Abstract
Phenobarbital (PB) promotes liver tumorigenesis in rodents, in part through activation of the constitutive androstane receptor (CAR) and the consequent changes in hepatic gene expression and increases in hepatocyte proliferation. A typical effect of CAR activation by PB is a marked induction of Cyp2b10 expression in the liver; the latter has been suspected to be vital for PB-induced hepatocellular proliferation. This hypothesis was tested here by using a Cyp2a(4/5)bgs-null (null) mouse model in which all Cyp2b genes are deleted. Adult male and female wild-type (WT) and null mice were treated intraperitoneally with PB at 50 mg/kg once daily for 5 successive days and tested on day 6. The liver-to-body weight ratio, an indicator of liver hypertrophy, was increased by 47% in male WT mice, but by only 22% in male Cyp2a(4/5)bgs-null mice, by the PB treatment. The fractions of bromodeoxyuridine-positive hepatocyte nuclei, assessed as a measure of the rate of hepatocyte proliferation, were also significantly lower in PB-treated male null mice compared with PB-treated male WT mice. However, whereas few proliferating hepatocytes were detected in saline-treated mice, many proliferating hepatocytes were still detected in PB-treated male null mice. In contrast, female WT mice were much less sensitive than male WT mice to PB-induced hepatocyte proliferation, and PB-treated female WT and PB-treated female null mice did not show significant difference in rates of hepatocyte proliferation. These results indicate that CYP2B induction plays a significant, but partial, role in PB-induced hepatocyte proliferation in male mice.
Introduction
Many studies have shown that phenobarbital (PB) and related compounds can promote liver tumorigenesis in rodents (Whysner et al., 1996; IARC, 2001). In mode-of-action evaluations of PB-induced rodent liver tumor formation, the key event was considered to be activation of the constitutive androstane receptor (CAR) (Yamamoto et al., 2004), which leads to a multitude of downstream events, including altered expression of CAR target genes and cellular signaling, increased cell proliferation, and the development of pathologic changes, in the liver (Elcombe et al., 2014). Some of the PB-activated CAR target genes, such as Mmd2, Foxm1b, and Cyclins, have been suggested to be important in PB-induced liver hyperplasia and the subsequent development of liver tumors (Ledda-Columbano et al., 2002; Huang et al., 2005; Blanco-Bose et al., 2008); but the molecular mechanism of the CAR-mediated activation of hepatocyte proliferation is still not fully understood.
A number of cytochrome P450 genes, particularly the Cyp2b genes, are among the most highly induced hepatic CAR target genes; hepatic induction of Cyp2b is a characteristic downstream event of CAR activation by PB (Honkakoski et al., 1998). However, it is unknown whether the induction of Cyp2b is necessary for CAR-mediated activation of hepatocyte proliferation and tumorigenesis. There has been no study that directly examined the possible role of CYP2B enzymes in PB-induced hepatocyte proliferation.
The CYP2B enzymes metabolize many drugs, such as cyclophosphamide, ifosfamide, bupropion, nicotine, and propofol, and a large number of environmental chemicals, such as aflatoxin B1, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, and chlorpyrifos (Dicke et al., 2005; Wang and Tompkins, 2008; Turpeinen and Zanger, 2012). Humans have a single CYP2B gene, CYP2B6; whereas mice have five Cyp2b genes, Cyp2b9, Cyp2b10, Cyp2b13, Cyp2b19, and Cyp2b23 (Nelson et al., 2004). In mice, Cyp2b9, Cyp2b10, and Cyp2b13 are the forms primarily expressed in the liver (Finger et al., 2011). Cyp2b10 is transcriptionally regulated by CAR (Honkakoski et al., 1998; Zhang et al., 2002; Wang et al., 2003; Kretschmer and Baldwin, 2005) and it is highly inducible by PB treatment (Honkakoski et al., 1998; Li-Masters and Morgan, 2001). Cyp2b9 and Cyp2b13 are female predominant in most mouse strains (Damiri et al., 2012). There was conflicting evidence as to whether Cyp2b9 is inducible by PB (Rivera-Rivera et al., 2003), but Cyp2b13 has been shown to be inducible by PB (Stupans et al., 1984).
We recently reported the generation and characterization of a Cyp2a(4/5)bgs-null mouse model, in which Cyp2a4, Cyp2a5, all five Cyp2b genes, Cyp2g1, and Cyp2s1 are deleted (Li et al., 2013; Wei et al., 2013). Among the deleted genes, only Cyp2a4/5 and Cyp2b9/10/13 are expressed in liver, and Cyp2a4 and Cyp2b9/13 are female predominant (Damiri et al., 2012; Li et al., 2013). The deletion of the gene cluster did not lead to any notable developmental or morphologic changes, and there was no significant compensatory change in the expression of CPR or various other major P450 enzymes, including CYP2C, CYP3A, CYP2E1, and CYP1A1/2 (Wei et al., 2013). A report of a Cyp2b-knockdown mouse also showed absence of any notable biologic phenotypes (Damiri et al., 2012). The Cyp2a(4/5)bgs-null mouse, in which an induction of the Cyp2b genes would not occur upon PB treatment, was used in the present study to test the hypothesis that Cyp2b induction is mechanistically important for PB-induced hepatocyte proliferation. We first confirmed that Cyp2b10 is induced by PB in wild-type (WT) mice but not in Cyp2a(4/5)bgs-null mice and that the Cyp2a(4/5)bgs gene deletion did not change the inducibility of other Cyp genes in the liver. We then assessed the PB-induced hepatic hypertrophy (weight increase) and hyperplasia [bromodeoxyuridine (BrdU) incorporation] in Cyp2a(4/5)bgs-null and WT mice (both male and female). Our results indicate that CYP2B plays a significant, but partial, role in PB-induced hepatocyte proliferation in male mice.
Materials and Methods
Animals and Treatments.
All studies with mice were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center. WT mice and Cyp2a(4/5)bgs-null mice, male and female, all on C57BL/6 background, were allowed free access to water and food. To induce hepatocyte proliferation, 2- to 3-month-old mice were treated with five consecutive daily injections of phenobarbital sodium (Sigma-Aldrich, St. Louis, MO; 50 mg/kg/day in saline) or saline alone. Mice were weighed and then euthanized by CO2 overdose at 24 hours after the last injection. The whole liver was removed carefully and weighed. A portion of liver tissue was stored at 10% formalin for histologic analysis and the remainder was stored at −80°C until use.
RNA-Polymerase Chain Reaction Analysis.
Total RNA was prepared using Trizol reagent (ThermoFisher, Waltham, MA) and stored at −80°C. Reverse transcription of RNA was carried out using the SuperScript III first-strand synthesis system (ThermoFisher), with use of 5 µg of total RNA, pretreated with DNase I (ThermoFisher) at room temperature for 15 minutes, and 0.5 µg of oligo(dT) in a final volume of 20 µl. Real-time polymerase chain reaction (PCR) was performed on an ABI StepOne Plus PCR system (Applied Biosystems, Foster City, CA) using SYBR Green PCR core reagent (Applied Biosystem), essentially as described previously (Zhang et al., 2007). Reactions were performed in duplicate in a total volume of 10 µl, with 2 µl of diluted (1:15) first-strand cDNA as template. Reactions were initiated at 50°C for 2 minutes (to allow degradation of any potential contaminating PCR products by the AmpErase UNG), followed by denaturation at 95°C for 10 minutes, and then 45 cycles of amplifications (95°C for 15 seconds, 62°C for 1 minute). The final melting curve analysis was carried out at 95°C for 15 seconds, 60°C for 1 minute, and 95°C for 15 seconds. The following primers were used: glyceraldehyde 3-phosphate dehydrogenase, forward 5′-tgtgaacggatttggccgta-3′ and reverse 5′-tcgctcctggaagatggtga-3′ (Wei et al., 2012); CYP3A11, forward 5′-ggatgagatcgatgaggctctg-3′ and reverse: 5′-caggtattccatctccatcacagt-3′; CYP2B10, forward 5′-caggtgatcggctcacacc-3′ and reverse: 5′-tgactgcatctgagtatggcatt-3′ (Pan et al., 2000); CYP2A5, forward 5′-TCTGTTGCTCATGAAGTACC-3′ and reverse 5′-TTGTCATCTAGGAAGTGCTT-3′; and CYP2C29, forward 5′-GGGCTCAAAGCCTACTGTCA-3′ and reverse 5′-AACGCCAAAACCTTTAA-3′ (Zhang et al., 2003).
Histology and BrdU Assay.
Mice were injected intraperitoneally with BrdU (Sigma-Aldrich) once at 100 mg/kg body weight at 90 minutes before the first saline or PB treatment, and they were also maintained on BrdU-containing drinking water (0.8 mg/ml) for the duration of the experiment to achieve continuous labeling (Blanco-Bose et al., 2008). Liver tissues were fixed in 10% neutral buffered formalin and then sectioned at 4 µm for hematoxylin-eosin staining or BrdU immunostaining. BrdU assay was performed as described previously (Moser et al., 2009). Briefly, the sections were deparaffinized, soaked in H2O2 for blocking endogenous peroxidase, and subjected to heat-induced epitope retrieval. Subsequently, the sections were stained with mouse anti-BrdU (1:500; Abcam, Cambridge, MA), and the slides were counterstained with hematoxylin, dehydrated, and placed under a coverslip. The numbers of BrdU-positive and BrdU-negative hepatocyte nuclei were tallied microscopically using ten randomly selected sections, three section per animal (at least 1000 hepatocyte nuclei), at ×200 magnification. The threshold for identification of BrdU-positive nuclei was set empirically, and the independent results from two experienced researchers were averaged to produce the final data.
Data Analysis.
Statistical significance of differences among groups in various parameters was examined with two-way analysis of variance, followed by Bonferroni or Sidak's multiple comparisons post test, using GraphPad Prism.
Results and Discussion
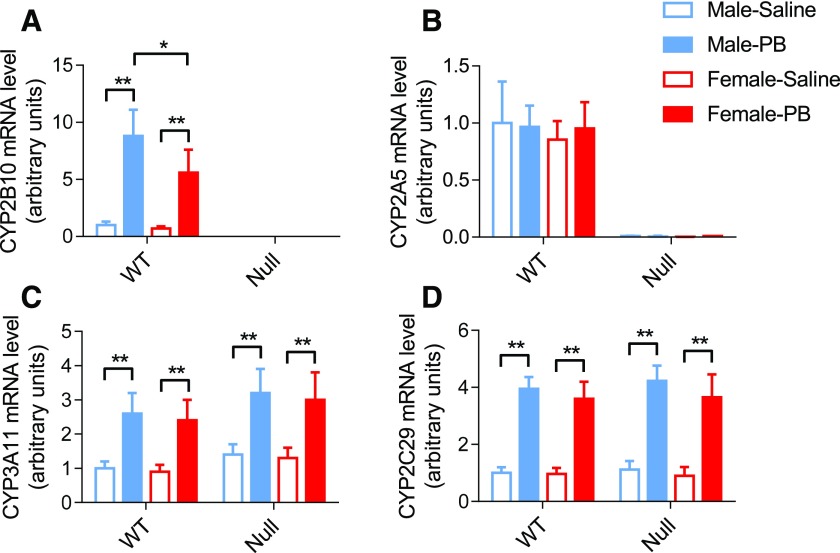
To confirm the induction of Cyp2b10 by PB in WT mice and the lack thereof in the null mice, we compared CYP2B10 mRNA levels in the livers of saline- or PB-treated WT and Cyp2a(4/5)bgs-null mice. The levels of CYP2A5, CYP2C29, and CYP3A11 mRNAs were also determined as controls. As shown in Fig. 1, at 24 hours after 5 consecutive daily injection of PB (50 mg/kg/day, i.p.), CYP2B10 mRNA levels were remarkably increased in WT mice compared with the saline-treated control group. In contrast, CYP2B10 mRNA could not be detected in the Cyp2a(4/5)bgs-null mice in either saline or PB group, which confirms the gene deletion. As a control, the levels of CYP3A11 and CYP2C29 mRNA were also increased by the PB treatment, as reported previously (Zhang et al., 2003), in both WT and the Cyp2a(4/5)bgs-null mice, thus confirming PB-mediated activation of CAR in the Cyp2a(4/5)bgs-null mice. CYP2A5 mRNA was not induced by PB in WT mice and it was not detected in the Cyp2a(4/5)bgs-null mice. These findings were consistent in males and females, except for a lower extent of CYP2B10 induction in WT female mice (Fig. 1A). Taken together, these results confirm that PB is a CYP2B10 inducer but not CYP2A5 inducer and that the deletion of Cyp2a(4/5)bgs genes did not affect the regulation of other major CYPs (CYP3A and CYP2C) by PB. Thus, the Cyp2a(4/5)bgs-null mouse is useful for subsequent studies on the role of CYP2B in PB-induced hepatocyte proliferation in the WT mice.
Fig. 1.
Effects of PB on hepatic CYP2A5, CYP2B10, CYP2C29, and CYP3A11 mRNA expression. Male and female WT and Cyp2a(4/5)bgs-null mice (2 to 3 months old) were treated with PB (50 mg/kg/day, i.p.) or saline, once daily for 5 consecutive days. Livers from individual mice were obtained 24 hours after the last dose for RNA isolation and PCR analysis. Data represent means ± S.D. (n = 3 or 4) and were normalized by the levels of glyceraldehyde 3-phosphate dehydrogenase. *P < 0.05; **P < 0.01 [2-way analysis of variance (ANOVA) with Bonferroni post test].
Phenobarbital treatment is known to induce hepatocyte proliferation in male mice, an event that could further develop into hepatocellular carcinogenesis (Blanck et al., 1986; El-Serag and Rudolph, 2007). Both hypertrophy and hyperplasia occur during hepatocyte proliferation. To examine the role of CYP2B in PB-induced hepatic hypertrophy, we measured the liver weight and liver-to-body weight ratio in WT and Cyp2a(4/5)bgs-null mice, both male and female, after PB or saline treatment. As shown in Table 1, the liver weights were greater in PB-treated groups than in saline-treated groups, for both WT and Cyp2a(4/5)bgs-null mice, male or female. The liver-to-body weight ratios were also significantly higher in PB-treated male WT (by ∼47%), but not in PB-treated male or female Cyp2a(4/5)bgs-null or PB-treated female WT mice, relative to the corresponding saline-treated mice. The liver-to-body weight ratio was also significantly greater in PB-treated male WT than in PB-treated male Cyp2a(4/5)bgs-null mice (P < 0.01), which indicated that the PB-induced hepatic hypertrophy in male mice was partially dependent on the presence of the Cyp2a(4/5)bgs genes.
TABLE 1.
Effects of PB on hepatic hypertrophy in WT and Cyp2a(4/5)bgs-null mice
Liver weight and liver-to-body weight ratio were determined for 2- to 3-month-old WT and Cyp2a(4/5)bgs-null mice after PB or saline treatment. Data represent means ± S.D. (n=3 or 4).
| Sex | Strain | Liver weight (g) |
Liver-to-Body Weight Ratio |
||||
|---|---|---|---|---|---|---|---|
| Saline | PB | (PB-Saline)/Saline | Saline | PB | (PB-Saline)/Saline | ||
| Male | WT | 1.47 ± 0.15 | 2.54 ± 0.27a | 73% | 0.048 ± 0.006 | 0.069 ± 0.004a | 47% |
| Null | 1.25 ± 0.08 | 1.69 ± 0.25b,c | 35% | 0.046 ± 0.004 | 0.056 ± 0.002c | 22% | |
| Female | WT | 1.04 ± 0.22 | 1.43 ± 0.13d | 38% | 0.045 ± 0.005 | 0.051 ± 0.004d | 13% |
| Null | 1.13 ± 0.07 | 1.39 ± 0.08 | 23% | 0.046 ± 0.003 | 0.049 ± 0.003 | 6.5% | |
P < 0.01, compared with corresponding saline group (2-way ANOVA with Sidak's multiple comparisons posttest).
P < 0.05, compared with corresponding saline group.
P < 0.01, compared with corresponding WT group.
P < 0.01, compared with corresponding male group.
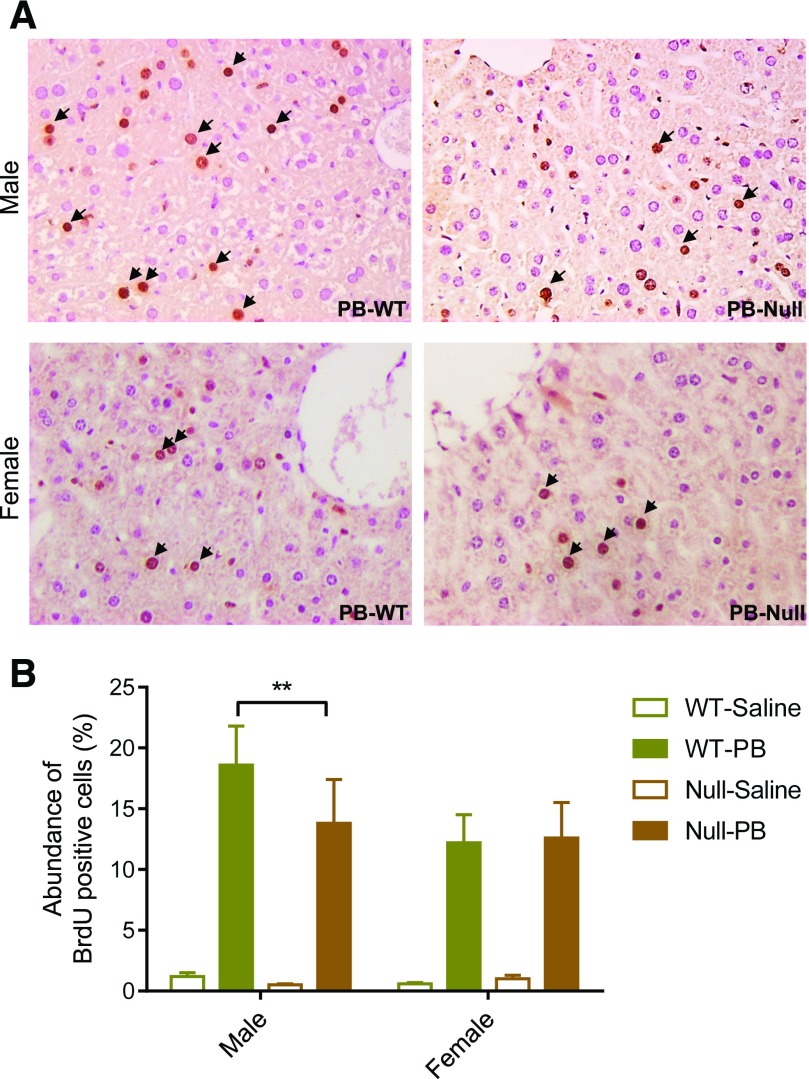
To examine the role of CYP2B in PB-induced hepatic hyperplasia, we examined hepatic BrdU incorporation in WT and Cyp2a(4/5)bgs-null mice treated concomitantly with BrdU and PB for five consecutive days. As shown in Fig. 2, A and B, the numbers of BrdU-positive hepatocytes were considerably greater in the livers of PB-treated WT and Cyp2a(4/5)bgs-null mice, male or female, than in the corresponding saline-treated groups; the latter had very few BrdU-positive cells. Among the PB-treated groups, the abundance of BrdU-positive cells was significantly greater in male WT mice than in male Cyp2a(4/5)bgs-null mice, which indicates that the PB-induced increase in BrdU incorporation in WT male mice was partly dependent on the presence of the Cyp2a(4/5)bgs genes. Consistent with the sex difference in PB-induced hepatic hypertrophy, female mice also showed a lower response to PB-induced hyperplasia than male mice did, and the abundance of BrdU-positive hepatocytes in females was not different between WT and Cyp2a(4/5)bgs-null mice.
Fig. 2.
Effects of PB on hepatic hyperplasia. (A) Immunohistochemical staining of BrdU-positive cells (brown color; arrow) in paraffin sections of livers from WT and Cyp2a(4/5)bgs-null mice after 5 days of treatment with PB or saline, as described in Materials and Methods. Typical results are shown. (B) Relative abundance of BrdU-positive nuclei among nuclei of all hepatocytes. The percentage of BrdU-positive nuclei in hepatocytes was compared among various groups (male or female, PB or saline, WT or Cyp2a(4/5)bgs-null). Data represent means ± S.D. (n = 3 or 4). **P < 0.01 (2-way ANOVA with Bonferroni post test).
Taken together, these results indicate that the Cyp2a(4/5)bgs genes play a significant, although partial, role in PB-induced hepatocyte proliferation in male mice. Given that the Cyp2b genes are the only ones induced by PB among the genes deleted in the Cyp2a(4/5)bgs-null mice, our results support the hypothesis that induction of CYP2B is important for PB-induced hepatocyte proliferation. Of the Cyp2b genes, whereas Cyp2b9, 2b10, and 2b13 are all expressed in the liver, Cyp2b9 and 2b13 are female predominant (Damiri et al., 2012; Li et al., 2013) and Cyp2b10 is the most highly induced by PB (Li-Masters and Morgan, 2001). Thus, it can be deduced that Cyp2b10 was involved in PB-induced hepatocyte proliferation in male mice. Notably, the association of hepatocyte proliferation with CYP2B induction has also been observed for other CYP2B inducers, such as the environmental pollutant potassium perfluorooctanesulfonate and the synthetic pyrethroid metofluthrin (Deguchi et al., 2009; Elcombe et al., 2012). It remains to be determined whether CYP2B induction contributes to the hepatic hypertrophy induced by these other compounds.
The mechanistic basis for the sex difference in the extent of PB-induced hepatocyte proliferation and the apparent noninvolvement of CYP2B in the proliferative response in females are currently not understood, but they may be partly explained by the lower extent of hepatic CYP2B induction by PB in females than in males. In that regard, our present result was consistent with previous reports, that PB induced CYP2B10 to a larger extent in males than in females (Li-Masters and Morgan, 2001; Stamou et al., 2014) and that male mice were more susceptible to PB-induced hepatocarcinogenesis than female mice (Heindryckx et al., 2009; Maronpot, 2009). This sex difference in CYP2B inducibility by PB does not appear to be due to a difference in CAR expression, because cytosolic (Hernandez et al., 2009) or nuclear (Saito et al., 2013) CAR protein level was found to be similar between untreated male and female mice. There was also no sex difference in nuclear CAR protein level after PB treatment (Saito et al., 2013). Our finding, that the PB-inducibility of two CAR target genes, Cyp3a11 and Cyp2c29, was similar in male and female WT mice and it was not altered in the Cyp2a(4/5)bgs-null mice, further confirmed the absence of a sex difference in hepatic CAR activation and the notion that CAR activity was not changed by the loss of the Cyp2b genes. The latter finding was consistent with results from a Cyp2b-knockdown mouse (Damiri et al., 2012).
The mechanistic link between CYP2B induction and hepatocyte proliferation remains to be determined. In one possible scenario, the large induction of CYP2B may promote hepatocyte proliferation through induction of reactive oxygen species and increased oxidative stress (Imaoka et al., 2004; Dostalek et al., 2007,2008). Reactive oxygen species and oxidative stress can activate various signaling pathways, including mitogen-activated protein kinase and phosphatidylinositol 3-kinase pathways, to promote hepatocyte proliferation (Galli et al., 2005; Dragin et al., 2006). Other studies have shown possible roles of β-catenin and the two-pore K+ channel Kcnk1 in the sex difference in PB-induced the hepatocyte proliferation (Braeuning et al., 2011; Saito et al., 2013). PB is not a preferred substrate for CYP2B (Pacifici, 2016), although the clearance of another barbiturate, pentobarbital, in mice appeared to involve enzymes encoded by the Cyp2a(4/5)bgs gene cluster (Wei et al., 2013). It is unclear whether the loss of the Cyp2abgs genes would cause a change in PB metabolism, but there has been no report demonstrating a role for PB metabolites in stimulating hepatocyte proliferation.
In summary, we confirmed that PB induces hepatic Cyp2b10 expression and hepatocyte proliferation to greater extents in male mice than in female mice. In male mice, CYP2B plays a significant, but partial, role in PB-induced hepatocyte proliferation.
Acknowledgments
We gratefully acknowledge the use of the services of the Pathology Core and the Advanced Light Microscopy and Image Analysis Core Facilities of the Wadsworth Center. We thank Ms. Weizhu Yang for assistance with mouse breeding.
Abbreviations
- BrdU
bromodeoxyuridine
- CAR
constitutive androstane receptor
- PB
phenobarbital
- PCR
polymerase chain reaction
- WT
wild type
Authorship Contributions
Participated in research design: Li, Zhang, Negishi, Ding.
Conducted experiments: Li, Bao.
Performed data analysis: Li, Bao, Ding.
Wrote or contributed to the writing of the manuscript: Li, Bao, Zhang, Negishi, Ding.
Footnotes
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant CA092596], National Institute of Environmental Health Sciences [Grant ES020867], National Institute of General Medical Sciences [Grant GM082978], and in part by the Intramural Research Program of the National Institutes of Health [National Institute of Environmental Health Sciences].
Parts of this work were previously presented as a poster at the following workshop: Li L, Bao X, Zhang QY, Negishi M, Ding X (2015) Role of CYP2B in phenobarbital-induced hepatocyte proliferation in mice. 54th Annual Meeting of the Society of Toxicology; 22–26 Mar 2015; San Diego, CA.
References
- Blanck A, Hansson T, Gustafsson JA, Eriksson LC. (1986) Pituitary grafts modify sex differences in liver tumor formation in the rat following initiation with diethylnitrosamine and different promotion regimens. Carcinogenesis 7:981–985. [DOI] [PubMed] [Google Scholar]
- Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, Moore DD, Trumpp A. (2008) C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology 48:1302–1311. [DOI] [PubMed] [Google Scholar]
- Braeuning A, Heubach Y, Knorpp T, Kowalik MA, Templin M, Columbano A, Schwarz M. (2011) Gender-specific interplay of signaling through β-catenin and CAR in the regulation of xenobiotic-induced hepatocyte proliferation. Toxicol Sci 123:113–122. [DOI] [PubMed] [Google Scholar]
- Damiri B, Holle E, Yu X, Baldwin WS. (2012) Lentiviral-mediated RNAi knockdown yields a novel mouse model for studying Cyp2b function. Toxicol Sci 125:368–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deguchi Y, Yamada T, Hirose Y, Nagahori H, Kushida M, Sumida K, Sukata T, Tomigahara Y, Nishioka K, Uwagawa S, et al. (2009) Mode of action analysis for the synthetic pyrethroid metofluthrin-induced rat liver tumors: evidence for hepatic CYP2B induction and hepatocyte proliferation. Toxicol Sci 108:69–80. [DOI] [PubMed] [Google Scholar]
- Dicke KE, Skrlin SM, Murphy SE. (2005) Nicotine and 4-(methylnitrosamino)-1-(3-pyridyl)-butanone metabolism by cytochrome P450 2B6. Drug Metab Dispos 33:1760–1764. [DOI] [PubMed] [Google Scholar]
- Dostalek M, Brooks JD, Hardy KD, Milne GL, Moore MM, Sharma S, Morrow JD, Guengerich FP. (2007) In vivo oxidative damage in rats is associated with barbiturate response but not other cytochrome P450 inducers. Mol Pharmacol 72:1419–1424. [DOI] [PubMed] [Google Scholar]
- Dostalek M, Hardy KD, Milne GL, Morrow JD, Chen C, Gonzalez FJ, Gu J, Ding X, Johnson DA, Johnson JA, et al. (2008) Development of oxidative stress by cytochrome P450 induction in rodents is selective for barbiturates and related to loss of pyridine nucleotide-dependent protective systems. J Biol Chem 283:17147–17157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragin N, Smani M, Arnaud-Dabernat S, Dubost C, Moranvillier I, Costet P, Daniel JY, Peuchant E. (2006) Acute oxidative stress is associated with cell proliferation in the mouse liver. FEBS Lett 580:3845–3852. [DOI] [PubMed] [Google Scholar]
- El-Serag HB, Rudolph KL. (2007) Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology 132:2557–2576. [DOI] [PubMed] [Google Scholar]
- Elcombe CR, Elcombe BM, Foster JR, Chang SC, Ehresman DJ, Butenhoff JL. (2012) Hepatocellular hypertrophy and cell proliferation in Sprague-Dawley rats from dietary exposure to potassium perfluorooctanesulfonate results from increased expression of xenosensor nuclear receptors PPARα and CAR/PXR. Toxicology 293:16–29. [DOI] [PubMed] [Google Scholar]
- Elcombe CR, Peffer RC, Wolf DC, Bailey J, Bars R, Bell D, Cattley RC, Ferguson SS, Geter D, Goetz A, et al. (2014) Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: A case study with phenobarbital as a model constitutive androstane receptor (CAR) activator. Crit Rev Toxicol 44:64–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finger JH, Smith CM, Hayamizu TF, McCright IJ, Eppig JT, Kadin JA, Richardson JE, Ringwald M. (2011) The mouse Gene Expression Database (GXD): 2011 update. Nucleic Acids Res 39:D835–D841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A, Svegliati-Baroni G, Ceni E, Milani S, Ridolfi F, Salzano R, Tarocchi M, Grappone C, Pellegrini G, Benedetti A, et al. (2005) Oxidative stress stimulates proliferation and invasiveness of hepatic stellate cells via a MMP2-mediated mechanism. Hepatology 41:1074–1084. [DOI] [PubMed] [Google Scholar]
- Heindryckx F, Colle I, Van Vlierberghe H. (2009) Experimental mouse models for hepatocellular carcinoma research. Int J Exp Pathol 90:367–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez JP, Mota LC, Huang W, Moore DD, Baldwin WS. (2009) Sexually dimorphic regulation and induction of P450s by the constitutive androstane receptor (CAR). Toxicology 256:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M. (1998) The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol 18:5652–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Zhang J, Washington M, Liu J, Parant JM, Lozano G, Moore DD. (2005) Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol 19:1646–1653. [DOI] [PubMed] [Google Scholar]
- IARC (2001) Some thyrotropic agents. IARC Monogr Eval Carcinog Risks Hum 79:i–iv, 1–725. [PMC free article] [PubMed] [Google Scholar]
- Imaoka S, Osada M, Minamiyama Y, Yukimura T, Toyokuni S, Takemura S, Hiroi T, Funae Y. (2004) Role of phenobarbital-inducible cytochrome P450s as a source of active oxygen species in DNA-oxidation. Cancer Lett 203:117–125. [DOI] [PubMed] [Google Scholar]
- Kretschmer XC, Baldwin WS. (2005) CAR and PXR: xenosensors of endocrine disrupters? Chem Biol Interact 155:111–128. [DOI] [PubMed] [Google Scholar]
- Ledda-Columbano GM, Pibiri M, Concas D, Cossu C, Tripodi M, Columbano A. (2002) Loss of cyclin D1 does not inhibit the proliferative response of mouse liver to mitogenic stimuli. Hepatology 36:1098–1105. [DOI] [PubMed] [Google Scholar]
- Li-Masters T, Morgan ET. (2001) Effects of bacterial lipopolysaccharide on phenobarbital-induced CYP2B expression in mice. Drug Metab Dispos 29:252–257. [PubMed] [Google Scholar]
- Li L, Jia K, Zhou X, McCallum SE, Hough LB, Ding X. (2013) Impact of nicotine metabolism on nicotine’s pharmacological effects and behavioral responses: insights from a Cyp2a(4/5)bgs-null mouse. J Pharmacol Exp Ther 347:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maronpot RR. (2009) Biological basis of differential susceptibility to hepatocarcinogenesis among mouse strains. J Toxicol Pathol 22:11–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser GJ, Foley J, Burnett M, Goldsworthy TL, Maronpot R. (2009) Furan-induced dose-response relationships for liver cytotoxicity, cell proliferation, and tumorigenicity (furan-induced liver tumorigenicity). Exp Toxicol Pathol 61:101–111. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. (2004) Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics 14:1–18. [DOI] [PubMed] [Google Scholar]
- Pacifici GM. (2016) Clinical pharmacology of phenobarbital in neonates: effects, metabolism and pharmacokinetics. Curr Pediatr Rev 12:48–54. [DOI] [PubMed] [Google Scholar]
- Pan J, Xiang Q, Ball S. (2000) Use of a novel real-time quantitative reverse transcription-polymerase chain reaction method to study the effects of cytokines on cytochrome P450 mRNA expression in mouse liver. Drug Metab Dispos 28:709–713. [PubMed] [Google Scholar]
- Rivera-Rivera I, Kim J, Kemper B. (2003) Transcriptional analysis in vivo of the hepatic genes, Cyp2b9 and Cyp2b10, by intravenous administration of plasmid DNA in mice. Biochim Biophys Acta 1619:254–262. [DOI] [PubMed] [Google Scholar]
- Saito K, Moore R, Negishi M. (2013) Nuclear receptor CAR specifically activates the two-pore K+ channel Kcnk1 gene in male mouse livers, which attenuates phenobarbital-induced hepatic hyperplasia. Toxicol Sci 132:151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamou M, Wu X, Kania-Korwel I, Lehmler HJ, Lein PJ. (2014) Cytochrome p450 mRNA expression in the rodent brain: species-, sex-, and region-dependent differences. Drug Metab Dispos 42:239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupans I, Ikeda T, Kessler DJ, Nebert DW. (1984) Characterization of a cDNA clone for mouse phenobarbital-inducible cytochrome P-450b. DNA 3:129–137. [DOI] [PubMed] [Google Scholar]
- Turpeinen M, Zanger UM. (2012) Cytochrome P450 2B6: function, genetics, and clinical relevance. Drug Metabol Drug Interact 27:185–197. [DOI] [PubMed] [Google Scholar]
- Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, LeCluyse EL. (2003) A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem 278:14146–14152. [DOI] [PubMed] [Google Scholar]
- Wang H, Tompkins LM. (2008) CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab 9:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Li L, Zhou X, Zhang QY, Dunbar A, Liu F, Kluetzman K, Yang W, Ding X. (2013) Generation and characterization of a novel Cyp2a(4/5)bgs-null mouse model. Drug Metab Dispos 41:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Wu H, Li L, Liu Z, Zhou X, Zhang QY, Weng Y, D’Agostino J, Ling G, Zhang X, et al. (2012) Generation and characterization of a CYP2A13/2B6/2F1-transgenic mouse model. Drug Metab Dispos 40:1144–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whysner J, Ross PM, Williams GM. (1996) Phenobarbital mechanistic data and risk assessment: enzyme induction, enhanced cell proliferation, and tumor promotion. Pharmacol Ther 71:153–191. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Goldsworthy TL, Negishi M, Maronpot RR. (2004) The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res 64:7197–7200. [DOI] [PubMed] [Google Scholar]
- Zhang J, Huang W, Chua SS, Wei P, Moore DD. (2002) Modulation of acetaminophen-induced hepatotoxicity by the xenobiotic receptor CAR. Science 298:422–424. [DOI] [PubMed] [Google Scholar]
- Zhang QY, Dunbar D, Kaminsky LS. (2003) Characterization of mouse small intestinal cytochrome P450 expression. Drug Metab Dispos 31:1346–1351. [DOI] [PubMed] [Google Scholar]
- Zhang X, D’Agostino J, Wu H, Zhang QY, von Weymarn L, Murphy SE, Ding X. (2007) CYP2A13: variable expression and role in human lung microsomal metabolic activation of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. J Pharmacol Exp Ther 323:570–578. [DOI] [PubMed] [Google Scholar]