Abstract
In the brain, microglia continuously scan the surrounding extracellular space in order to respond to damage or infection by becoming activated and participating in neuroinflammation. When activated, microglia increase the expression of translocator protein (TSPO) 18 kDa, thereby making the TSPO expression a marker for neuroinflammation. We used the radiotracer [11C]DAA1106 (a ligand for TSPO) and positron emission tomography (PET) to determine the effect of smoking on availability of this marker for neuroinflammation. Forty-five participants (30 smokers and 15 non-smokers) completed the study and had usable data. Participants underwent a dynamic PET scanning session with bolus injection of [11C]DAA1106 (with smokers in the satiated state) and blood draws during PET scanning to determine TSPO affinity genotype and plasma nicotine levels. Whole-brain standardized uptake values (SUVs) were determined, and analysis of variance was performed, with group (smoker vs non-smoker) and genotype as factors, thereby controlling for genotype. Smokers and non-smokers differed in whole-brain SUVs (P=0.006) owing to smokers having 16.8% lower values than non-smokers. The groups did not differ in injected radiotracer dose or body weight, which were used to calculate SUV. An inverse association was found between whole-brain SUV and reported cigarettes per day (P<0.05), but no significant relationship was found for plasma nicotine. Thus, smokers have less [11C]DAA1106 binding globally than non-smokers, indicating less microglial activation. Study findings are consistent with much prior research demonstrating that smokers have impaired inflammatory functioning compared with non-smokers and that constituents of tobacco smoke other than nicotine affect inflammatory processes.
Introduction
Inflammation is a critical component of normal tissue repair and is fundamental to the body’s defense against infection (Goncalves et al, 2011). In the brain, microglia continuously scan the surrounding extracellular space (Nayak et al, 2014) in order to respond swiftly to damage or infection by becoming activated and participating in neuroinflammation (Anthony and Pitossi, 2012). In this context, activated microglia participate in functions such as clearance of apoptotic cells and extracellular pathogens, removal of degenerating neurons and extracellular proteins, and cytokine/chemokine production (Anthony and Pitossi, 2012). When activated, microglial cellular morphology changes and the expression of the translocator protein (TSPO) 18 kDa is increased, thereby making the expression of TSPO a marker for neuroinflammation.
The radioligand N-(2,5-dimethoxybenzyl)-N-(5-fluoro-2-phenoxyphenyl) acetamide labeled with carbon-11 (abbreviated as [11C]DAA1106) has emerged as a reliable second-generation radiotracer for labeling TSPO (Maeda et al, 2004; Okubo et al, 2004; Zhang et al, 2003) with high affinity (Chaki et al, 1999; Chauveau et al, 2008; Venneti et al, 2007a, 2008) for positron emission tomography (PET) scanning in vivo. Because [11C]DAA1106 and other newer radiotracers have higher affinity for TSPO than previously used radiotracers (eg, [11C]PK11195), they are more useful for quantifying PET data by having the sensitivity to account for genetic TSPO predispositions (discussed in more detail below) (Owen et al, 2011) and smaller changes in neuroinflammation (Venneti et al, 2008). TSPO was originally called the ‘peripheral benzodiazepine receptor’ (Zhang et al, 2003) because it was identified by benzodiazepine binding but was renamed to acknowledge its many potential functions and location in the central nervous system (as well as in the periphery) (Papadopoulos et al, 2006). Specific binding of DAA1106 correlates with the presence of activated microglia identified by immunohistochemistry in situ (Venneti et al, 2008) and immunohistochemistry combined with autoradiography in brain tissue (Venneti et al, 2007a).
PET studies using [11C]DAA1106 and similar radiotracers have examined a range of conditions thought to be associated with neuroinflammation. This method was used recently to demonstrate increases in radiotracer binding in patients with Alzheimer’s disease (Fan et al, 2015; Kreisl et al, 2013; Suridjan et al, 2015; Varrone et al, 2015; Yasuno et al, 2012, 2008), Lewy body dementia (Surendranathan et al, 2015), amyotrophic lateral sclerosis (Zurcher et al, 2015), stroke (Lartey et al, 2014), and non-smokers with major depression (Setiawan et al, 2015), but not Parkinson’s disease (Koshimori et al, 2015) or normal aging (Suridjan et al, 2014). Increases in this marker have also been demonstrated in animal models of brain injury (Sandiego et al, 2015; Venneti et al, 2007b; Wang et al, 2014; Yu et al, 2010) and stroke (Walberer et al, 2014), along with subsequent normalization with time after a brain insult (Ory et al, 2015; Walberer et al, 2014; Wang et al, 2014). In contrast, a decrease in the marker for neuroinflammation was found with administration of propofol anesthesia (Hines et al, 2013).
Over the past 30+ years, a large body of research has addressed the effects of cigarette smoking on inflammation in the body (Goncalves et al, 2011; Towler, 2000). A driving force behind this research is the known impairment of wound healing by smoking. Comprehensive literature reviews have recommended preoperative and postoperative abstinence periods of ⩾4 weeks in smokers undergoing surgical procedures (Pluvy et al, 2015; Rinker, 2013). Though the mechanism by which smoking impairs wound healing has not been fully elucidated, cigarette smoke contains >250 toxins, many of which are known to affect healing (Rinker, 2013), and studies of laboratory animals exposed to cigarette smoke have demonstrated significant alterations (both decreases and increases) in markers of neuroinflammation (Khanna et al, 2013). Reviews of this literature indicate that the inflammatory healing response is attenuated in smokers by reduced inflammatory cell chemotactic responsiveness, diminished migratory function, and increased oxidative stress (Reuther and Brennan, 2014; Sorensen, 2012).
In the absence of studies directly examining the effect of human cigarette smoking on neuroinflammation in vivo, we used PET scanning to determine whether cigarette smokers have altered binding of [11C]DAA1106, a marker for neuroinflammation, compared with non-smokers. We hypothesized that non-smoker vs smoker effects would occur globally throughout the brain, as prior research by our group (Brody et al,2009a,2011, 2006a, 2013) and others (Cosgrove et al, 2009; Staley et al, 2006) demonstrates widespread effects of smoking when studying systems (eg, the nicotinic cholinergic system) that are widely distributed. We also sought to examine the effect of menthol, as menthol cigarette smoking is common (~1/3 of US smokers) (SAMHSA, 2009) and menthol smokers have more difficulty quitting in standard treatment programs (Gandhi et al, 2009; Okuyemi et al, 2007; Pletcher et al, 2006), elevated serum nicotine/cotinine/exhaled carbon monoxide (CO) levels (in some (Williams et al, 2007), but not all (Abobo et al, 2012; Muscat et al, 2009), studies), and more severe upregulation of brain nicotinic acetylcholine receptors (Brody et al, 2013) when compared with non-menthol cigarette smokers. Therefore, we also hypothesized that effects of smoking on [11C]DAA1106 binding would be greater in menthol than in non-menthol smokers.
Materials and methods
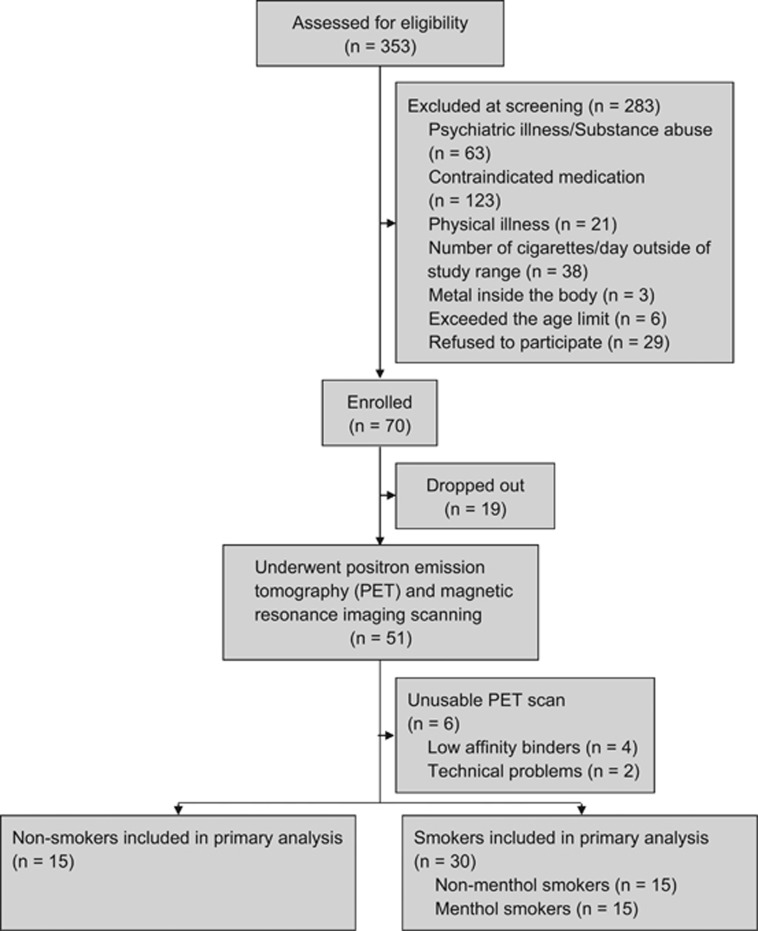
Forty-five participants (30 smokers and 15 non-smokers) completed the study and had usable data. These participants underwent telephone and in-person screening, a bolus [11C]DAA1106 PET scanning session, blood draws during PET to determine TSPO affinity genotype and plasma nicotine (and metabolite) levels, and a structural magnetic resonance imaging (MRI) scan, as described below. An additional 6 participants underwent PET scanning but were excluded due to genotype (n=4, see below) or technical PET scanning issues (n=2) (Figure 1).
Figure 1.
Flow diagram showing the number of potential and actual participants at each step of the study, including reasons for potential participants being screened out of participation.
Participants were veterans who were recruited through Internet (eg, Craigslist) advertisements and posted flyers. Inclusion criteria were: (1) healthy adult (18–65 years) cigarette smokers (10–40 cigarettes per day) who met DSM-IV criteria (First et al, 1995) for Nicotine Dependence or non-smokers (<100 cigarettes lifetime and none within the past year), (2) smoking primarily (>80%) either menthol or non-menthol cigarettes (for the smoker group), (3) ability to read, write, and give voluntary informed consent, and (4) an exhaled CO ⩾ or <8 ppm (and urine cotinine ⩾ or <200 ng/ml) during the study screening visit to support smoking or non-smoking status, respectively. Exclusion criteria were: (1) any Axis I diagnosis (including mood, anxiety, psychotic, and substance abuse disorders) within the past year, (2) any current medication or history of a medical condition that might affect the central nervous system at the time of scanning (eg, current treatment with a psychotropic medication or history of severe head trauma with loss of consciousness, epilepsy, or other neurological diseases), (3) regular use (>1 × /week) of anti-inflammatory medication, such as steroidal or non-steroidal anti-inflammatory medications (eg, corticosteroids, ibuprofen, naproxen, aspirin, or celecoxib (Celebrex)), (4) unstable cardiovascular disease, severe liver disease, or renal insufficiency, which might make tolerating study procedures difficult, or (5) pregnancy. Occasional drug/alcohol use not meeting criteria for abuse or dependence was not exclusionary, but participants were instructed to abstain from drug/alcohol use for at least 48 h prior to PET scanning.
For the telephone screening, a thorough smoking history, including age of first cigarette, maximum smoking habit, menthol or non-menthol cigarette use, length and dates of abstinence periods, previous treatments used, and current smoking habit, was obtained. A brief medical, psychiatric, and substance use history was also obtained during the telephone screening. During a subsequent in-person visit, eligibility criteria were confirmed and general demographics, smoking history, and symptom ratings were obtained with screening questions from the SCID for DSM-IV (First et al, 1995), the Smoker’s Profile Form (Brody et al, 2006a), the Fagerström Test for Nicotine Dependence (FTND) (Fagerstrom, 1978; Heatherton et al, 1991) (to assess severity of Nicotine Dependence), Shiffman–Jarvik Withdrawal Scale (SJWS) (Shiffman and Jarvik, 1976) (to measure craving and withdrawal), and Spielberger State Trait Anxiety Index (STAI) (Spielberger, 1983) and Beck Depression Inventory (BDI) (Beck et al, 1996) (to confirm the absence of potentially confounding psychiatric symptoms). A brief medical review of systems and chart review were also performed by a study physician (ALB or MSM), along with an exhaled CO measurement (Micro+ Smokerlyzer Breath CO Monitor; Bedfont Scientific, UK), urine cotinine screen (The Accutest NicAlert; Jant Pharmacal, Encino, CA), breathalyzer (AlcoMatePro), urine toxicology screen (Test Country I-Cup Urine Toxicology Kit), and urine pregnancy test (Test Country Cassette Urine Pregnancy Test) to verify inclusion/exclusion criteria.
Participants meeting inclusion/exclusion criteria who wished to participate underwent a [11C]DAA1106 PET scanning session 1 week later, using a procedure similar to the one developed in previous studies (Ikoma et al, 2007; Takano et al, 2010; Yasuno et al, 2012, 2008). At 1400 hours on the day of PET scanning, participants arrived at the VA Greater Los Angeles Healthcare System PET Center and underwent a brief clinical interview, breathalyzer, and urine cotinine, toxicology, and pregnancy screens, in order to verify continued meeting of inclusion/exclusion criteria (including confirmation of reports of drug abstinence at the time of scanning). From 1430 to 1445 hours, smokers smoked to satiety (2–3 cigarettes, favorite brand) in an outdoor area adjacent to the PET center. From 1445 to 1500 hours, participants were positioned on the PET scanner and a venous line was placed. At 1500 hours, participants received a bolus injection of 377 (±62) MBq of [11C]DAA1106 and underwent dynamic PET scanning of the brain for the next 90 min. PET scans were obtained using the Philips Gemini TruFlight PET Scanner (Koninklijke Philips Electronics N.V., Eindhoven, The Netherlands). [11C]DAA1106 was prepared by an established method (Wang et al, 2012). An investigational new drug (IND) approval from the Food and Drug Administration (IND 122041) was obtained to use the radiotracer [11C]DAA1106 for the study described here.
A 5-ml blood sample was drawn prior to the initiation of PET scanning for genotyping of each individual’s TSPO affinity subtype (high [C/C], medium [C/T], or low [T/T]), because these affinity subtypes have been shown to affect radiotracer binding for all currently used radiotracers determining TSPO availability (Owen et al, 2011; Owen et al, 2012; Yoder et al, 2013). For this sample, venous blood was drawn via a port in the catheter placed for radiotracer injection. Genomic DNA was extracted from whole blood using the QiaAmp DNA Blood Mini Kits (Qiagen, Valencia, CA) by study collaborators (EN and LS) and TSPO single-nucleotide polymorphism (rs6971) genotyping using the TaqMan Allelic Discrimination (Thermo Fisher Scientific, Canoga Park, CA) platform was performed in duplicate, according to the manufacturer’s specified protocol. Quality control was ensured by perfect concordance of replicate samples, expected minor allele frequencies, and adherence to Hardy–Weinberg equilibrium. Only scans from participants with the high- or medium-affinity genotypes (known to be >90% of North Americans; Mizrahi et al, 2012) were included in study analyses in order to avoid a potential confound. The exclusion of low-affinity binders from data analysis is standard practice in recent research in this field (Hafizi et al, 2016; Hannestad et al, 2013; Koshimori et al, 2015; Zurcher et al, 2015).
In addition, blood samples were drawn 10 and 60 min after the initiation of PET scanning for determination of plasma nicotine/cotinine levels. Afternoon plasma cotinine has been shown to be a good measure of nicotine exposure for the past 24 h (Benowitz and Jacob, 1994). Samples were centrifuged to obtain plasma, packed on dry ice, and shipped to the Clinical Pharmacology Laboratory at the University of California, San Francisco for assay by gas chromatography by Peyton Jacob and colleagues.
One week after the PET scanning session, an MRI scan of the brain was obtained on a 3.0-T scanner (Signa; GE Medical Systems, Milwaukee, WI) in order to aid in localization of regions on the PET scans. The MRI had the following specifications: three-dimensional Fourier-transform spoiled-gradient-recalled acquisition with TR=30 ms, TE=7 ms, 30-degree angle, 2 acquisitions, and 256 × 192 view matrix. The acquired volume was reconstructed as roughly 90 contiguous 1.5-mm thick transaxial slices.
As in previous research by our group (Brody et al2009a, 2002, 2006a, 2009b, 2006b, 2004), MRI/PET co-registration was performed using the Statistical Parametric Mapping software (FIL Methods Group, UK), and automated volumes of interest (VOIs) were determined on MRI using FSL tools for structural MRI. These automated VOIs were transferred from each participant’s MRI to his/her co-registered PET scan and visually inspected using PMOD (PMOD Technologies, Zurich, Switzerland). The primary VOI was whole brain (including gray and white matter) for reasons cited in the Introduction section. However, as automated volumes are easily attained and regional differences are possible, VOIs were also determined for the amygdala, caudate, hippocampus, nucleus accumbens, putamen, and thalamus, similar to VOIs obtained in prior research (Takano et al, 2010; Yasuno et al, 2012).
In order to obtain a quantitative measurement of VOI binding to TSPO in the brain, standardized uptake values (SUVs) were calculated using the standard definition of SUV=mean tissue activity concentration (Bq/ml)/(injected dose (Bq)/body weight (g)). Mean tissue activity concentration from 20 to 40 min postinjection was used, based on time activity curves demonstrating stable activity during this time period. SUV was used as the primary outcome measure because it avoids invasive arterial blood sampling and has been shown to strongly correlate with total volume of distribution (Vt) values (Toth et al, 2015; Walker et al, 2015), has good test–retest reproducibility (Toth et al, 2015), and has less intersubject variability than Vt (Walker et al, 2015) for a similar radiotracer.
For statistical analysis of data, an analysis of variance (ANOVA) was performed, with whole-brain SUV as the measure of interest and both group (smokers vs non-smoker) and TSPO genotype (mixed or high affinity) as between-subject factors (Suridjan et al, 2015; Varrone et al, 2015). To determine whether group differences were due to differences in particular brain regions, a multivariate ANOVA (MANOVA), using the smaller automated VOIs, was performed with the same structure as the preceding ANOVA, followed by univariate ANOVAs for the individual VOIs. To quantify between-group differences, percentage of difference was calculated as: 100 × (SUVnon-smokers−SUVsmokers)/SUVnon-smokers. Based on prior research reporting greater brain exposure to cigarette smoke in menthol than in non-menthol cigarette smokers, we also performed an ANOVA for whole-brain SUV with the same structure as the above test, using non-smoker vs menthol vs non-menthol cigarette preference as a between-subject factor. As an exploratory analysis, linear analyses were performed for the smoker group, with whole-brain SUV value as the dependent variable and independent variables related to smoking, controlling for TSPO genotype. Statistical tests were performed using the statistical software program SPSS/PASW version 24 (SPSS, Chicago, IL).
Results
Study groups had no significant differences in age, sex, race/ethnicity, height, weight, depression/anxiety levels, or caffeine, alcohol, or marijuana use (Table 1). On average, the groups were middle-aged, mostly male, and had generally low levels of depression/anxiety and drug/alcohol use. No significant between-group differences were present for body weight or injected dose of radiotracer, which were used to calculate SUV.
Table 1. Baseline Demographics and Rating Scale Scores for the Non-Smoker and Smoker Groups.
| Variable | Non-smoker group (n=15) | Whole smoker group (n=30) | Non-menthol smoker subgroup (n=15) | Menthol smoker subgroup (n=15) |
|---|---|---|---|---|
| Age, years | 47.6 (±13.8) | 52.1 (±8.1) | 49.9 (±8.4) | 54.4 (±7.4) |
| Sex (% female) | 26.7 | 20.0 | 20.0 | 20.0 |
| Race/ethnicity (%) | ||||
| African American | 26.7 | 46.7 | 33.3 | 60.0 |
| Asian | 26.7 | 10.0 | 6.7 | 13.3 |
| Hispanic | 26.7 | 13.3 | 13.3 | 13.3 |
| White | 20.0 | 30.0 | 46.7 | 13.3 |
| Height (inches) | 68.9 (±4.0) | 68.2 (±4.0) | 68.3 (±4.7) | 68.2 (±3.5) |
| Weight (kg) | 88.1 (±23.4) | 84.0 (±16.2) | 83.9 (±17.9) | 84.1 (±14.9) |
| Cigarettes per day | 0 (±0) | 13.9 (±3.8) | 13.5 (±3.8) | 14.4 (±3.9) |
| Exhaled carbon monoxide (ppm) | 1.6 (±0.6) | 13.3 (±4.7) | 13.0 (±4.2) | 13.5 (±5.2) |
| Fagerström Test for Nicotine Dependence (FTND) | 0 (±0) | 4.0 (±2.3) | 4.1 (±2.1) | 3.9 (±2.4) |
| Beck Depression Inventory | 1.0 (±1.3) | 1.7 (±2.3) | 1.3 (±1.8) | 2.1 (±2.8) |
| State Trait Anxiety Inventory | 58.5 (±15.0) | 66.7 (±17.8) | 68.3 (±18.1) | 65.1 (±18.0) |
| Caffeine use (coffee cup equivalents/day) | 1.1 (±1.3) | 2.0 (±1.6) | 2.0 (±1.7) | 1.9 (±1.5) |
| Alcohol drinks per day | 0.6 (±1.4) | 1.0 (±2.1) | 0.7 (±1.6) | 1.3 (±2.5) |
| Marijuana cigarettes per week | 0.0 (±0.0) | 0.3 (±1.3) | 0.5 (±1.8) | 0.1 (±0.4) |
All values are presented as means (±SD) or percentages. Using χ2 tests for categorical variables and Student’s t-tests for continuous variables, no between-group (or between-subgroup) tests were significant, other than differences in measures of smoking (cigarettes per day, exhaled carbon monoxide, and FTND scores) between the smoker groups/subgroups and the non-smoker group (all P-values<0.0005).
PET data analysis comparing smokers and non-smokers revealed a significant effect of group for whole-brain SUV values (ANOVA, F=8.3; df=1,41; P=0.006), due to smokers having mean 16.8% lower values than non-smokers (Table 2 and Figure 2). Consistent with this global finding, in the analysis of the smaller VOIs, a significant multivariate effect of group was found (MANOVA; F=2.8, df=12,30; P=0.01), with all VOIs having a significant (or trend-level) between-group effect on univariate analysis (Table 2), owing to smokers having lower SUV values than non-smokers (range 14.6–19.7%) in all VOIs studied.
Table 2. Standardized Uptake Values (SUVs) for the Whole Brain and Smaller Regions of Interest for Non-smokers and Smokers (and the Non-Menthol Smoker and Menthol Smoker Subgroups).
| Brain region | SUV values—non-smokers (n=15) | SUV values—smokers (n=30) | SUV values—non-menthol smoker subgroup (n=15) | SUV values—menthol smoker subgroup (n=15) |
|---|---|---|---|---|
| Whole brain | 0.20 (±0.03) | 0.17 (±0.04) | 0.18 (±0.04) | 0.16 (±0.02) |
| Accumbens | ||||
| R | 0.20 (±0.03) | 0.17 (±0.04) | 0.17 (±0.04) | 0.16 (±0.03) |
| L | 0.21 (±0.03) | 0.17 (±0.04) | 0.17 (±0.04) | 0.16 (±0.03) |
| Amygdala | ||||
| R | 0.18 (±0.03) | 0.15 (±0.04) | 0.16 (±0.04) | 0.13 (±0.02) |
| L | 0.17 (±0.03) | 0.15 (±0.04) | 0.16 (±0.04) | 0.14 (±0.02) |
| Caudate | ||||
| R | 0.18 (±0.04) | 0.14 (±0.03) | 0.15 (±0.03) | 0.14 (±0.02) |
| L | 0.18 (±0.03) | 0.15 (±0.03) | 0.15 (±0.03) | 0.14 (±0.02) |
| Hippocampus | ||||
| R | 0.19 (±0.03) | 0.16 (±0.03) | 0.17 (±0.04) | 0.15 (±0.02) |
| L | 0.19 (±0.03) | 0.15 (±0.03) | 0.16 (±0.04) | 0.14 (±0.02) |
| Putamen | ||||
| R | 0.23 (±0.03) | 0.19 (±0.05) | 0.21 (±0.06) | 0.18 (±0.03) |
| L | 0.23 (±0.03) | 0.19 (±0.04) | 0.20 (±0.05) | 0.18 (±0.03) |
| Thalamus | ||||
| R | 0.22 (±0.04) | 0.19 (±0.04) | 0.20 (±0.04) | 0.18 (±0.03) |
| L | 0.22 (±0.03) | 0.18 (±0.04) | 0.19 (±0.04) | 0.17 (±0.03) |
Abbreviations: L=left; R=right. All values are mean±SD. All regions were analyzed using analysis of variance, with group (non-smoker vs smoker or non-smoker vs non-menthol smoker vs menthol smoker) and genotype as between-subject factors. All regions were significant for the non-smoker vs smoker comparison at the P⩽0.05 level, except for the right and left accumbens, which approached significance (P-values=0.08 and 0.06, respectively). Similarly, all regions were significant for the three group comparisons (non-smoker vs non-menthol smoker vs menthol smoker) at the P⩽0.05 level. The automated regions listed here were generated using the FSL toolkit.
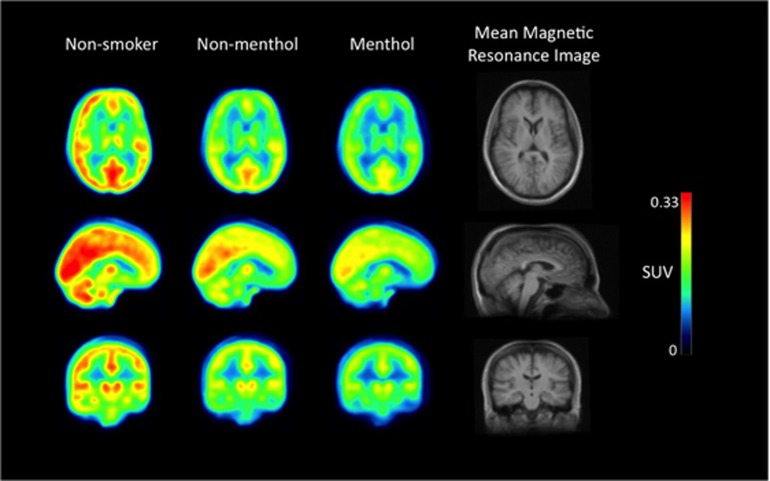
Figure 2.
Mean positron emission tomography (PET) images from the study subgroups (non-smokers, non-menthol cigarette smokers, and menthol cigarette smokers) demonstrating higher [11C]DAA1106 standardized uptake values (SUVs) for non-smokers than the two smoker subgroups. The first three columns consist of mean SUV PET images (transaxial, sagittal, and coronal from top to bottom) for the three study groups/subgroups (n=15 each) and the far right column shows the group mean magnetic resonance image. For this figure representation of study results, the PET scans were spatially normalized into standard Montreal Neurological Institute (MNI) template space.
For the three-group comparison (non-smokers vs non-menthol cigarette smokers vs menthol cigarette smokers), the whole-brain SUV comparison was significant (ANOVA, F=6.1; df=2,39; P=0.005), owing to a range of values from non-smokers (highest) to non-menthol cigarette smokers (middle) to menthol cigarette smokers (lowest) (Table 2). In the multivariate analysis of smaller VOIs, a significant effect of group was found (MANOVA; F=1.8, df=24,56; P=0.03), with all VOIs having a significant between-group effect, owing to the range (from high to low) of SUV values from smokers to non-menthol smokers to menthol smokers (Table 2). In comparing only the non-menthol with the menthol cigarette smokers, the whole-brain SUV comparison did not reach significance (ANOVA; F=3.6; df=1,26; P=0.07), and similar results were found for the smaller VOIs (ANOVAs; Ps=0.03–0.21), possibly owing to the smaller samples used for comparing the non-menthol with the menthol cigarette smoker subgroups.
In the exploratory analysis of smoking-related variables, a significant relationship was found between cigarettes per day and whole-brain SUV (F=6.3; P=0.02), indicating that higher levels of reported smoking were associated with lower levels of TSPO availability. Similarly, a significant relationship between the stimulation subscale scores of the SJWS and whole-brain SUV was also found (F=5.6; P=0.03), indicating that higher levels of withdrawal stimulation were associated with lower levels of TSPO availability. No significant associations were found for FTND scores, CO levels, plasma nicotine/cotinine levels, or other subscales on the SJWS.
Discussion
Cigarette smokers have less [11C]DAA1106 binding than non-smokers throughout the brain, indicating less TSPO availability. Though several explanations for this finding are possible, a straightforward one is that smoking results in global impairment of microglial activation. This explanation is consistent with much prior research demonstrating that smokers have impaired inflammatory functioning in other parts of the body, which leads to compromised wound healing (Goncalves et al, 2011; Towler, 2000). Furthermore, the inverse correlation between [11C]DAA1106 binding and participant reports of cigarette use per day indicates that the severity of impaired microglial activation may be related to the amount of current cigarette usage. Of note, the fact that study results were global (rather than regional) is also consistent with prior research demonstrating widespread effects of smoking on brain receptors (Brody et al2009a, 2011, 2006a, 2013; Cosgrove et al, 2009; Staley et al, 2006). These global effects of smoking are in line with known properties of cigarette smoke, namely, that it rapidly enters the body and brain due to high permeability through lung, vasculature, and brain cells (Henderson and Lester, 2015). Taken together, study results may demonstrate a significant widespread brain abnormality in smokers in the satiated state.
The negative association between SUV values and cigarettes per day, but not plasma nicotine levels (or other measures of smoking behavior), may indicate that components of cigarette smoke other than nicotine are responsible for the low level of microglial activation found here. Laboratory studies support this theory, with several studies demonstrating that whole tobacco smoke administration results in greater alterations in inflammatory markers than nicotine alone (Arimilli et al, 2015; Tilp et al, 2016). However, given the evidence that nicotine indeed impairs (Kalra et al, 2004; Piao et al, 2009) or attenuates (Gao et al, 2014) some inflammatory processes, and the relatively small sample of smokers studied here in the correlational analysis, the exact relationship between nicotine and neuroinflammation in human smokers remains to be confirmed.
Although impairment of neuroinflammation by smoking is a straightforward explanation of the study results, other explanations are possible, given the complex effects of cigarette smoking on the brain. Cigarette smoke contains thousands of constituents (Green and Rodgman, 1996), with hundreds having known toxic effects (Baker et al, 2004; Fowles and Dybing, 2003). It is possible that one or more of these constituents directly interfered with [11C]DAA1106 binding to TSPO, which would have resulted in the difference in binding between smokers in the satiated state and non-smokers found here. Additionally, acute smoking is known to disrupt blood–brain barrier function (Sajja et al, 2016), which could have created differences in radiotracer binding for smokers and non-smokers for the PET time period of interest used here.
In addition to the overall difference between smokers and non-smokers, the menthol cigarette smoker subgroup had less [11C]DAA1106 binding than the non-menthol cigarette smoker subgroup. This finding is consistent with prior research by our group (Brody et al, 2013) showing greater upregulation of nicotinic acetylcholine receptors throughout almost all brain regions in menthol than in non-menthol cigarette smokers. Also, research by others demonstrates that menthol cigarette smoking is associated with more severe biological abnormalities in some (Williams et al, 2007), but not all (Abobo et al, 2012; Muscat et al, 2009), studies that have examined this issue. Therefore, as in prior research, the present finding may be due to greater brain exposure to cigarette smoke (leading to greater impairment of microglial activation) in menthol cigarette smokers, a direct effect of menthol flavoring, or some other mechanism.
The primary limitation of this study was the absence of arterial blood sampling such that total distribution volume (Vt) was not ascertained. Vt may control for the potential confounds of between-subject differences in radiotracer metabolism and binding to vascular endothelium and plasma protein (Koshimori et al, 2015; Rizzo et al, 2014; Turkheimer et al, 2015). Although Vt is a common outcome measure in PET studies examining TSPO in conditions other than tobacco dependence (Colasanti et al, 2016; Haarman et al, 2016; Narendran et al, 2014), recent research demonstrates that the less invasive SUV measure tends to correlate well with Vt within individual PET studies (Toth et al, 2015; Yoder et al, 2015) and has high test–retest reliability (Nair et al, 2016; Toth et al, 2015). Other similar studies have used pseudo-reference regions for PET data analysis (Colasanti et al, 2016; Coughlin et al, 2014; Hamelin et al, 2016; Kreisl et al, 2016; Lyoo et al, 2015; Zurcher et al, 2015) to minimize potential confounds, but this method would not have been appropriate here due to the hypothesized and confirmed effect of smoking throughout the brain. Additional limitations included a modest sample size and the fact that smokers were scanned in the satiated state, such that we did not determine whether results were due to acute or chronic cigarette smoking. Future research could examine smokers in the abstinent state to determine the relationship between decreased [11C]DAA1106 binding and recency of smoking.
In summary, cigarette smokers in the satiated state have decreased TSPO availability, which is related to participants’ current smoking level (higher levels of smoking were associated with less TSPO availability). This effect appeared to be greater for menthol than for non-menthol cigarette smokers. Future research could examine the time course of recovery of TSPO availability upon smoking cessation and the interplay between smoking, neuroinflammation, and the progression of diseases thought to be mediated by neuroinflammation.
Funding and disclosure
This study was supported by the Tobacco-Related Disease Research Program (to ALB (23XT-0002)), the National Institute on Drug Abuse (to ALB (R01 DA20872)), and the Department of Veterans Affairs, Office of Research and Development (CSR&D Merit Review Award I01 CX000412 (to ALB)). This research was also supported, in part, by the DOMONKAI fund from the Department of Psychiatry, Graduate School of Medicine, at Chiba University (to KO), endowments from the Thomas P. and Katherine K. Pike Chair in Addiction Studies (to EDL) and Marjorie M. Green Trust (to EDL), and the National Institutes of Health (T32 DA024635 (to LCS)). The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript. The authors declare no conflict of interest.
Acknowledgments
We thank Josephine Ribe and Thienthe Vu for performing positron emission tomography and magnetic resonance imaging scans, respectively, for the study.
References
- Abobo CV, Ma J, Liang D (2012). Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine Tob Res 14: 801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony DC, Pitossi FJ (2012). Special issue commentary: the changing face of inflammation in the brain. Mol Cell Neurosci 53: 1–5. [DOI] [PubMed] [Google Scholar]
- Arimilli S, Damratoski BE, G LP (2015). Methods to evaluate cytotoxicity and immunosuppression of combustible tobacco product preparations. J Vis Exp 95: 52351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker RR, Massey ED, Smith G (2004). An overview of the effects of tobacco ingredients on smoke chemistry and toxicity. Food Chem Toxicol 42(Suppl)): S53–S83. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W (1996). Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 67: 588–597. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P 3rd (1994). Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther 56: 483–493. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Costello MR, Abrams AL, Scheibal D, Farahi J et al (2009. a). Brain nicotinic acetylcholine receptor occupancy: effect of smoking a denicotinized cigarette. Int J Neuropsychopharmacol 12: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Childress AR, Bota RG, Ho ML et al (2002). Brain metabolic changes during cigarette craving. Arch Gen Psychiatry 59: 1162–1172. [DOI] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Khan A, Kozman D, Costello MR et al (2011). Effect of secondhand smoke on occupancy of nicotinic acetylcholine receptors in brain. Arch Gen Psychiatry 68: 953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, London ED, Olmstead RE, Farahi J, Scheibal D et al (2006. a). Cigarette smoking saturates brain alpha4beta2 nicotinic acetylcholine receptors. Arch Gen Psychiatry 63: 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Allen-Martinez Z, Scheibal D, Abrams AL et al (2009. b). Ventral striatal dopamine release in response to smoking a regular vs a denicotinized cigarette. Neuropsychopharmacology 34: 282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mandelkern MA, Olmstead RE, Scheibal D, Hahn E, Shiraga S et al (2006. b). Gene variants of brain dopamine pathways and smoking-induced dopamine release in the ventral caudate/nucleus accumbens. Arch Gen Psychiatry 63: 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mukhin AG, La Charite J, Ta K, Farahi J, Sugar CA et al (2013). Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol 16: 957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P et al (2004). Smoking-induced ventral striatum dopamine release. Am J Psychiatry 161: 1211–1218. [DOI] [PubMed] [Google Scholar]
- Chaki S, Funakoshi T, Yoshikawa R, Okuyama S, Okubo T, Nakazato A et al (1999). Binding characteristics of [3H]DAA1106, a novel and selective ligand for peripheral benzodiazepine receptors. Eur J Pharmacol 371: 197–204. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Boutin H, Van Camp N, Dolle F, Tavitian B (2008). Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging 35: 2304–2319. [DOI] [PubMed] [Google Scholar]
- Colasanti A, Guo Q, Giannetti P, Wall MB, Newbould RD, Bishop C et al (2016). Hippocampal neuroinflammation, functional connectivity, and depressive symptoms in multiple sclerosis. Biol Psychiatry 80: 62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T et al (2009). beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry 66: 666–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV et al (2014). Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neurovirol 20: 219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO (1978). Measuring the degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addict Behav 3: 235–241. [DOI] [PubMed] [Google Scholar]
- Fan Z, Okello AA, Brooks DJ, Edison P (2015). Longitudinal influence of microglial activation and amyloid on neuronal function in Alzheimer's disease. Brain 138(Pt 12)): 3685–3698. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBWStructured Clinical Interview for DSM-IV Axis I Disorders Patient Edition (SCID-I/P, Version 2.0). Biometrics Research, New York State Psychiatric Institute: New York, NY, USA,1995.
- Fowles J, Dybing E (2003). Application of toxicological risk assessment principles to the chemical constituents of cigarettesmoke. Tob Control 12: 424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi KK, Foulds J, Steinberg MB, Lu SE, Williams JM (2009). Lower quit rates among African American and Latino menthol cigarette smokers at a tobacco treatment clinic. Int J Clin Pract 63: 360–367. [DOI] [PubMed] [Google Scholar]
- Gao Z, Nissen JC, Ji K, Tsirka SE (2014). The experimental autoimmune encephalomyelitis disease course is modulated by nicotine and other cigarette smoke components. PLoS ONE 9: e107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves RB, Coletta RD, Silverio KG, Benevides L, Casati MZ, da Silva JS et al (2011). Impact of smoking on inflammation: overview of molecular mechanisms. Inflamm Res 60: 409–424. [DOI] [PubMed] [Google Scholar]
- Green CR, Rodgman A (1996). The Tobacco Chemists' Research Conference; a half-century of advances in analytical methodology of tobacco and its products. Recent Adv Tob Sci 22: 131–304. [Google Scholar]
- Haarman BC, Burger H, Doorduin J, Renken RJ, Sibeijn-Kuiper AJ, Marsman JB et al (2016). Volume, metabolites and neuroinflammation of the hippocampus in bipolar disorder - a combined magnetic resonance imaging and positron emission tomography study. Brain Behav Immun 56: 21–33. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Tseng HH, Rao N, Selvanathan T, Kenk M, Bazinet RP et al (2016). Imaging microglial activation in untreated first-episode psychosis: a PET study with [18F]FEPPA. Am J Psychiatry 174: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelin L, Lagarde J, Dorothee G, Leroy C, Labit M, Comley RA et al (2016). Early and protective microglial activation in Alzheimer's disease: a prospective study using 18F-DPA-714 PET imaging. Brain 139(Pt 4)): 1252–1264. [DOI] [PubMed] [Google Scholar]
- Hannestad J, DellaGioia N, Gallezot JD, Lim K, Nabulsi N, Esterlis I et al (2013). The neuroinflammation marker translocator protein is not elevated in individuals with mild-to-moderate depression: a [(1)(1)C]PBR28 PET study. Brain Behav Immun 33: 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Henderson BJ, Lester HA (2015). Inside-out neuropharmacology of nicotinic drugs. Neuropharmacology 96(Pt B)): 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines CS, Fujita M, Zoghbi SS, Kim JS, Quezado Z, Herscovitch P et al (2013). Propofol decreases in vivo binding of 11C-PBR28 to translocator protein (18 kDa) in the human brain. J Nucl Med 54: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikoma Y, Yasuno F, Ito H, Suhara T, Ota M, Toyama H et al (2007). Quantitative analysis for estimating binding potential of the peripheral benzodiazepine receptor with [(11)C]DAA1106. J Cereb Blood Flow Metab 27: 173–184. [DOI] [PubMed] [Google Scholar]
- Kalra R, Singh SP, Pena-Philippides JC, Langley RJ, Razani-Boroujerdi S, Sopori ML (2004). Immunosuppressive and anti-inflammatory effects of nicotine administered by patch in an animal model. Clin Diagn Lab Immunol 11: 563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna A, Guo M, Mehra M, Royal W 3rd (2013). Inflammation and oxidative stress induced by cigarette smoke in Lewis rat brains. J Neuroimmunol 254: 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshimori Y, Ko JH, Mizrahi R, Rusjan P, Mabrouk R, Jacobs MF et al (2015). Imaging striatal microglial activation in patients with Parkinson's disease. PLoS ONE 10: e0138721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, Liow JS, Wei M, Snow J, Page E et al (2016). 11C-PBR28 binding to translocator protein increases with progression of Alzheimer's disease. Neurobiol Aging 44: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisl WC, Lyoo CH, McGwier M, Snow J, Jenko KJ, Kimura N et al (2013). In vivo radioligand binding to translocator protein correlates with severity of Alzheimer's disease. Brain 136(Pt 7)): 2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lartey FM, Ahn GO, Shen B, Cord KT, Smith T, Chua JY et al (2014). PET imaging of stroke-induced neuroinflammation in mice using [18F]PBR06. Mol Imaging Biol 16: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo CH, Ikawa M, Liow JS, Zoghbi SS, Morse CL, Pike VW et al (2015). Cerebellum can serve as a pseudo-reference region in Alzheimer disease to detect neuroinflammation measured with PET radioligand binding to translocator protein. J Nucl Med 56: 701–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda J, Suhara T, Zhang MR, Okauchi T, Yasuno F, Ikoma Y et al (2004). Novel peripheral benzodiazepine receptor ligand [11C]DAA1106 for PET: an imaging tool for glial cells in the brain. Synapse 52: 283–291. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Rusjan PM, Kennedy J, Pollock B, Mulsant B, Suridjan I et al (2012). Translocator protein (18 kDa) polymorphism (rs6971) explains in vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J Cereb Blood Flow Metab 32: 968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat JE, Chen G, Knipe A, Stellman SD, Lazarus P, Richie JP Jr (2009). Effects of menthol on tobacco smoke exposure, nicotine dependence, and NNAL glucuronidation. Cancer Epidemiol Biomarkers Prev 18: 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A, Veronese M, Xu X, Curtis C, Turkheimer F, Howard R et al (2016). Test-retest analysis of a non-invasive method of quantifying [(11)C]-PBR28 binding in Alzheimer's disease. EJNMMI Res 6: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Lopresti BJ, Mason NS, Deuitch L, Paris J, Himes ML et al (2014). Cocaine abuse in humans is not associated with increased microglial activation: an 18-kDa translocator protein positron emission tomography imaging study with [11C]PBR28. J Neurosci 34: 9945–9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB (2014). Microglia development and function. Annu Rev Immunol 32: 367–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo T, Yoshikawa R, Chaki S, Okuyama S, Nakazato A (2004). Design, synthesis and structure-affinity relationships of aryloxyanilide derivatives as novel peripheral benzodiazepine receptor ligands. Bioorg Med Chem 12: 423–438. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Faseru B, Sanderson Cox L, Bronars CA, Ahluwalia JS (2007). Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction 102: 1979–1986. [DOI] [PubMed] [Google Scholar]
- Ory D, Planas A, Dresselaers T, Gsell W, Postnov A, Celen S et al (2015). PET imaging of TSPO in a rat model of local neuroinflammation induced by intracerebral injection of lipopolysaccharide. Nucl Med Biol 42: 753–761. [DOI] [PubMed] [Google Scholar]
- Owen DR, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC et al (2011). Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med 52: 24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A et al (2012). An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab 32: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P et al (2006). Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci 27: 402–409. [DOI] [PubMed] [Google Scholar]
- Piao WH, Campagnolo D, Dayao C, Lukas RJ, Wu J, Shi FD (2009). Nicotine and inflammatory neurological disorders. Acta Pharmacol Sin 30: 715–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher MJ, Hulley BJ, Houston T, Kiefe CI, Benowitz N, Sidney S (2006). Menthol cigarettes, smoking cessation, atherosclerosis, and pulmonary function: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Arch Int Med 166: 1915–1922. [DOI] [PubMed] [Google Scholar]
- Pluvy I, Garrido I, Pauchot J, Saboye J, Chavoin JP, Tropet Y et al (2015). Smoking and plastic surgery, part I. Pathophysiological aspects: update and proposed recommendations. Ann Chir Plast Esthet 60: e3–e13. [DOI] [PubMed] [Google Scholar]
- Reuther WJ, Brennan PA (2014). Is nicotine still the bad guy? Summary of the effects of smoking on patients with head and neck cancer in the postoperative period and the uses of nicotine replacement therapy in these patients. Br J Oral Maxillofac Surg 52: 102–105. [DOI] [PubMed] [Google Scholar]
- Rinker B (2013). The evils of nicotine: an evidence-based guide to smoking and plastic surgery. Ann Plast Surg 70: 599–605. [DOI] [PubMed] [Google Scholar]
- Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE, Bertoldo A (2014). Kinetic modeling without accounting for the vascular component impairs the quantification of [(11)C]PBR28 brain PET data. J Cereb Blood Flow Metab 34: 1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajja RK, Rahman S, Cucullo L (2016). Drugs of abuse and blood-brain barrier endothelial dysfunction: a focus on the role of oxidative stress. J Cereb Blood Flow Metab 36: 539–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMHSASubstance Abuse and Mental Health Services Administration. Office of Applied Studies. The NSDUH Report: Use of Menthol Cigarettes. US Department of Health and Human Services: Rockville, MD, USA, 2009.
- Sandiego CM, Gallezot JD, Pittman B, Nabulsi N, Lim K, Lin SF et al (2015). Imaging robust microglial activation after lipopolysaccharide administration in humans with PET. Proc Natl Acad Sci USA 112: 12468–12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G et al (2015). Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman SM, Jarvik ME (1976). Smoking withdrawal symptoms in two weeks of abstinence. Psychopharmacology (Berl) 50: 35–39. [DOI] [PubMed] [Google Scholar]
- Sorensen LT (2012). Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg 147: 373–383. [DOI] [PubMed] [Google Scholar]
- Spielberger C (1983) Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press: Palo Alto, CA, USA. [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E et al (2006). Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci 26: 8707–8714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surendranathan A, Rowe JB, O'Brien JT (2015). Neuroinflammation in Lewy body dementia. Parkinsonism Relat Disord 21: 1398–1406. [DOI] [PubMed] [Google Scholar]
- Suridjan I, Pollock BG, Verhoeff NP, Voineskos AN, Chow T, Rusjan PM et al (2015). In vivo imaging of grey and white matter neuroinflammation in Alzheimer's disease: a positron emission tomography study with a novel radioligand, [18F]-FEPPA. Mol Psychiatry 20: 1579–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suridjan I, Rusjan PM, Voineskos AN, Selvanathan T, Setiawan E, Strafella AP et al (2014). Neuroinflammation in healthy aging: a PET study using a novel Translocator Protein 18kDa (TSPO) radioligand, [(18)F]-FEPPA. Neuroimage 84: 868–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R et al (2010). Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol 13: 943–950. [DOI] [PubMed] [Google Scholar]
- Tilp C, Bucher H, Haas H, Duechs MJ, Wex E, Erb KJ (2016). Effects of conventional tobacco smoke and nicotine-free cigarette smoke on airway inflammation, airway remodelling and lung function in a triple allergen model of severe asthma. Clin Exp Allergy 46: 957–972. [DOI] [PubMed] [Google Scholar]
- Toth M, Doorduin J, Haggkvist J, Varrone A, Amini N, Halldin C et al (2015). Positron emission tomography studies with [11C]PBR28 in the healthy rodent brain: validating SUV as an outcome measure of neuroinflammation. PLoS ONE 10: e0125917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler J (2000). Cigarette smoking and its effects on wound healing. J Wound Care 9: 100–104. [DOI] [PubMed] [Google Scholar]
- Turkheimer FE, Rizzo G, Bloomfield PS, Howes O, Zanotti-Fregonara P, Bertoldo A et al (2015). The methodology of TSPO imaging with positron emission tomography. Biochem Soc Trans 43: 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrone A, Oikonen V, Forsberg A, Joutsa J, Takano A, Solin O et al (2015). Positron emission tomography imaging of the 18-kDa translocator protein (TSPO) with [18F]FEMPA in Alzheimer's disease patients and control subjects. Eur J Nucl Med Mol Imaging 42: 438–446. [DOI] [PubMed] [Google Scholar]
- Venneti S, Lopresti BJ, Wang G, Slagel SL, Mason NS, Mathis CA et al (2007. a). A comparison of the high-affinity peripheral benzodiazepine receptor ligands DAA1106 and (R)-PK11195 in rat models of neuroinflammation: implications for PET imaging of microglial activation. J Neurochem 102: 2118–2131. [DOI] [PubMed] [Google Scholar]
- Venneti S, Wagner AK, Wang G, Slagel SL, Chen X, Lopresti BJ et al (2007. b). The high affinity peripheral benzodiazepine receptor ligand DAA1106 binds specifically to microglia in a rat model of traumatic brain injury: implications for PET imaging. Exp Neurol 207: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneti S, Wang G, Nguyen J, Wiley CA (2008). The positron emission tomography ligand DAA1106 binds with high affinity to activated microglia in human neurological disorders. J Neuropathol Exp Neurol 67: 1001–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walberer M, Jantzen SU, Backes H, Rueger MA, Keuters MH, Neumaier B et al (2014). In vivo detection of inflammation and neurodegeneration in the chronic phase after permanent embolic stroke in rats. Brain Res 1581: 80–88. [DOI] [PubMed] [Google Scholar]
- Walker MD, Dinelle K, Kornelsen R, Lee NV, Miao Q, Adam M et al (2015). [11C]PBR28 PET imaging is sensitive to neuroinflammation in the aged rat. J Cereb Blood Flow Metab 35: 1331–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gao M, Zheng QH (2012). Fully automated synthesis of PET TSPO radioligands [11C]DAA1106 and [18F]FEDAA1106. Appl Radiat Isot 70: 965–973. [DOI] [PubMed] [Google Scholar]
- Wang Y, Yue X, Kiesewetter DO, Niu G, Teng G, Chen X (2014). PET imaging of neuroinflammation in a rat traumatic brain injury model with radiolabeled TSPO ligand DPA-714. Eur J Nucl Med Mol Imaging 41: 1440–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Gandhi KK, Steinberg ML, Foulds J, Ziedonis DM, Benowitz NL (2007). Higher nicotine and carbon monoxide levels in menthol cigarette smokers with and without schizophrenia. Nicotine Tob Res 9: 873–881. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Kosaka J, Ota M, Higuchi M, Ito H, Fujimura Y et al (2012). Increased binding of peripheral benzodiazepine receptor in mild cognitive impairment-dementia converters measured by positron emission tomography with [(11)C]DAA1106. Psychiatry Res 203: 67–74. [DOI] [PubMed] [Google Scholar]
- Yasuno F, Ota M, Kosaka J, Ito H, Higuchi M, Doronbekov TK et al (2008). Increased binding of peripheral benzodiazepine receptor in Alzheimer's disease measured by positron emission tomography with [11C]DAA1106. Biol Psychiatry 64: 835–841. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Nho K, Risacher SL, Kim S, Shen L, Saykin AJ (2013). Influence of TSPO genotype on 11C-PBR28 standardized uptake values. J Nucl Med 54: 1320–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder KK, Territo PR, Hutchins GD, Hannestad J, Morris ED, Gallezot JD et al (2015). Comparison of standardized uptake values with volume of distribution for quantitation of [(11)C]PBR28 brain uptake. Nucl Med Biol 42: 305–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu I, Inaji M, Maeda J, Okauchi T, Nariai T, Ohno K et al (2010). Glial cell-mediated deterioration and repair of the nervous system after traumatic brain injury in a rat model as assessed by positron emission tomography. J Neurotrauma 27: 1463–1475. [DOI] [PubMed] [Google Scholar]
- Zhang MR, Kida T, Noguchi J, Furutsuka K, Maeda J, Suhara T et al (2003). [(11)C]DAA1106: radiosynthesis and in vivo binding to peripheral benzodiazepine receptors in mouse brain. Nucl Med Biol 30: 513–519. [DOI] [PubMed] [Google Scholar]
- Zurcher NR, Loggia ML, Lawson R, Chonde DB, Izquierdo-Garcia D, Yasek JE et al (2015). Increased in vivo glial activation in patients with amyotrophic lateral sclerosis: assessed with [(11)C]-PBR28. Neuroimage Clin 7: 409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]