dermatology: Mechanisms of action. Eur J Dermatol. 2002;12:618–22. [PubMed] [Google Scholar]
- 59.Zonneveld IM, Rubins A, Jablonska S, Dobozy A, Ruzicka T, Kind P, et al. Topical tacrolimus is not effective in chronic plaque psoriasis. A pilot study. Arch Dermatol. 1998;134:1101–2. doi: 10.1001/archderm.134.9.1101. [DOI] [PubMed] [Google Scholar]
- 60.Gupta AK, Chow M. Pimecrolimus: A review. J Eur Acad Dermatol Venereol. 2003;17:493–503. doi: 10.1046/j.1468-3083.2003.00692.x. [DOI] [PubMed] [Google Scholar]
- 61.Wang C, Lin A. Efficacy of topical calcineurin inhibitors in psoriasis. J Cutan Med Surg. 2014;18:8–14. doi: 10.2310/7750.2013.13059. [DOI] [PubMed] [Google Scholar]
- 62.Kreuter A, Sommer A, Hyun J, Bräutigam M, Brockmeyer NH, Altmeyer P, et al. 1% pimecrolimus, 0.005% calcipotriol, and 0.1% betamethasone in the treatment of intertriginous psoriasis: A double-blind, randomized controlled study. Arch Dermatol. 2006;142:1138–43. doi: 10.1001/archderm.142.9.1138. [DOI] [PubMed] [Google Scholar]
- 63.Ortonne JP, van de Kerkhof PC, Prinz JC, Bieber T, Lahfa M, Rubins A, et al. 0.3% tacrolimus gel and 0.5% tacrolimus cream show efficacy in mild to moderate plaque psoriasis: Results of a randomized, open-label, observer-blinded study. Acta Derm Venereol. 2006;86:29–33. doi: 10.1080/00015550510039817. [DOI] [PubMed] [Google Scholar]
- 64.Lebwohl M, Gower T. A safety assessment of topical calcineurin inhibitors in the treatment of atopic dermatitis. MedGenMed. 2006;8:8. [PMC free article] [PubMed] [Google Scholar]
- 65.Tauscher AE, Fleischer AB, Jr, Phelps KC, Feldman SR. Psoriasis and pregnancy. J Cutan Med Surg. 2002;6:561–70. doi: 10.1007/s10227-001-0147-1. [DOI] [PubMed] [Google Scholar]
- 66.Brune A, Miller DW, Lin P, Cotrim-Russi D, Paller AS. Tacrolimus ointment is effective for psoriasis on the face and intertriginous areas in pediatric patients. Pediatr Dermatol. 2007;24:76–80. doi: 10.1111/j.1525-1470.2007.00341.x. [DOI] [PubMed] [Google Scholar]
- 67.Ormerod AD, Shah SA, Copeland P, Omar G, Winfield A. Treatment of psoriasis with topical sirolimus: Preclinical development and a randomized, double-blind trial. Br J Dermatol. 2005;152:758–64. doi: 10.1111/j.1365-2133.2005.06438.x. [DOI] [PubMed] [Google Scholar]
- 68.Duvic M, Asano AT, Hager C, Mays S. The pathogenesis of psoriasis and the mechanism of action of tazarotene. J Am Acad Dermatol. 1998;39(4 Pt 2):S129–33. doi: 10.1016/s0190-9622(98)70309-3. [DOI] [PubMed] [Google Scholar]
- 69.Weinstein GD, Koo JY, Krueger GG, Lebwohl MG, Lowe NJ, Menter MA, et al. Tazarotene cream in the treatment of psoriasis: Two multicenter, double-blind, randomized, vehicle-controlled studies of the safety and efficacy of tazarotene creams 0.05% and 0.1% applied once daily for 12 weeks. J Am Acad Dermatol. 2003;48:760–7. doi: 10.1067/mjd.2003.103. [DOI] [PubMed] [Google Scholar]
- 70.Lebwohl M, Ast E, Callen JP, Cullen SI, Hong SR, Kulp-Shorten CL, et al. Once-daily tazarotene gel versus twice-daily fluocinonide cream in the treatment of plaque psoriasis. J Am Acad Dermatol. 1998;38(5 Pt 1):705–11. doi: 10.1016/s0190-9622(98)70594-8. [DOI] [PubMed] [Google Scholar]
- 71.Mehta BH, Amladi ST. Evaluation of topical 0.1% tazarotene cream in the treatment of palmoplantar psoriasis: An observer-blinded randomized controlled study. Indian J Dermatol. 2011;56:40–3. doi: 10.4103/0019-5154.77550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaur I, Dogra S, Jain R, Kumar B. Comparative study of calcipotriol (0.005%) ointment and tazarotene (0.05% and 0.1%) gel in the treatment of stable plaque psoriasis. Indian J Dermatol Venereol Leprol. 2008;74:471–4. doi: 10.4103/0378-6323.44302. [DOI] [PubMed] [Google Scholar]
- 73.Kumar U, Kaur I, Dogra S, De D, Kumar B. Topical tazarotene vs. coal tar in stable plaque psoriasis. Clin Exp Dermatol. 2010;35:482–6. doi: 10.1111/j.1365-2230.2009.03610.x. [DOI] [PubMed] [Google Scholar]
- 74.Koo JY, Martin D. Investigator-masked comparison of tazarotene gel q.d. plus mometasone furoate cream q.d. vs. mometasone furoate cream b.i.d. in the treatment of plaque psoriasis. Int J Dermatol. 2001;40:210–2. doi: 10.1046/j.1365-4362.2001.01167.x. [DOI] [PubMed] [Google Scholar]
- 75.Handa S. Newer trends in the management of psoriasis at difficult to treat locations: Scalp, palmoplantar disease and nails. Indian J Dermatol Venereol Leprol. 2010;76:634–44. doi: 10.4103/0378-6323.72455. [DOI] [PubMed] [Google Scholar]
- 76.Veraldi S, Caputo R, Pacifico A, Peris K, Soda R, Chimenti S. Short contact therapy with tazarotene in psoriasis vulgaris. Dermatology. 2006;212:235–7. doi: 10.1159/000091250. [DOI] [PubMed] [Google Scholar]
- 77.Wollina U. Genital ulcers in a psoriasis patient using topical tazarotene. Br J Dermatol. 1998;138:713–4. doi: 10.1046/j.1365-2133.1998.02199.x. [DOI] [PubMed] [Google Scholar]
- 78.Breneman D, Sheth P, Berger V, Naini V, Stevens V. Phase II clinical trial of bexarotene gel 1% in psoriasis. J Drugs Dermatol. 2007;6:501–6. [PubMed] [Google Scholar]
- 79.Magliocco MA, Pandya K, Dombrovsky V, Christiansen L, Wong Y, Gottlicb AB. A randomized double blinded, vehicle- controlled, bilateral comparison trail of baxarotene gel 1% versus vehicle gel in combination with NBUVB phototreatment for moderate to severe psoriasis vulgaris. J Am Assoc Dermatol. 2006;54:115–8. doi: 10.1016/j.jaad.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 80.Vickers CF. Existence of reservoir in the stratum corneum. Experimental proof. Arch Dermatol. 1963;88:20–3. doi: 10.1001/archderm.1963.01590190026002. [DOI] [PubMed] [Google Scholar]
- 81.Feldmann RJ, Maibach HI. Regional variation in percutaneous penetration of 14C cortisol in man. J Invest Dermatol. 1967;48:181–3. doi: 10.1038/jid.1967.29. [DOI] [PubMed] [Google Scholar]
- 82.Bernhard J, Whitmore C, Guzzo C, Kantor I, Kalb RE, Ellis C, et al. Evaluation of halobetasol propionate ointment in the treatment of plaque psoriasis: Report on two double-blind, vehicle-controlled studies. J Am Acad Dermatol. 1991;25(6 Pt 2):1170–4. doi: 10.1016/0190-9622(91)70320-2. [DOI] [PubMed] [Google Scholar]
- 83.Blum G, Yawalkar S. A comparative, multicenter, double blind trial of 0.05% halobetasol propionate ointment and 0.1% betamethasone valerate ointment in the treatment of patients with chronic, localized plaque psoriasis. J Am Acad Dermatol. 1991;25(6 Pt 2):1153–6. doi: 10.1016/0190-9622(91)70315-s. [DOI] [PubMed] [Google Scholar]
- 84.Olsen EA. Efficacy and safety of fluticasone propionate 0.005% ointment in the treatment of psoriasis. Cutis. 1996;57(2 Suppl):57–61. [PubMed] [Google Scholar]
- 85.Katz HI, Prawer SE, Medansky RS, Krueger GG, Mooney JJ, Jones ML, et al. Intermittent corticosteroid maintenance treatment of psoriasis: A double-blind multicenter trial of augmented betamethasone dipropionate ointment in a pulse dose treatment regimen. Dermatologica. 1991;183:269–74. doi: 10.1159/000247698. [DOI] [PubMed] [Google Scholar]
- 86.Svartholm H, Larsson L, Frederiksen B. Intermittent topical treatment of psoriasis with clobetasol propionate. Curr Med Res Opin. 1982;8:154–7. doi: 10.1185/03007998209112377. [DOI] [PubMed] [Google Scholar]
- 87.Olsen EA, Cram DL, Ellis CN, Hickman JG, Jacobson C, Jenkins EE, et al. A double-blind, vehicle-controlled study of clobetasol propionate 0.05% (Temovate) scalp application in the treatment of moderate to severe scalp psoriasis. J Am Acad Dermatol. 1991;24:443–7. doi: 10.1016/0190-9622(91)70069-e. [DOI] [PubMed] [Google Scholar]
- 88.Franz TJ, Parsell DA, Halualani RM, Hannigan JF, Kalbach JP, Harkonen WS. Betamethasone valerate foam 0.12%: A novel vehicle with enhanced delivery and efficacy. Int J Dermatol. 1999;38:628–32. doi: 10.1046/j.1365-4362.1999.00782.x. [DOI] [PubMed] [Google Scholar]
- 89.Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol. 2006;54:1–15. doi: 10.1016/j.jaad.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 90.Chi CC, Wang SH, Kirtschig G, Wojnarowska F. Systematic review of the safety of topical corticosteroids in pregnancy. J Am Acad Dermatol. 2010;62:694–705. doi: 10.1016/j.jaad.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 91.Friedlander SF, Hebert AA, Allen DB. Fluticasone Pediatrics Safety Study Group. Safety of fluticasone propionate cream 0.05% for the treatment of severe and extensive atopic dermatitis in children as young as 3 months. J Am Acad Dermatol. 2002;46:387–93. doi: 10.1067/mjd.2002.118337. [DOI] [PubMed] [Google Scholar]
- 92.Feldman SR, Yentzer BA. Topical clobetasol propionate in the treatment of psoriasis: A review of newer formulations. Am J Clin Dermatol. 2009;10:397–406. doi: 10.2165/11311020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 93.Poulin Y, Papp K, Bissonnette R, Guenther L, Tan J, Lynde C, et al. Clobetasol propionate shampoo 0.05% is efficacious and safe for long-term control of moderate scalp psoriasis. J Dermatolog Treat. 2010;21:185–92. doi: 10.3109/09546630903493311. [DOI] [PubMed] [Google Scholar]
- 94.Binderup L, Bramm E. Effects of a novel Vitamin D analogue MC903 on cell proliferation and differentiation in vitro and on calcium metabolism in vivo. Biochem Pharmacol. 1988;37:889–95. doi: 10.1016/0006-2952(88)90177-3. [DOI] [PubMed] [Google Scholar]
- 95.Highton A, Quell J. Calcipotriene ointment 0.005% for psoriasis: A safety and efficacy study. Calcipotriene study group. J Am Acad Dermatol. 1995;32:67–72. doi: 10.1016/0190-9622(95)90186-8. [DOI] [PubMed] [Google Scholar]
- 96.Ramsay CA. Management of psoriasis with calcipotriol used as monotherapy. J Am Acad Dermatol. 1997;37(3 Pt 2):S53–4. [PubMed] [Google Scholar]
- 97.Mason AR, Mason J, Cork M, Dooley G, Edwards G. Topical treatments for chronic plaque psoriasis. Cochrane Database Syst Rev. 2009;2:CD005028. doi: 10.1002/14651858.CD005028.pub2. [DOI] [PubMed] [Google Scholar]
- 98.Green C, Ganpule M, Harris D, Kavanagh G, Kennedy C, Mallett R, et al. Comparative effects of calcipotriol (MC903) solution and placebo (vehicle of MC903) in the treatment of psoriasis of the scalp. Br J Dermatol. 1994;130:483–7. doi: 10.1111/j.1365-2133.1994.tb03382.x. [DOI] [PubMed] [Google Scholar]
- 99.Lebwohl M, Siskin SB, Epinette W, Breneman D, Funicella T, Kalb R, et al. A multicenter trial of calcipotriene ointment and halobetasol ointment compared with either agent alone for the treatment of psoriasis. J Am Acad Dermatol. 1996;35(2 Pt 1):268–9. doi: 10.1016/s0190-9622(96)90349-7. [DOI] [PubMed] [Google Scholar]
- 100.Taraska V, Tuppal R, Olesen M, Bang Pedersen C, Papp K. A novel aerosol foam formulation of calcipotriol and betamethasone has no impact on HPA axis and calcium homeostasis in patients with extensive psoriasis vulgaris. J Cutan Med Surg. 2016;20:44–51. doi: 10.1177/1203475415597094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gerritsen MJ, Van De Kerkhof PC, Langner A. Long-term safety of topical calcitriol 3 microg/g ointment. Br J Dermatol. 2001;144(Suppl 58):17–9. doi: 10.1046/j.1365-2133.2001.144s58017.x. [DOI] [PubMed] [Google Scholar]
- 102.van de Kerkhof PC, Berth-Jones J, Griffiths CE, Harrison PV, Hönigsmann H, Marks R, et al. Long-term efficacy and safety of tacalcitol ointment in patients with chronic plaque psoriasis. Br J Dermatol. 2002;146:414–22. doi: 10.1046/j.1365-2133.2002.04567.x. [DOI] [PubMed] [Google Scholar]
- 103.Veien NK, Bjerke JR, Rossmann-Ringdahl I, Jakobsen HB. Once daily treatment of psoriasis with tacalcitol compared with twice daily treatment with calcipotriol. A double-blind trial. Br J Dermatol. 1997;137:581–6. doi: 10.1111/j.1365-2133.1997.tb03790.x. [DOI] [PubMed] [Google Scholar]
- 104.Trémezaygues L, Reichrath J. Vitamin D analogs in the treatment of psoriasis: Where are we standing and where will we be going? Dermatoendocrinol. 2011;3:180–6. doi: 10.4161/derm.3.3.17534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laws PM, Young HS. Topical treatment of psoriasis. Expert Opin Pharmacother. 2010;11:1999–2009. doi: 10.1517/14656566.2010.492778. [DOI] [PubMed] [Google Scholar]
- 106.Pèrez A, Chen TC, Turner A, Raab R, Bhawan J, Poche P, et al. Efficacy and safety of topical calcitriol (1,25-dihydroxyvitamin d3) for the treatment of psoriasis. Br J Dermatol. 1996;134:238–46. [PubMed] [Google Scholar]
- 107.Kragbelle K. Vitamin D3 analogues. Dermatol Clin. 1996;13:835–8. [PubMed] [Google Scholar]
- 108.Patel B, Siskin S, Krazmien R, Lebwohl M. Compatibility of calcipotriene with other topical medications. J Am Acad Dermatol. 1998;38(6 Pt 1):1010–1. doi: 10.1016/s0190-9622(98)70171-9. [DOI] [PubMed] [Google Scholar]
- 109.Darley CR, Cunliffe WJ, Green CM, Hutchinson PE, Klaber MR, Downes N. Safety and efficacy of calcipotriol ointment (Dovonex) in treating children with psoriasis vulgaris. Br J Dermatol. 1996;135:390–3. [PubMed] [Google Scholar]
- 110.Takahashi H, Ibe M, Kinouchi M, Ishida-Yamamoto A, Hashimoto Y, Iizuka H. Similarly potent action of 1,25-dihydroxyvitamin D3 and its analogues, tacalcitol, calcipotriol, and maxacalcitol on normal human keratinocyte proliferation and differentiation. J Dermatol Sci. 2003;31:21–8. doi: 10.1016/s0923-1811(02)00136-6. [DOI] [PubMed] [Google Scholar]
- 111.Durakovic C, Ray S, Holick MF. Topical paricalcitol (19-nor-1 alpha, 25-dihydroxyvitamin D2) is a novel, safe and effective treatment for plaque psoriasis: A pilot study. Br J Dermatol. 2004;151:190–5. doi: 10.1111/j.1365-2133.2004.06002.x. [DOI] [PubMed] [Google Scholar]
- 112.Helfrich YR, Kang S, Hamilton TA, Voorhees JJ. Topical becocalcidiol for the treatment of psoriasis vulgaris: A randomized, placebo-controlled, double-blind, multicentre study. Br J Dermatol. 2007;157:369–74. doi: 10.1111/j.1365-2133.2007.08037.x. [DOI] [PubMed] [Google Scholar]


