Abstract
Heart position relative to total body length (TL) varies among snakes, with anterior hearts in arboreal species and more centrally located hearts in aquatic or ground-dwelling species. Anterior hearts decrease the cardiac work associated with cranial blood flow and minimize drops in cranial pressure and flow during head-up climbing. Here, we investigate whether heart position shifts intraspecifically during ontogenetic increases in TL. Insular Florida cottonmouth snakes, Agkistrodon conanti, are entirely ground-dwelling and have a mean heart position that is 33.32% TL from the head. In contrast, arboreal rat snakes, Pantherophis obsoleta, of similar lengths have a mean heart position that is 17.35% TL from the head. In both species, relative heart position shifts craniad during ontogeny, with negative slopes = −.035 and −.021% TL/cm TL in Agkistrodon and Pantherophis, respectively. Using a large morphometric data set available for Agkistrodon (N = 192 individuals, 23–140 cm TL), we demonstrate there is an anterior ontogenetic shift of the heart position within the trunk (= 4.56% trunk length from base of head to cloacal vent), independent of head and tail allometry which are both negative. However, in longer snakes > 100 cm, the heart position reverses and shifts caudally in longer Agkistrodon but continues toward the head in longer individuals of Pantherophis. Examination of data sets for two independent lineages of fully marine snakes (Acrochordus granulatus and Hydrophis platurus), which do not naturally experience postural gravity stress, demonstrate both ontogenetic patterns for heart position that are seen in the terrestrial snakes. The anterior migration of the heart is greater in the terrestrial species, even if TL is standardized to that of the longer P. obsoleta, and compensates for about 5 mmHg gravitational pressure head if they are fully upright.
Keywords: gravity, habitat, heart position, ontogenetic shifts, ontogeny, snakes
Graphical Abstract
Migration of heart (% total body length, TL) toward head in 4 species of snakes. The migration of heart reverses direction in older individuals of Acrochordus granulatus (aquatic) and Agkistrodon conanti (ground-dwelling), but not in the aquatic Hydrophis platurus or semi-terrestrial Pantherophis obsoleta.
1 INTRODUCTION
Animals that are long and adopt upright postures are susceptible to gravitational disturbances to the circulation of blood. In the upright posture, the heart must eject blood against the prevailing arterial pressure (“afterload”), which passively increases at heart level corresponding to the gravitational pressure associated with the absolute height of the blood column above the heart. Simultaneously, venous return of blood to the heart may decrease transiently owing to collection or “pooling” of blood in vessels distended below the heart. The magnitude of the gravitational disturbance to cardiac output depends on the extent of blood pooling, the length of blood columns during upright posture, and the efficacy of cardiovascular reflexes involved with circulatory homeostasis (Lillywhite, 1996). The hearts of animals with longer blood columns above the head must expend work to pump blood against the gravitational blood column as well as a higher peripheral resistance that reflexively acts to maintain arterial blood pressure (Lilly-white & Donald, 1994; Lillywhite & Gallagher, 1985; White & Seymour, 2013). Hence, the maintenance of arterial pressure and blood flow to the brain is related to posture, behavior, and evolutionary adjustments to gravity.
Snakes are especially instructive animals for understanding gravity’s influence on blood circulation (Lillywhite, 2005). Snakes have a body form that is sensitive to gravity stress, and they have evolved numerous specializations related to evolutionary diversification of behavior and the demands of habitat (Lillywhite, Albert, Sheehy, & Seymour, 2012; Lillywhite & Henderson, 1993; Lillywhite & Smits, 1992). Moreover, unlike terrestrial birds and mammals, the body cavity of snakes does not constrain the heart to a bony enclosure of thoracic cavity. Therefore, considerable variation of heart position has been documented in various species and appears to be correlated with gravitational stress related to habitat. Hearts of entirely aquatic species are significantly closer to the body center than are those of arboreal or scansorial terrestrial species, while semiaquatic and relatively nonclimbing terrestrial species are intermediate (Lillywhite et al., 2012; Seymour, 1987; Seymour & Lillywhite, 1976). At the extremes, heart positions as little as 15% of body length from the head have been recorded in arboreal species, while the heart position in aquatic species is nearly 45% of body length from the head (Lillywhite et al., 2012).
Interspecifically, the relative position of the heart is independent of body mass and body length, and there is no tendency for a reduction of head–heart distance in longer snakes within a category of habitat (Seymour, 1987). That is, the anatomic differences in heart position among species of snakes appear to be strongly related to habitat and behavior, acknowledging phylogenetic constraints that have been demonstrated in certain taxa (Gartner et al., 2010; Lillywhite et al., 2012). The importance of heart position in a physiological context is demonstrated by experiments which emphasize the effects of heart–head distance on cephalic blood pressure (Seymour & Arndt, 2004). Both interspecifically and intraspecifically, mean arterial blood pressure increases with body mass and the distance between the heart and head within, but not between, categories of habitat (Enok, Slay, Abe, Hicks, & Wang, 2014).
The question arises whether there is a cephalad shift of the heart during ontogeny of snakes, because the magnitude of gravitational disturbance to blood circulation is related to the absolute distance between the heart and head and is thus greater in longer individuals. Data germane to this question were published recently by Anderson and Secor (2015), who documented anterior shifts of the heart as body size increased from neonate to adult in ten species of snakes. These authors also demonstrated (2016) that internal organs in Diamondback water snakes (Nerodia rhombifer) did not exhibit any size-dependent changes in their alignment to body segments, and they concluded that observed anterior shifts in relative organ positions (including the heart) are the result of regional differences in the growth of body segments.
Here, we examine the ontogenetic shifts of heart position in four species of snake for which we have large data sets of anatomical measurements. Especially useful are data for the Florida cottonmouth, Agkistrodon conanti. These data were collected from a large number of insular snakes collected from Seahorse Key (Levy County, Florida). These snakes have been the subjects of many years’ study, and their behaviors are well known. They are completely ground-dwelling and are never observed in full vertical posture. We compare these data for cottonmouths with those available for Yellow rat snakes, Pantherophis obsoleta quadrivittata, which are scansorial and climb frequently with the body fully upright. Both of these species grow to similar maximum body lengths, but their behavioral tendencies to climb are entirely different. Each is representative of taxa having different heart position that covaries with gravitational stress, independent of phylogeny (Lillywhite et al., 2012). Finally, because of the outcome of the comparison, we also examined available data for two entirely marine taxa (Acrochordus granulatus, Hydrophis platurus) having independent evolutionary histories and a sufficient sample size to justify inclusion in the study. These are representative of the extreme end of the environmental spectrum with respect to gravity stress, which is essentially absent for snakes having long evolutionary association with completely aquatic habitats (Lillywhite & Pough, 1983; Lillywhite, 1987, 1996). Fully aquatic species were not included in the recently published studies of Anderson and Secor (2015, 2016). We test the hypothesis that ontogenetic changes in heart position in aquatic snakes are more similar to that of ground-dwelling cottonmouths than to scansorial rat snakes.
2 MATERIAL AND METHODS
2.1 Snake species and anatomic data
This study is based largely on noninvasive measurements of heart position and body lengths of large numbers of snakes. The data were collected over many years in conjunction with various investigations unrelated to the focus of this paper. In the case of the cottonmouths, each snake was anesthetized with isoflurane to the point of lacking muscle tone. The snake was laid out straight on a table, and the following measurements were taken: total length (TL) from tip of the snout to the tip of the tail; snout to vent length (SVL) measured from the tip of the snout to the center of the vent; head to heart distance (HH) measured from the tip of the snout to the center of the ventricle; head width (HW) measured from outer cheek to outer cheek, widest point of measurement, with the head relaxed; and head length (HL) measured from the tip of the snout to the posterior tip of the mandibular compound bone. Body length and HH measurements were made to the nearest mm using a meter stick. Head measurements were made to the nearest 0.1 mm using vernier calipers. The position of the cardiac ventricle (center) was determined by visual sighting of ventral scute movements corresponding to the heart beats, confirmed by palpation and enhancement of the visual signal when the ventral skin was pressed flat.
Measurements from rat snakes and two aquatic species were made similarly, except that head measurements were not taken. Some of the earlier data for these snakes were obtained while snakes were anesthetized during surgical procedures or euthanized for anatomical dissections subsequent to physiological investigations. In these cases the ventricle was observed directly. Whether the ventricle was observed directly or indirectly, the measurements were identical.
Measurements were obtained for 192 Florida cottonmouths (Agkistrodon conanti), 43 Yellow rat snakes (Pantherophis obsoleta), 62 Little file snakes (Acrochordus granulatus), and 190 Yellow-bellied sea snakes (Hydrophis [Pelamis] platurus; see Supporting Information, All research in connection with the measurements reported herein was conducted within guidelines and approval of institutional animal care and usage, and with approvals of collecting and export permits (nos. 018-2009-ACAT, DNOP-002-2010, DGT-013-04-2010, ACG-PI-012-2010, 129-2011—SINAC, 069-2012-ACAT, PI-ACAT-053-2012, DGVS-171-2013).).
2.2 Statistical methods
Statistical analysis of data were conducted using simple and logarithmic regression, ANCOVA, model specification tests, and segmented regression, using Statview SAS 5.0.1, R package np, and programming with Python (see Supporting Information). Measurements are presented as means ± SE.
We use regressions with ratios for the practical purpose of providing the best means of visualizing graphical changes in heart position that occur during ontogeny, noting that the use of ratios has sometimes been considered controversial (Freckleton, 2002; Garcia-Berthou, 2001; Packard & Boardman, 1988; Tracy & Sugar, 1989). We examined models wherein the dependent variable is the ratio of the HH length divided by TL. The independent regression variables were functions of body length. There is no problem using ratios in regressions, as has been shown in great detail by Firebaugh and Gibbs (1985) as well as numerous other articles (see also Anderson & Secor, 2015). Our analyses of data reported herein demonstrate that for all snakes the residuals from regressions of the form (where ε is an error term) have a clear pattern of increasing variance with increasing x (see Supporting Information). Dividing by x was shown to largely normalize these variances, which is a further statistical justification for using ratio regressions.
3 RESULTS
We present the results from conventional, simple line regression and ratio regression analyses and use this approach to illustrate patterns of morphometric data we obtained in the four species of snake. More models are considered and subjected to model selection tests in the Supporting Information. For two species of snakes, we find that segmented regression models provide a significantly better fit to the data.
In a recent analysis reported by Anderson and Secor (2015), neither absolute nor relative heart position was different between males and females of Nerodia rhombifer, the species for which the sample of individuals was largest (n = 434). Similarly, we did not find a significant difference between sexes for relative heart position in Agkistrodon conanti (t-test, p = .0723). Because of these findings in the two respective studies, and because sex can be difficult to determine noninvasively in smaller individuals, we do not consider sex as a variable in further analyses that we discuss herein.
3.1 Terrestrial snakes
3.1.1 Agkistrodon conanti
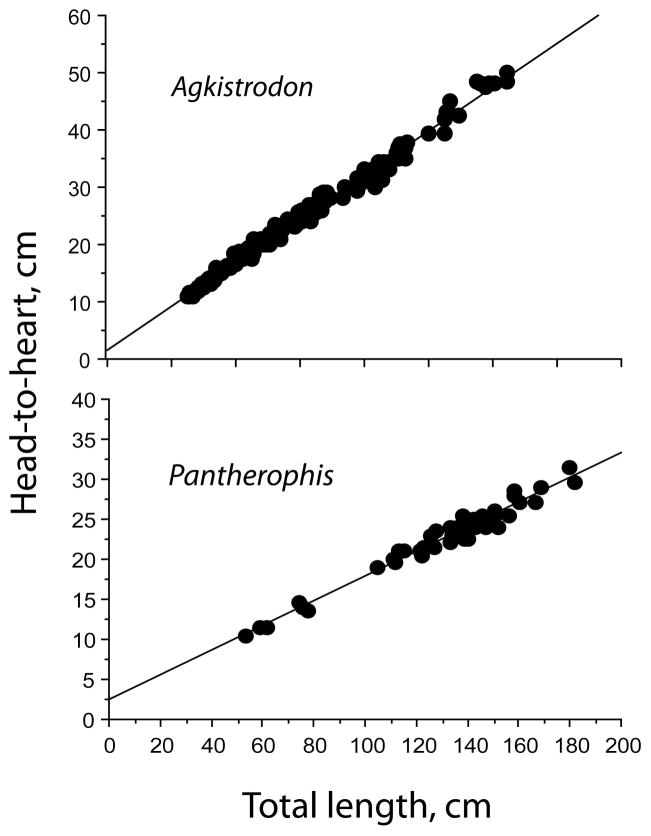
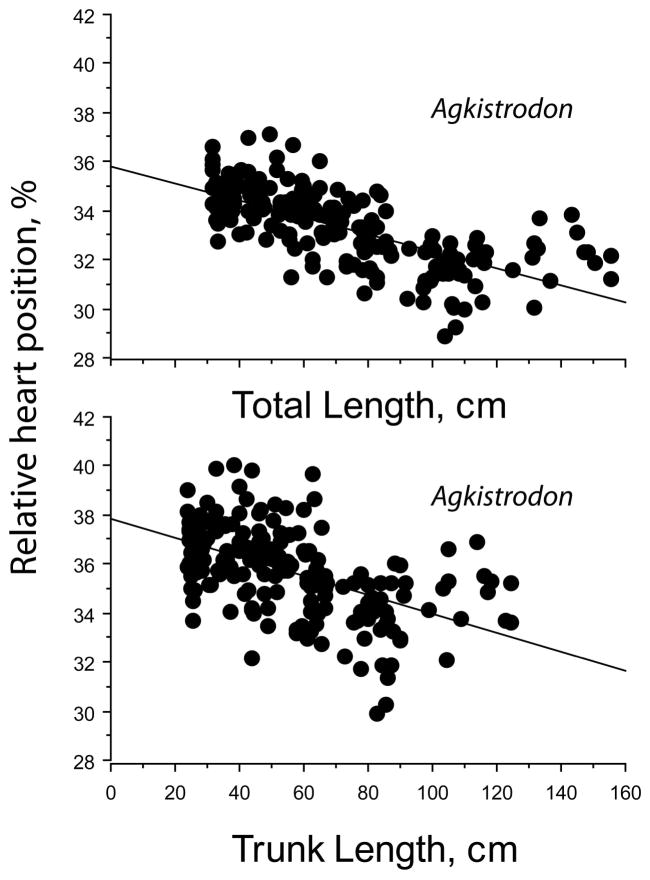
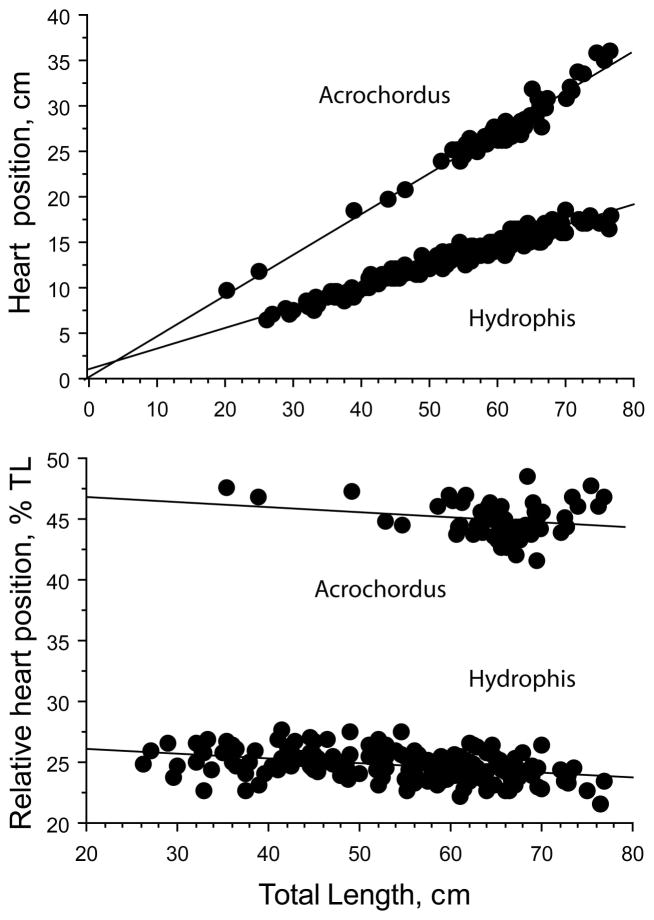
The absolute HH distance increased with TL (slope = 0.305, Figure 1). When HL was subtracted from SVL, heart position was defined in relation to the length of the body trunk, BL, as (HH −HL). Heart position within the trunk increased with BL (slope = 0.331), and R2 = 0.99 for both TL and BL regressions. The relative heart position, expressed as a percentage of the total body length, (HH/TL × 100), decreased with TL and the slope was significantly different from zero (p < .0001; Figure 2). Similarly, the relative heart position expressed as a percentage of the trunk length decreased with BL, and the slope was significantly different from zero (p < .0001; Figure 2). Thus, heart position is relatively more anterior in longer (and older) snakes.
FIGURE 1.
Relationship between heart-to-head distance (HH) and total body length (TL) in Agkistrodon conanti and Pantherophis o. quadrivittata. Regression equations are HH = 1.692 + 0.305 TL, R2 = 0.99, in Agkistrodon and HH = 2.455 + 0.154 TL, R2 = 0.966, in Pantherophis
FIGURE 2.
Regressions of relative heart position in Agkistrodon conanti expressed as distance to snout percentage of total body length (upper graph) and as distance to posterior mandibular bone percentage of trunk length (lower graph). Regression equations are % TL = 35.794–.035 TL, R2 = 0.465 and % trunk = 37.819–.039 trunk length, R2 = 0.279
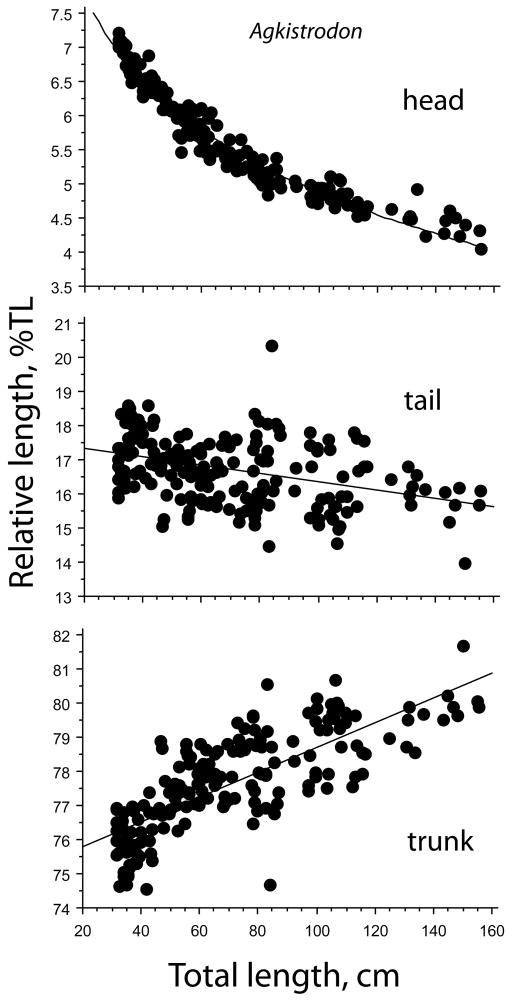
There was a tendency for heart position to stabilize or reverse position at body lengths greater than about 90 cm BL and 100 cm TL (Figure 2, and see below). The overall mean heart position was 35.7 ± 0.1%BL and 33.3 ± 0.1%TL. The length of both head and tail exhibited negative allometry with respect to total body length in Agkistrodon, whereas trunk length varied positively with TL (Figure 3).
FIGURE 3.
Regressions of relative head, tail, and trunk lengths, expressed as percentages of total body length in Agkistrodon conanti. Regression equations are % TL = 13.112–1.789 ln TL (R2 = 0.955) for head length; % TL = 17.56–.012 TL (R2 = 0.146) for tail length; and %TL = −1.832 + 0.807 TL (R2 = 0.999) for trunk length. Note that the relationship for trunk length is essentially isometric
3.1.2 Pantherophis obsoleta
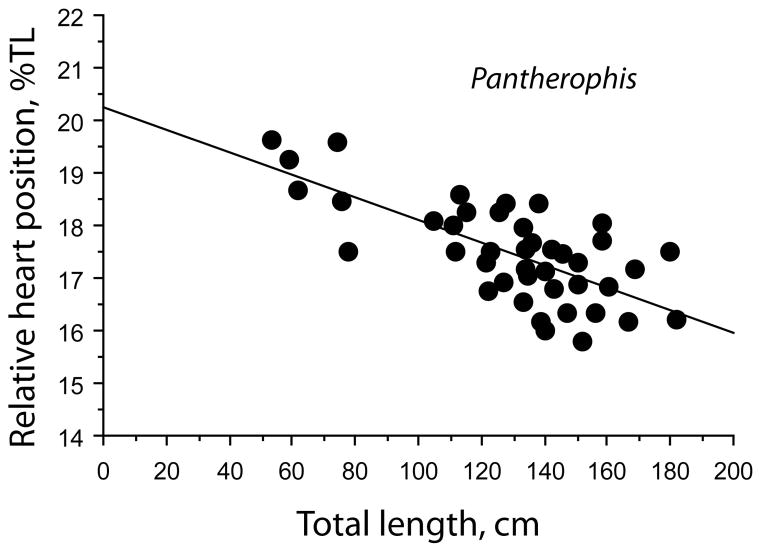
Heart position changed with TL according to the equation HH = 2.455 + 0.154 TL, with R2 = 0.966 (Figure 1). The relative heart position, expressed as a percentage of the total body length, decreased with TL and the slope was significantly different from zero (p < .001; Figure 4). There was no significant tendency for heart position to stabilize at greater body lengths. Heart position was relatively more anterior in longer and older snakes, and the overall mean position was 17.5 ± 0.1%TL.
FIGURE 4.
Regressions of relative heart position in Pantherophis o. quadrivittata expressed as distance to snout percentage of total body length. Regression equation, % TL = 20.232–.021, R2 = 0.5
3.2 Marine snakes
3.2.1 Acrochordus granulatus
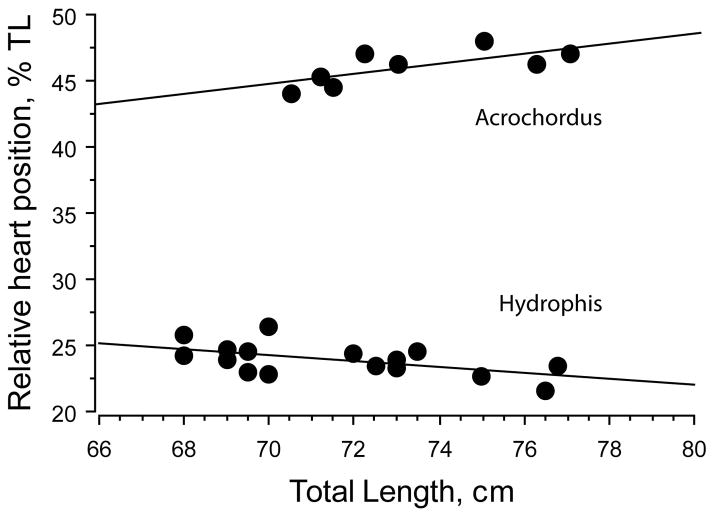
The overall mean heart position in A. granulatus was 45.1 ± 0.2%TL. The position of the heart changed with TL according to the equation HH = 0.126 + 0.448 TL (Figure 5). The relative heart position, expressed as a percentage of the total body length, decreased with TL, but the slope was not significantly different from zero (p = 0.1087; Figure 5). There was a tendency for heart position to stabilize or reverse at greater body lengths (TL > 70 cm; Figures 5 and 6). The reversal was marginally significant judging from simple regression of data for snakes > 70 cm in TL (p = .0596; Figure 6).
FIGURE 5.
Regressions for heart position (upper panel) and relative heart position (lower panel) in aquatic snakes. Regression equations for heart position are HH = 0.126 + 0.448 TL, R2 = 0.961 for Acrochordus granulatus and HH = 1.142 + 0.226 TL, R2 = 0.951 for Hydrophis platurus. Regression equations for relative heart position are %TL = 46.957–.031 TL, R2 = .042 for A. granulatus and % TL = 26.923–.04 TL, R2 = 0.159 for H. platurus
FIGURE 6.
Regressions of relative heart position in larger individuals of Acrochordus granulatus (>70 cm TL) and Hydrophis platurus (≥68 cm TL). Regression equations are % TL = 18.396 + 0.377 TL, R2 = 0.472 and % TL = 40.096–0.226 TL, R2 = 0.305, respectively. Respective p values are .0596 and .0174
3.2.2 Hydrophis platurus
The overall mean heart position in Hydrophis was 24.8 ± 0.1%TL. The position of the heart changed with TL according to the equation HH = 1.142 + 0.226 TL (Figure 5). The relative heart position, expressed as a percentage of the total body length, decreased with TL, and the slope was significantly different from zero (p < .001; Figure 5). There was no significant tendency for heart position to stabilize or reverse at greater body lengths (Figure 6).
3.3 Species comparisons
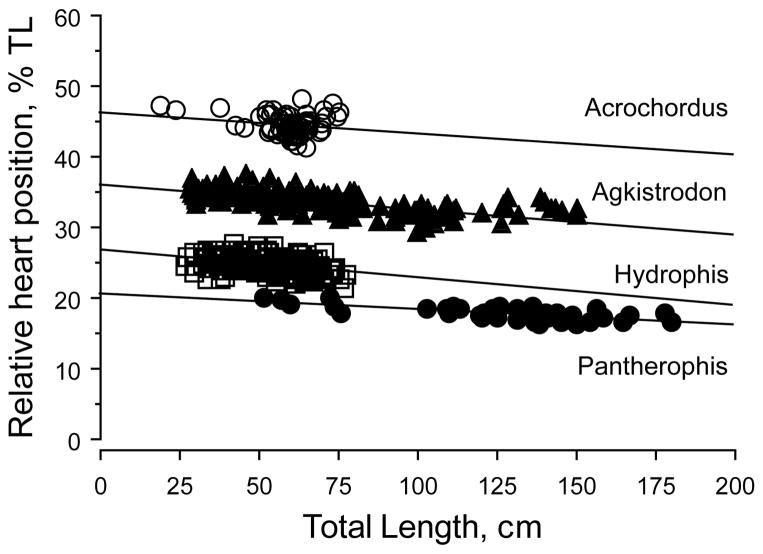
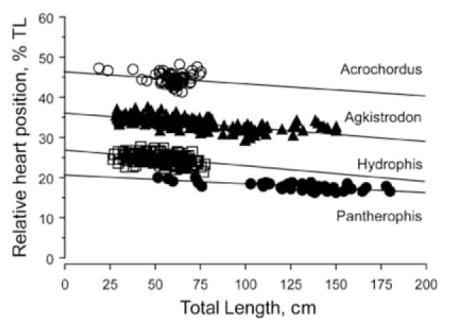
To reinforce our analysis and conclusions, we tested further for species differences in heart position using ANCOVA with heart position as the dependent variable, species as factor, and TL as a covariate. The position of the heart (controlling for body length) varied significantly among the four species (F3, 482 = 6619.87, p < .0001). However, we add the caveat that slopes of the species’ regressions were significantly different, so the ANCOVA can be considered to be invalid. Analysis using ANOVA, with log of relative heart position as the dependent variable and species as factor, demonstrates that relative heart position differs significantly among the four species (F3, 483 = 4833.4, p < .0001). Moreover, Fisher’s PLSD for log relative heart position indicates that all species comparisons are equally significantly different (p < .0001). Figure 7 illustrates comparisons of heart position across body lengths for all four species. The tendencies for heart reversal in longer individuals can be seen for Agkistrodon and Acrochordus.
FIGURE 7.
Simple linear regression of relative heart position across all body lengths for all four species of snakes
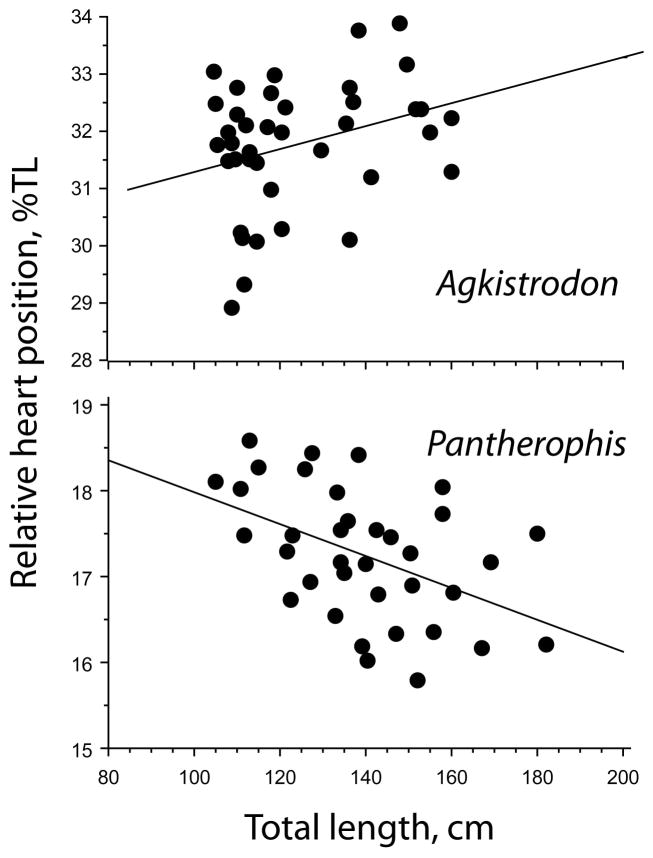
Simple linear regression of full data sets does not represent the true pattern in older individuals of Agkistrodon. Considering data for relative heart position in snakes longer than 100 cm TL, the regressions comparing Agkistrodon and Pantherophis are entirely different (Figure 8). The heart continues to move relatively closer to the head with increasing length in Pantherophis (F = 9.834, p = .0035) whereas it moves farther away from the head in Agkistrodon (F = 3.841, p = .0576). The latter relation is marginally not significant; however, simple regression analysis based on data for snakes longer than 90 cm was significant at p = .0252. The regressions for the two species have opposite slopes (.019 in Agkistrodon; −.019 in Pantherophis), suggesting there is a real directional change of heart migration in longer individuals of Agkistrodon.
FIGURE 8.
Regressions of relative heart position for snakes ≥90 cm in Agkistrodon conanti and Pantherophis o. quadrivittata. The respective regression equations are % TL = 29.329 + .02 TL, R2 = .094 and % TL = 19.851–.019 TL, R2 = 0.219. Respective p values are .0576 and .0035
With respect to changes in regression lines at longer body lengths, we estimated segmented regression models with one breakpoint. The functional form of these models is continuous piece-wise linear with two segments, and we informally refer to them as “V-fits.” For estimating these models, we employed a well-known exact numerical algorithm that we implemented in Python. We used bootstrap techniques for computing confidence intervals and for testing hypotheses for some parameter restrictions. For further details see Supporting Information.
We chose models based on specification tests for six regression model choices. Both ordinary and segmented regression models were considered. For all the snakes, the model selection results are qualitatively similar to the nonparametric regression fits.
The line model without a V pattern was the best fit for Pantherophis, whereas data for Agkistrodon supported a V-fit with right-hand slope positive (Figures 2–4, and 8 and Supporting Information). We rejected the restriction that the right-hand slope is zero or negative, and therefore show statistical support for the hypothesis that the heart migration changes direction in longer snakes.
With respect to the aquatic species, there was a tendency for heart position to reverse migration at longer body lengths in Acrochordus but not in Hydrophis (Figure 6).
The statistical results for Acrochordus are similar to those for Agkistrodon. The V pattern is inverted for Hydrophis relative to Agkistrodon and Acrochordus, although model specification tests do not reject the ratio line model. All species exhibit a significant migration of the heart across the full range of body sizes available (Figure 7).
A summary of information related to heart migration is given in Table 1. The cephalad migration of heart position per 10-cm increase in body length varied from highest in Acrochordus granulatus to lowest in Pantherophis obsoleta and correlated with the overall distance of the heart from the head (r = 0.998, p = .0006). That is, with increased lengthening of the body (TL), species with a heart position farthest from the head demonstrated a greater amount of migration toward the head with ontogenetic increases of body length.
Table 1.
Summary of data for heart migration in terrestrial and aquatic species of snakes.
| Species | Slopea | Heart Migration cm HH/10 cm TL | Relative Heart Position, % TL Mean ± SE | range TL cm | G Maxb mm HG | Ontogenetic Heart Migrationc | G Ontogenyd mm HG | G Max Ontogenye mm Hg | Mean Arterial Pressuref mm Hg |
|---|---|---|---|---|---|---|---|---|---|
| Aquatic | |||||||||
| Acrochordus granulatus | 0.45 | 4.48 | 45.1 ± 1.47 | 20.7–76.2 (55.5) | 26.57 | 0.34 | 0.26 | 0.60 | 27 |
| Hydrophis platurus | 0.23 | 2.26 | 24.75 ± 1.16 | 26.2–76.5 (50.3) | 14.64 | 2.19 | 1.69 | 4.32 | |
| Terrestrial | |||||||||
| Agkistrodon piscivorus | 0.31 | 3.05 | 33.32 ± 0.11 | 31.5–155.3 (123.8) | 39.62 | 6.52 | 5.04 | 5.23 | 32 |
| Arboreal | |||||||||
| Pantherophis obsoleta | 0.15 | 1.54 | 17.48 ± 0.14 | 53.5–182 (128.5) | 24.59 | 5.89 | 4.55 | 4.55 | 62 |
Slopes of data plots based on overall simple linear regression of HH on TL.
Gravitational pressure head of blood above heart in fully vertical, head-up position, for longest adult snake in sample.
Headward migration of heart, in cm, from smallest snake in sample to largest snake in sample.
Gravitational pressure head of vertical blood column represented by migration of the heart during ontogeny
Gravitational pressure head of vertical blood column represented by migration of the heart during ontogeny, standardized to maximum ontogenetic increase of TL equivalent to that of Pantherophis obsoleta (128.5 cm).
Mean resting arterial pressure in horizontal position (Lillywhite, 1987)
The range of TL during ontogeny within each species was greater in the two terrestrial species, which achieve a longer maximum body length. Using the measured length of the smallest (shortest) snake of each species, we calculated the heart position using the respective regression equation for all individuals of the species. Next we expressed the HH distance for the smallest snake of each species as a decimal fraction of its total length and, using this information, calculated the heart position for the largest (longest) snake represented in the respective ontogenetic series (for each species) assuming the HH remained as this constant percentage of the TL. We then compared the heart position of the longest snake of each species calculated in this manner with the heart position of the respective largest snake using the regression equation for all individuals and incorporating the respective heart migration (Figures 1 and 5). The distance between these two heart positions represents the distance of actual anterior migration. The absolute migration expressed in these terms was greater in the terrestrial species, even if the ontogenetic lengthening of all species was standardized to that of the longer species, P. obsoleta (Table 1).
4 DISCUSSION
Various traits related to the cardiovascular system must coevolve to meet the requirements for cardiovascular performance in response to challenges attributed to gravity in elongate animals, such as snakes (Lillywhite, 2005). A central heart position (comparatively mid-body location) tends to minimize the work of the heart perfusing the body (Seymour, 1987) and tends to be characteristic of strictly aquatic species of snakes where the surrounding water mimics an environment of microgravity (Lillywhite et al., 2012). Counteracting selection pressures related to gravity’s effect on blood circulation appears to account for more anterior heart positions in snakes that climb or assume upright postures outside of water. The anterior position (i) favors perfusion of the brain at all body angles, (ii) minimizes the pressure against which the heart must work when the head is up (proportional to the absolute vertical distance between heart and head) (Seymour, Hargens, & Pedley, 1993), and (iii) may locate baroreceptors anterior to the hydrostatic indifferent point to detect shifts of gravitational pressures whenever the postural attitude departs from horizontal (Lillywhite, 2005; Lillywhite & Donald, 1994).
Compelling evidence for the importance of heart position is that from the study of Seymour and Arndt (2004) who examined the independent effects of heart–head distance and dependent (inferior, or lower body) blood pooling on blood pressure regulation in aquatic and terrestrial snakes. The data demonstrated that vertical distance between heart and head had 2–4 times as much influence on cephalic blood pressures than did blood pooling in terrestrial pythons and in aquatic file snakes, respectively. Moreover, changes in cephalic blood pressure during head–up tilts involving only part of the body summed cumulatively to equal the total pressure change during full-body tilt.
That is, cephalic blood pressure is influenced by postural behaviors involving even partial elevation of the head. Accordingly, terrestrial and arboreal species of snakes, in contrast with strictly aquatic taxa, have evolved more effective baroreflexes in response to tilting and have more effective mechanisms to counteract dependent blood pooling (Conklin, Lillywhite, Olson, Ballard, & Hargens, 1996; Lillywhite, 1985, 1987; Lillywhite & Donald, 1994; Lillywhite & Gallagher, 1985; Lillywhite & Seymour, 1978; Young, Wassersug, & Pinder, 1997). However, the conclusion from the study of Seymour and Arndt (2004) is that heart position is more effective in counteracting gravity or postural disturbance to cephalic blood pressure than is the mitigation of blood pooling in the dependent vasculature.
Heart position is clearly different between the four species of snakes investigated here (Figure 7). The anterior heart position of Pantherophis is characteristic of scansorial species in general (Lillywhite et al., 2012). Rat snakes are proficient climbers and are known to climb to great heights on pine trees where they can be fully vertical against the trunk. In contrast, cottonmouths are terrestrial/amphibious snakes that rarely climb. All of the measurements for Agkistrodon conanti reported here represented specimens from the island of Seahorse Key where these snakes are completely ground-dwelling (Lillywhite & McCleary, 2008). Consequently, we surmise these snakes are not experiencing selection for cardiovascular competence related to gravitational influence of upright postures. The heart position in A. conanti is characteristic of viperids in general (Lillywhite & Smits, 1992), which are largely ground-dwelling as a group. The heart position in these snakes appears to be constrained somewhat by phylogeny insofar as those members of the family that have evolved arboreal habits are comparatively short, so an anterior position is not required owing to the relative absence of elongated blood columns in these shorter species (Lillywhite et al., 2012).
In spite of the ecological, behavioral, and phylogenetic differences between the scansorial Pantherophis and ground-dwelling Agkistrodon, both exhibited ontogenetic shifts of the heart toward the head (Figures 2 and 4). The data set for Agkistrodon allows further evaluation of causality in this phenomenon. Both the head and the tail scale negatively with body size (Figure 3), and thus growth slows in both of these characters in adult snakes (see also Vincent, Herrel, & Irschick, 2004). Thus the trunk elongates more so than either of these segments during growth, and the scaling is positive (Figure 3). Our data demonstrate that the anterior shift of the heart position occurs with respect to the trunk elongation as well as that of the total body (Figure 2). This is probably true in Pantherophis as well, although we do not have data for growth allometry of the head in this or other species.
The relative extent of heart displacement during ontogeny, representing just a few percent of total body length (Table 1; Figures 2 and 4; see also Anderson & Secor, 2015, 2016), is small compared with the difference of mean heart position between species (e.g., 32%TL vs. 17%TL in Agkistrodon and Pantherophis, respectively). It seems clear there is selection for heart position related to gravity and postural behaviors in Pantherophis but not in Agkistrodon, and this is an interspecific phenomenon (Lillywhite, 1985, 2005; Lillywhite & Donald, 1994; Lillywhite & Gallagher, 1985; Lillywhite & Smits, 1992). The small shifts of heart position during ontogeny (in all four species) complement the suggestion that anterior displacement within the trunk is related largely, or entirely, to differential growth of body segments presumably independent of gravitational influence (Anderson & Secor, 2016). This, however, is a tentative conclusion related to a complex phenomenon wherein causality has not been demonstrated in multiple species.
Evidence for a component of selection related to gravity in Pantherophis is suggested by the continuation of heart migration toward the head in longer individuals, whereas the heart location reverses direction in the longer individuals of Agkistrodon (Figure 8). We do not have sufficient data to examine ontogenetic shifts of heart position within a broader phylogenetic context.
Data for the entirely marine species of file snake, Acrochordus granulatus, demonstrate a trend that is similar to that of Agkistrodon for the larger individuals (Figures 5 and 6). Both of these taxa are not subjected to postural gravity stress, so these data favor the hypothesis that a component of selection favors the anterior heart movement in Pantherophis, the only scansorial species for which we have data. Conversely, a robust set of data from the pelagic sea snake Hydrophis platurus fails to expose the same pattern, and there appears to be no change in the heart migration at longer body lengths, similar to the data for Pantherophis. The V-fit pattern appears to be inverted relative to that of Agkistrodon conanti and Acrochordus granulatus. However, two separate model specification tests do not reject the ratio line model (Supporting Information). Perhaps the reason for the different patterns in the two marine species is related to differences in evolutionary history (Rasmussen, Murphy, Ompi, Gibbons, & Uetz, 2011; Sanders, Lee, Mumpuni, Bertozzi, & Rasmussen, 2013). Acrochordus has a much earlier evolutionary origin (and thus exposure to marine environment) than does Hydrophis.
Calculated migration distances of the heart in the four species we studied, from small to large individuals, demonstrate that if longer living adults are at fully vertical head-up posture, the ontogenetic migration of the heart tends to compensate for passive gravitational losses of arterial pressure at the head (attributable to height of the blood column between the heart and head) by several mmHg in the terrestrial species, but only much smaller changes in the marine species (Table 1). These differences are partly attributable to the (maximal) size differences of adults among these species (Table 1). Importantly, data in Table 1 illustrate that the arboreal species P. obsoleta compensates for gravitational loss of arterial pressure at the level of the head (say, during climbing) by having (1) comparatively high level of resting arterial pressure, (2) comparatively anterior position of the heart, and (3) anterior migration of the heart during ontogeny. See Lillywhite (2005), Seymour and Arndt (2004), and White and Seymour (2013) for further explanation of gravitational challenges to arterial pressure and blood circulation during upright posture.
There must be significance in the facts that the direction of heart migration is consistently anterior in snakes (and therefore not random), and the pattern for heart migration in longer individuals appears to vary among species. Further detailed investigation of the position of hearts in robust ontogenetic series of snakes representing more species (including longer body lengths) will be required to further understand the constraints and selective pressures that are acting on the hearts of these vertebrates. Two central questions arise: (1) How do the mechanisms for trunk elongation of snakes interact with selection to produce different heart positions among species (Lillywhite et al., 2012), presumably derived from differential genomic rearrangements of regulators and gene expression (Aires et al., 2016)? (2) Is there adaptive significance to the fact that patterns of heart migration during ontogeny also vary in relation to trunk elongation?
Supplementary Material
Acknowledgments
We are grateful to numerous individuals who assisted with collection of animals, assistance in the laboratory, and discussions of issues related to the nature of the data reported herein. These persons include A. Smits, C. Sheehy III, R. Seymour, A. Hargens, R. Ballard, J. Donald, R. McCleary, K. Zippel, F. Brischoux, J. Pfaller, H. Heatwole, M.-C. Tu, M. Edwards, J. Wixson, and J. Lillywhite. We thank Tobias Wang and an anonymous reviewer for comments on the manuscript. We thank various funding sources including the National Institutes of Health (HL 33821), National Science Foundation (IOS-0926802), the National Research Council and NASA for support of prior work that contributed morphological data.
Footnotes
Additional Supporting Information may be found in the online version of this article.
References
- Aires R, Jurberg AD, Leal F, Nóvoa A, Cohn MJ, Mallo M. Oct4 is a key regulator of vertebrate trunk length diversity. Developmental Cell. 2016;38:262–274. doi: 10.1016/j.devcel.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Anderson GE, Secor SM. Ontogenetic shifts and spatial associations in organ positions for snakes. Zoology. 2015;118:403–412. doi: 10.1016/j.zool.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Anderson GE, Secor SM. Differential growth of body segments explains ontogenetic shifts in organ position for Diamondback Water Snake (Nerodia rhombifera) Canadian Journal of Zoology. 2016;94:651–660. [Google Scholar]
- Conklin DJ, Lillywhite HB, Olson KR, Ballard RE, Hargens AR. Blood vessel adaptation to gravity in a semi-arboreal snake. Journal of Comparative Physiology. 1996;165:B518–B526. doi: 10.1007/BF00387512. [DOI] [PubMed] [Google Scholar]
- Enok S, Slay C, Abe AS, Hicks JW, Wang T. Intraspecific scaling of arterial blood pressure in the Burmese python. Journal of Experimental Biology. 2014;217:2232–2234. doi: 10.1242/jeb.099226. [DOI] [PubMed] [Google Scholar]
- Firebaugh G, Gibbs JP. User’s guide to ratio variables. American Sociological Review. 1985;50:713–722. [Google Scholar]
- Freckleton RP. On the misuse of residuals in ecology: Regression of residuals vs. multiple regression. Journal of Animal Ecology. 2002;71:542–545. [Google Scholar]
- Garcia-Berthou E. On the misuse of residuals in ecology: Testing regression residuals vs. the analysis of covariance. Journal of Animal Ecology. 2001;70:708–711. [Google Scholar]
- Gartner GEA, Hicks JW, Manzani PR, Andrade DV, Abe AS, Wang T, Secor SM, Garland T., Jr Phylogeny, ecology, and heart position in snakes. Physiological and Biochemical Zoology. 2010;83:43–54. doi: 10.1086/648509. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB. Postural edema and blood pooling in snakes. Physiological Zoology. 1985;58:759–766. [Google Scholar]
- Lillywhite HB. Circulatory adaptations of snakes to gravity. American Zoologist. 1987;27:81–95. [Google Scholar]
- Lillywhite HB. Gravity, blood circulation, and the adaptation of form and function in lower vertebrates. Journal of Experimental Zoology. 1996;275:217–225. [PubMed] [Google Scholar]
- Lillywhite HB. Cardiovascular adaptation to gravity: Lessons from comparative studies of snakes. In: Hargens A, Takeda N, Singal PK, editors. Adaptation biology and medicine (Vol. 4: Current Concepts) New Delhi: Narosa Publishing House; 2005. pp. 68–81. [Google Scholar]
- Lillywhite HB, Albert JS, Sheehy CM, III, Seymour RS. Gravity and the evolution of cardiopulmonary morphology in snakes. Comparative Biochemistry and Physiology - Part A. 2012;161:230–242. doi: 10.1016/j.cbpa.2011.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillywhite HB, Donald JA. Neural regulation of arterial blood pressure in snakes. Physiological Zoology. 1994;67:1260–1283. [Google Scholar]
- Lillywhite HB, Gallagher KP. Hemodynamic adjustments to head-up posture in the partly arboreal snake, Elaphe obsoleta. Journal of Experimental Zoology. 1985;235:325–334. doi: 10.1002/jez.1402350303. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB, Henderson RW. Behavioral and functional ecology of arboreal snakes. In: Seigel RA, Collins JT, editors. Snakes. Ecology and evolution. New York: McGraw-Hill; 1993. pp. 1–48. [Google Scholar]
- Lillywhite HB, McCleary RJR. Trophic ecology of insular cottonmouth snakes: Review and perspective. South American Journal of Herpetology. 2008;3:175–185. [Google Scholar]
- Lillywhite HB, Pough FH. Control of arterial pressure in aquatic sea snakes. American Journal of Physiology. 1983;244:R66–R73. doi: 10.1152/ajpregu.1983.244.1.R66. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB, Seymour RS. Regulation of arterial blood pressure in Australian tiger snakes. Journal of Experimental Biology. 1978;75:65–79. doi: 10.1242/jeb.75.1.65. [DOI] [PubMed] [Google Scholar]
- Lillywhite HB, Smits AW. Cardiovascular adaptations of viperid snakes. In: Campbell JA, Brodie ED Jr, editors. Biology of pitvipers. Tyler Texas; Selva: 1992. pp. 143–153. [Google Scholar]
- Packard GC, Boardman TJ. The misuse of ratios, indices, and percentages in ecophysiological research. Physiological Zoology. 1988;61:1–9. [Google Scholar]
- Rasmussen AR, Murphy JC, Ompi M, Gibbons JW, Uetz P. Marine Reptiles. PLoS One. 2011;6(11):e27373. doi: 10.1371/journal.pone.0027373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KL, Lee MSY, Mumpuni Bertozzi T, Rasmussen AR. Multilocus phylogeny and recent rapid radiation of the viviparous sea snakes (Elapidae: Hydrophiinae) Molecular Phylogenetics and Evolution. 2013;66:575–591. doi: 10.1016/j.ympev.2012.09.021. [DOI] [PubMed] [Google Scholar]
- Seymour RS. Scaling of cardiovascular physiology in snakes. American Zoologist. 1987;27:97–109. [Google Scholar]
- Seymour RS, Arndt JO. Independent effects of heart-head distance and caudal blood pooling on blood pressure regulation in aquatic and terrestrial snakes. Journal of Experimental Biology. 2004;207:1305–1311. doi: 10.1242/jeb.00882. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Hargens AR, Pedley TJ. The heart works against gravity. American Journal of Physiology. 1993;265:R715–R720. doi: 10.1152/ajpregu.1993.265.4.R715. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Lillywhite HB. Blood pressure in snakes from different habitats. Nature. 1976;264:664–666. doi: 10.1038/264664a0. [DOI] [PubMed] [Google Scholar]
- Tracy CR, Sugar J. Potential misuse of ANCOVA: Comment on Packard and Boardman. Physiological Zoology. 1989;62:993–997. [Google Scholar]
- Vincent SE, Herrel A, Irschick DJ. Ontogeny of intersexual head shape and prey selection in the pitviper Agkistrodon piscivorus. Journal of the Linnean Society. 2004;81:151–159. [Google Scholar]
- White CR, Seymour RS. The role of gravity in the evolution of mammalian blood pressure. Evolution. 2013;68:901–908. doi: 10.1111/evo.12298. [DOI] [PubMed] [Google Scholar]
- Young BA, Wassersug RJ, Pinder A. Gravitational gradients and blood flow patterns in specialized arboreal (Ahaetulla nasuta) and terrestrial (Crotalus adamanteus) snakes. Journal of Comparative Physiology. 1997;167:B481–B493. doi: 10.1007/s003600050100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.