Abstract
Behavioural and psychological symptoms of dementia (BPSD) occur in most patients with dementia. They cause great suffering in patients and caregivers, sometimes more so than the cognitive and functional decline inherent to dementia. The clinical features of BPSD include a wide variety of affective, psychotic and behavioural symptoms and signs. The causes and risk factors for BPSD are multiple and include biological, psychological and environmental variables. Frequently, their combination, rather than any specific factor, explains the occurrence of BPSD in an individual patient. Thus, a sound etiopathogenetic investigation including the patient and the family or care team is essential. The aim is to develop an individualized treatment plan using a therapeutic decision tree modified by the individual and environmental risk profile. Still, treatment may be difficult and challenging. Clinical empiricism often steps in where evidence from controlled studies is lacking. Psychosocial treatment approaches are pivotal for successful treatment of BPSD. Often a combination of different non-pharmacological approaches precedes drug treatment (most of which is off-label). Regular assessments of the treatment plan and any prescriptions must be carried out to detect signs of relapse and to stop any medicines that may have become inappropriate. Even with optimal management, BPSD will not disappear completely in some cases and will remain challenging for all involved parties. This article is a narrative review based closely on the interprofessional Swiss recommendations for the treatment of BPSD. To establish the recommendations, a thorough research of the literature has been carried out. Evidence-based data were provided through searches of Medline, Embase, ISI and Cochrane-Database research. Evidence categories of the World Federation of Biological Societies were used. Additionally, the clinical experience of Swiss medical experts was considered.
Keywords: attachment, BPSD, environmental factors, etiopathogenetic, individualized treatment, personality
Introduction
The term behavioural and psychological symptoms of dementia (BPSD; also termed neuropsychiatric symptoms) describes the heterogeneous group of symptoms and signs of disturbed perception, thought content, mood or behaviour that frequently occur in patients with dementia.1,2 Throughout the course of their dementia, the vast majority of patients will develop one or more BPSD.1–6 BPSD can have serious consequences. They are associated with worsening cognition and progression to more severe stages of dementia.7 BPSD also lead to individual suffering and impact the caregiver burden.8 Furthermore, they increase the risk for secondary complications such as falls and fractures leading to emergency room admissions,9 and ultimately institutionalization.10,11 Finally, BPSD result in higher costs of therapy and caregiving.12,13
The treatment of patients with BPSD can be challenging for physicians and healthcare teams. The etiopathogenesis of BPSD is often complex, with multiple contributing direct factors and indirect mediators. Biological factors (e.g. brain changes, comorbidities, medication) may interact with psychological (e.g. personal life history, personality) or social (support network, living arrangements) aspects. Consequently, treatment should be guided by a comprehensive etiopathogenetic assessment. Currently, there is limited evidence for symptomatic treatments and the available evidence-based options are only moderately effective. Psychosocial, that is non-pharmacological, approaches should be considered the mainstay of therapy and are complemented by psychotropic medication only if unavoidable. Ideally, the available treatment algorithms are used to devise an individualized treatment plan informed by a multifaceted understanding of the patient’s situation, clinical experience and expert knowledge.
Clinical presentation of BPSD
The clinical presentations of BPSD include apathy, depression, anxiety, delusions, hallucinations, sexual or social disinhibition, sleep–wake cycle disturbances, aggression, agitation and other behaviours considered inappropriate.14 There are several instruments to systematically assess the presence and severity of BPSD,15 among which the Neuropsychiatric Inventory (NPI)16 and Behavioral Pathology in Alzheimer’s Disease Rating Scale (BEHAVE-AD)17 are recommended.18 Some BPSD tend to cluster together, usually into four clusters – that is, the affective, psychotic, hyperactive and apathetic clusters.1,19 In a population-based study, the cumulative incidence of having one or more NPI-measured BPSD from the onset of cognitive symptoms was 80%,20 indicating that occurrence of BPSD has to be expected throughout the course of dementia. Apathy, depression, anxiety and agitation were found to be the most frequent forms of BPSD.2,20,21 However, a recent systematic review revealed substantial variation in the reported prevalence, incidence and longitudinal course between different studies.22 In an individual patient, the type and severity of BPSD tend to change over time, but some forms such as wandering seem to be more persistent.22 Overall, the ‘natural course’ of BPSD over time is still largely unknown.
Depression
Most patients with dementia have depressive symptoms and signs at some point in time over the course of dementia (nearly 80% over the past 5 years). Some patients may present with a major depressive disorder (10–20%).1 A history of depressive disorder is likely to increase the risk of major depressive disorder during dementia. Insomnia, changes in circadian rhythm and anxiety may accompany depressive symptoms. Abnormalities in the serotonin, dopamine or epinephrine systems, frontal atrophy, and amygdala reactivity may be some of the neurobiological underpinnings of depressive features.
Hallucinations
Another condition that should be considered is the Charles Bonnet syndrome, which is due to an eye disease. Visual hallucinations in Charles Bonnet syndrome are usually of short duration and patients are aware that they are not real; these hallucinations are often well tolerated by the patient and therefore may not need treatment other than that prompted by the underlying eye disease. In some instances, carbamazepine may be useful.23 Auditory hallucinations must evoke an underlying psychotic state not primarily explained by dementia, and usually need treatment.
Agitation
In an inpatient clinical setting, agitation is often the most challenging BPSD since it may severely disrupt patient care. Hence, most treatment trials for BPSD have been performed for agitation. Agitation refers to an ill-defined spectrum of aberrant hyperactive motor behaviours (such as wandering, leaving home) and physically or verbally aggressive behaviours such as rejection of care. Only recently a provisional consensus definition has been suggested for agitation in cognitive disorders.24 Beyond the behavioural phenotype, this new definition emphasizes the emotional distress and excess disability that is associated with agitation. Agitation may worsen during the evening hours, a phenomenon referred to as ‘sundowning’.25
Delusions
Since delusions in dementia are found to sometimes correspond to reality or to be neither incorrigible nor held with absolute certainty, they may not represent psychotic symptoms in a narrow sense and should certainly not preclude the attempt to understand their meaning.26 In terms of content, delusions in dementia are frequently persecutory in nature or revolve around theft – that is, lost objects,27 danger, abandonment and the idea that one’s house is not one’s home.26 Notably, only about half of the cases of delusions appear to lead to discomfort and are associated with behavioural disturbances.28 A frequent subtype of delusions in dementia is the misidentification syndromes in which a patient consistently misidentifies persons, places, objects or events (e.g. Capgras syndrome).29 Sensory impairment is widely considered a contributory factor in the development of certain delusions in dementia, most obviously in cases of Charles Bonnet syndrome in the context of visual impairment.
Apathy
Apathy is usually defined as loss of motivation and decreased interest in daily activities.30 In the most severe forms, the affected patients may be unable to initiate almost any kind of directed activity, thus spending most of the day in bed or sitting in a chair. Apathy is one of the most frequent forms of BPSD and is associated with poor prognosis and increased mortality.31,32 However, apathy rarely leads to hospital admissions since it is not usually as disruptive for caregivers as other BPSD.
Sleep problems and disturbances of circadian rhythms
Sleep problems are both risk factors and frequent symptoms of dementias and may also arise from comorbidities.33,34 Sleep problems are a major contributor to caregiver burden. Even though the clinical need for effective treatments is high, the evidence base for treatments is limited.35 In dementia with Lewy bodies (DLB), REM (rapid eye movement) sleep behaviour disorder (RBD) can be an early sign of the disease, with daytime fluctuations of attention, greater number of daytime naps and longer night sleeps.36,37 RBD can also be seen in other synucleopathies such as Parkinson’s disease and multiple system atrophy. In frontotemporal dementia (FTD), RBD is rare but may be confused with excessive nocturnal activity due to disturbed circadian rhythmicity.38
Etiopathogenesis of BPSD
More often than not the etiopathogenesis of BPSD is complex and multifactorial.1 For the sake of didactic simplicity and practicability, the causative and contributing factors can be divided into biological, psychological and social or environmental factors (Figure 1).
Figure 1.
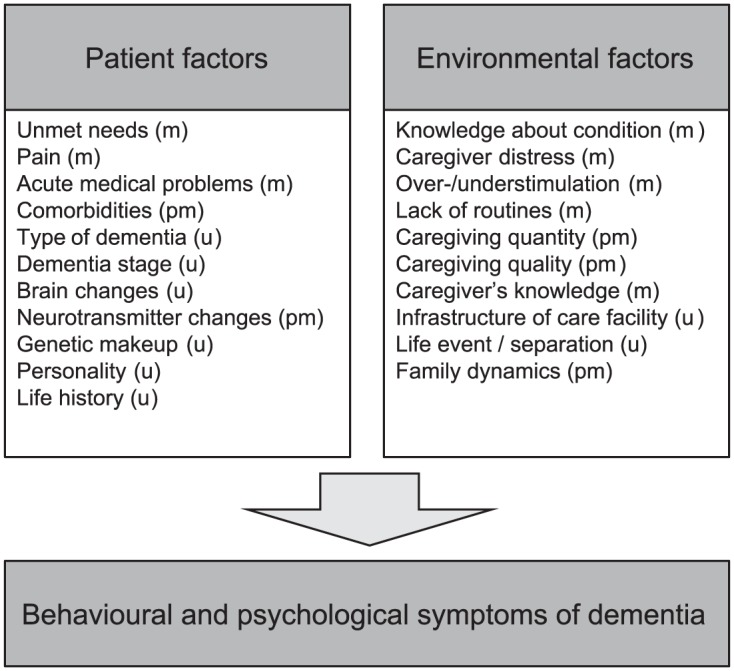
Simplified etiopathogenetic model of BPSD.
m, modifiable; pm, potentially modifiable; u, unmodifiable.
Based on this perspective, a model for etiopathogenetic treatment of BPSD can be derived.
Biological perspective
Brain lesions and type of dementia
Dementia-related brain lesions and changes in neurotransmission have been linked to specific BPSD.1 Their effect may be moderated by other biological factors such as comorbidity or treatment-related factors, as well as the individual’s genetic make-up.
In the course of Alzheimer’s disease (AD), psychotic symptoms were found to be associated with neuronal loss in several brain regions, including the hippocampus, parahippocampal gyrus and various brain stem nuclei.39 In mixed (vascular and AD) dementia, vascular factors may trigger hallucinations, illusions, anxiety, dysphoria, aggression and delirium.39–41
Besides the aphasic forms, FTD is defined by behavioural features (bv-FTD or behavioural variant of FTD). These features are present from the early stages of the disorder and include loss of interest and apathy, as well as disinhibition, including inappropriate sexual behaviour and impulsive behaviours that are more frequent than in other dementias.39 Both sporadic and familial forms of FTD have been described.42,43 Similar to AD, a number of neurotransmitter and cortico-limbic changes have been linked to behavioural disruptions in FTD.1,42,44 Clearly, FTD is an important differential diagnosis, particularly in the early dementia stages. It is different from the frontal variant of AD,45 and BPSD in FTD are probably less amenable to efficient treatment than in other forms of dementia.
Many neuroanatomical studies have been conducted that link BPSD to dementia-related brain lesions. For example, apathy was associated with hypoperfusion in the anterior cingulate cortex and fronto-subcortical structures,1,46 giving rise to a disconnection model of apathy between the prefrontal cortex and the mediodorsal and anterior thalamic nuclei.47 However, hypoperfusion in frontal or temporal lobes was also found to correlate with aggression and psychosis.1 A variety of functional and structural parameters including, for example, white matter changes,48 atrophy patterns and vascular damage contribute to BPSD.48,49
Changes in neurotransmission and neuromodulation
Changes in neurotransmission and neuromodulation have been found to correlate with BPSD. In AD, changes in cholinergic activity in the frontal and temporal cortices may be linked to aberrant motor activity and aggressive behaviour.50,51 Visual hallucinations in DLB seem to be linked with cholinergic deficits in the temporal cortex.50 In AD, aggression was reported to be related to reduced dopamine concentration in the temporal cortex.44 Decreased norepinephrine neurons in the locus coeruleus are accompanied by aggressive behaviour.1,52 Similarly, apathy was correlated with dopaminergic dysfunction in AD.31,53,54 Serotonin concentration correlated positively with aggressiveness, depressed mood, anxiety, agitation and restlessness.1 Interestingly, serotonin and, thus, SSRIs, may improve hippocampal neurogenesis,55 and synaptic plasticity and survival of neurons are linked to the glutamatergic pathway.1,56,57 Severe glutamate loss in AD may result in psychotic symptoms. Significant GABA decrease in the frontal and temporal cortex and high GABA plasma concentration were found in severe AD that correlated with depression and apathy.1 As drugs acting on the above-mentioned neurotransmitter systems are legion, it is evident that many of them can either cause or favour the development of BPSD58 or, on the contrary, have a potentially positive influence on them. Further pathway disorders related to neuromodulator and neuroendocrine systems have been proposed as correlates of BPSD, as well as circadian rhythms and sleep disorders,1,59,60 including those of the hypothalamic–pituitary–adrenal axis or the homocystein metabolism.61
A number of findings suggest a genetic vulnerability for BPSD, though this field of research is still in its infancy. As an example, AD subjects who are ApoEε4 carriers had more delusions and agitation/aggressive behaviours than non-ApoEε4 carriers.62 Given the implication in BPSD of neurotransmitter changes, it may come as no surprise that specific polymorphisms may predispose to BPSD.62–64
Physical disorders and pain
Physical disorders are often a central element in the understanding of BPSD and must be evaluated and treated accordingly.1,6,58,65 Among the most frequent somatic causes of BPSD are pain, infections, electrolyte imbalances or metabolic disorders, urinary retention, constipation, cerumen and others. Any of these may cause BPSD and a thorough medical examination is therefore a requirement. Especially pain often leads to BPSD of various types, such as insomnia, aggressiveness or agitation.66 Looking for pain-inducing factors and eliminating them is pivotal, as pain is too often undetected and therefore undertreated in people with dementia.
Psychological and environmental perspective
Considering BPSD as the result of stressors combined with variable degrees of vulnerability, it is easy to imagine a host of psychological and systemic factors (personality, environmental elements both physical and emotional that contribute to the occurrence of BPSD).
Personality traits
Personality changes occur when dementia develops. In AD, a predictable change seems to occur independently of the previous personality, in that neuroticism usually increases while extraversion, openness and conscientiousness tend to decrease, with agreeability remaining more stable.67 Furthermore and apart from its obvious face validity, there is increasing evidence that our personality – what we are as persons – contributes to the clinical expression of dementia. Thus, some personality traits may favour, or on the contrary protect against, BPSD. Thus, in AD, increased neuroticism as a premorbid personality trait may be associated with a higher risk for depression,1,65,68,69 and even be a risk factor for cognitive decline and AD.70,71 Patients who have been suspicious or aggressive before dementia starts are more likely to have BPSD than those without these traits.1,69 However, such correlations have not always been found68 and one of the more significant limitations of most of the studies currently available is the use of retrospective personality ratings, subjecting their findings to possible inaccuracies. Psychiatric diseases may be risk factors for dementia as this has been established for depression and for BPSD.72
Life events
Stressful life events in childhood or adulthood may favour BPSD in dementia through, among other etiopathogenic lines, increased vulnerability related to hippocampal hypotrophy and behavioural inhibition or insecure attachment.56,73–75 Thus, overt attachment behaviour towards a family member or stranger was pronounced in old nursing home residents depending on the degree of cognitive impairment, suggesting that dementia eroded feelings of security and activated attachment behaviours.76,77 Securely attached individuals with dementia displayed more positive affect than avoidantly attached individuals.78
Environmental risks
Environmental factors, both physical and social, are likely to precipitate or buffer BPSD. Thus, lower levels of BPSD are associated with well-being of nursing home residents, which is in turn related to environmental characteristics such as unobtrusive safety features, variety of spaces in environments with calm, single rooms available, small facility size, and optimization of levels of stimulation, taking into account the capacities of each patient.1 Similarly, caregiver distress can exacerbate BPSD and family discord or altered communication in the family need to be assessed.79,80
Treatment
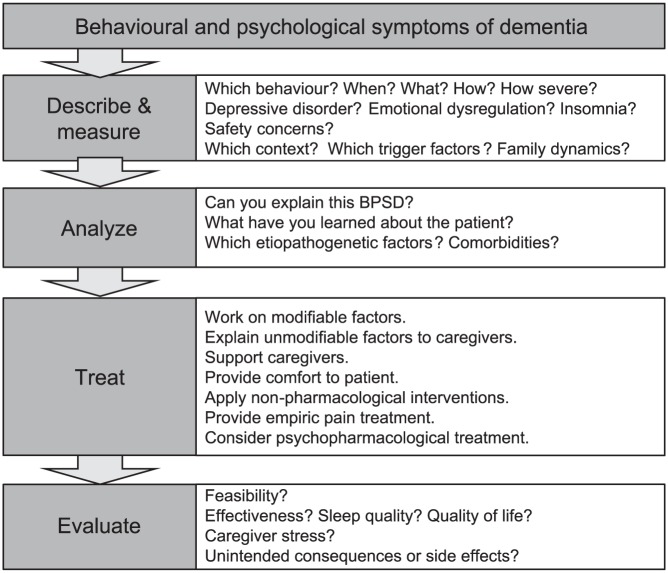
The treatment of BPSD is often highly challenging due to the complex etiopathogenesis of the symptoms and signs and the multi-morbidity of patients. BPSD management requires both a patient-centred and caregiver-centred focus and interventions to provide comfort to patients and alleviate caregiver burden are indispensable. Treating concomitant somatic diseases can reduce BPSD.6 Effective pain management is part of a successful BPSD treatment.81 Most expert recommendations and guidelines prefer non-pharmacological interventions as the first-line approach.6,82,83 Although the evidence for most non-pharmacological strategies is weak, their efficacy is supported by long-standing clinical experience. Pharmacotherapy for BPSD is frequently provided, but it carries the risk of serious side-effects. Therefore, non-pharmacological therapies are considered the first choice and should also be continued when pharmacotherapy is necessary. In order to measure treatment effects, frequency and severity of BPSD should be quantified at baseline, possibly using a validated scale or questionnaire, such as the NPI16 or BEHAVE-AD.17,18 Moreover, several algorithms have been published to guide the diagnostic and therapeutic process for BPSD.14 We suggest using the simplified BPSD-DATE algorithm (describe and measure, analyse, treat, evaluate; see Figure 2).
Figure 2.
BPSD-DATE interventional algorithm.
Non-pharmacological approaches
Due to the heterogeneous nature of non-pharmacological interventions, study designs vary widely in this area and call the generalizability of their results into question. Compared to pharmacological treatments of BPSD, the evidence base is much more limited. As of yet, it remains unclear whether or not heterogeneous non-pharmacological interventions are efficacious due to a common active but non-specific ingredient such as positive human interaction. At this point, the best scientific evidence exists for the use of home-based behavioural management techniques, caregiver-based interventions or staff training in communication skills, person-centred care, dementia care mapping against agitation84,85 and music therapy against agitation and anxiety.84
Psychosocial interventions
Psychoeducation for patients and caregivers can reduce BPSD.6,86–88 Multi-component single and group session programmes are effective if they focus on stressful events, provide both information about the disease and assistance, and allow for exchange on experiences as to how to deal with daily problems. Group sessions are more disburdening for caregivers when they include training in behavioural management techniques.87,88 Coping strategy-based family carer therapy and tailored activities for people with dementia and their caregivers were found to improve quality of life of people with dementia living at home.88 Psychoeducational interventions may be accompanied by social counselling, organizing assistance and supporting patients and caregivers. Psychosocial interventions, in general, decrease caregiver depression and can help delay the institutionalization of patients.89
Nursing care
The need-driven dementia-compromised behaviour model (NDB) can help understand BPSD as a dysfunctional expression of needs.90 Based on this model, behavioural analysis in nursing care may recognize the patient’s urgent needs and reveal their causes. Some factors causing BPSD, such as pain, hunger or thirst, can be satisfied immediately. However, personality characteristics and the biography of the patient, comorbidities and the lack of personal resources can complicate the course of the disease. The Serial Trial Intervention, based on the NDB model, uses systematic serial assessments and sequential trials of treatments to identify and treat unmet needs that may be the underlying cause of BPSD. It has been shown to reduce BPSD and the use of psychotropic drugs.91 Special nursing care interventions targeting vocalization92 and sexual disinhibition93 may be helpful to comfort patients.
Physical activity
Regular exercise improves physical fitness, behaviour, cognition and functioning in older people.94 There is strong evidence that regular physical activity improves physical, cognitive, functional and behavioural outcomes also in patients with dementia and can help reduce BPSD.94 Usually, training programmes are based on walking (mobility training) or they combine walking with different types of isotonic exercises.
Sensory stimulation and music therapy
Music therapy and multisensorial stimulation techniques such as snoezelen are effective in reducing agitation and disruptive behaviour during sessions and immediately after the intervention.6,86 However, there is no evidence for long-term effects. Biography-related music and combination with sensory stimulation seem to be more effective.
Reality orientation and cognitive stimulation therapy
These interventions are based on the idea that a better orientation in daily life to persons, time or surroundings can improve BPSD.86 Reality orientation therapy is more effective in combination with other techniques in improving mood and decreasing BPSD. Derived from reality orientation therapy, cognitive stimulation therapy addresses current problems in functioning using information processing.86 There are some immediate effects on BPSD, but the data are inconsistent.
Validation therapy
This patient-centred technique intends to resolve unfinished conflicts by encouraging and positively validating expression of feelings.86 There is some evidence that positively validating expression of feelings may reduce irritability.
Reminiscence therapy
Reminiscence therapy uses objects from daily life to stimulate memory and enable people to value their experiences.86 This intervention can improve mood.
Psychotherapeutic interventions
Psychological therapies have been investigated in mild to moderate dementia. The highest level of evidence of efficacy is available for cognitive behavioural techniques.6,86,95,96 Focusing on daily problems, psychotherapeutic interventions are more effective if caregivers are involved in the process. Combination with psychoeducation and family counselling improves effectiveness.96,97 Behavioural management techniques improve depression, anxiety, aggression and agitation in dementia.86 The effect is significant and lasts for months. Since caregivers can also develop depression during the care process, they can also benefit from individual psychotherapy.
Psychopharmacotherapy
Since patients with dementia are particularly vulnerable to adverse effects of drugs, the indication for psychopharmacotherapy in BPSD has to be discussed very critically. Multi-morbidity and polypharmacy are interacting factors complicating the use of pharmacotherapy. Most drugs are not approved for BPSD and their use is therefore off-label. A detailed clinical and laboratory examination including history of medication and an electrocardiogram should precede psychopharmacotherapy. Psychotropic medication use should be limited in time and stopped after a gradual reduction when BPSD improve. Drug metabolism is altered in elderly patients and compared to younger patients they usually need lower doses of psychotropic drugs.
Antidementia drugs
There is some evidence that cholinesterase inhibitors and memantine may be useful in the management of BPSD.6,14,98–101 Cholinesterase inhibitors may improve affective features in mild to moderate dementia. Cholinesterase inhibitors and memantine may be effective to treat BPSD. Indeed, donepezil may alleviate the following BPSD in mild to moderate dementia: apathy, depression, tension, irritability. There are similar findings for galantamine and rivastigmine. However, treatment of agitation in AD by donepezil appears to be inefficient. In conclusion, cholinesterase inhibitors have a certain efficacy on negative symptoms.6,102 Memantine may be more effective on positive symptoms including agitation, delusions and hallucinations, as well as aggression in moderate to severe AD.6,102 However, more recent trials specifically designed for treatment of agitation challenge these findings as they failed to demonstrate a benefit.99,103,104 Finally, antidementia drugs may reduce the incidence of BPSD. There are also data that provide some evidence for the preventive efficacy of Ginkgo biloba extract EGb 761® in the treatment of dementia patients with clinically relevant BPSD.105
Antidepressants
Depression and anxiety are among the most common BPSD and an effective antidepressive therapy in dementia can improve both cognition and affective symptoms as well as other forms of BPSD, such as agitation and aggressiveness.6,14,106 Tricyclic antidepressants are not recommended because of their anticholinergic adverse events. SSRIs have reasonable tolerability and favourable treatment response. In dementia, SSRIs (specifically citalopram) are as efficacious as atypical antipsychotics for treating agitation.107 SSRIs can be associated with severe adverse effects such as QT-prolongation and hyponatraemia.
Antipsychotics
First, it is important to state that antipsychotics have not been approved for clinical use in dementia, except for risperidone, at least in some countries. Thus, clinicians ought to refer to their country’s legislation before introducing an antipsychotic drug to treat BPSD. Atypical antipsychotics such as risperidone and aripiprazole are among the most often (and probably too often) prescribed drugs in BPSD. They are effective in the treatment of psychotic symptoms, agitation and aggression.2,14,108,109 Haloperidol may be considered in the treatment of delirium in dementia, but it is not recommended for a different use in dementia. Haloperidol is only recommended for delirium because of its high potential for side-effects. Adverse events associated with atypical antipsychotics include anticholinergic effects, orthostatic hypotension, seizures, metabolic syndrome, weight gain, extrapyramidal symptoms, sedation and QT-prolongation. The increased mortality and the risk for cerebrovascular incidents have led to a black box warning for the use of antipsychotics in dementia. Antipsychotics can be necessary and helpful in the treatment of certain BPSD, but their use must be limited in time. Regular evaluations of risks and benefits are necessary throughout the course of the treatment.110 While the evidence on the efficacy of quetiapine for BPSD is mixed, it is widely used clinically.111 Due to its favourable side-effect profile, particularly regarding extrapyramidal signs, quetiapine may be of particular value for BPSD, especially in patients with Parkinsonian features, despite conflicting evidence.112
Mood stabilizers
Although carbamazepine shows some benefit for agitation in dementia, mood stabilizers are often associated with severe side-effects.2,14,113 Thus, valproic acid is not recommended. There is some clinical experience and limited evidence for gabapentine and lamotrigine in the treatment of BPSD.
Benzodiazepines
Evidence for the efficacy of benzodiazepines in BPSD is lacking. Benzodiazepines are associated with sedation, dizziness, falls, worsening cognition, respiratory depression, dependency and paradoxical disinhibition in the elderly. They are thus only recommended for the management of an acute crisis,6,14 if other methods fail. Their use must be limited in time and they should not be prescribed as hypnotics.
Other substances
Hypnotics such as zopiclone, zolpidem or zaleplone can have similar side-effects as benzodiazepines.6 They are used for sleep disorders in dementia over a limited period of time and at small doses. Sedative antidepressants such as trazodone seem to improve sleep duration. Melatonin and melatonin receptor agonists can be effective in treating circadian sleep disorders.34,35
Biological therapies
Light therapy (in the morning) and light therapy in combination with melatonin (at bedtime) may be useful to treat sleep or circadian rhythm disorders, ‘sundowning’ and day sleepiness,114 but sleep deprivation is not recommended in BPSD.6 Indeed, there is a higher risk of agitation and other BPSD may appear due to insomnia.65
Electroconvulsive therapy may be helpful in individual situations. Similarly, repeated transcranial magnetic stimulation may become a useful method, but the study of this method to treat BPSD is still in its infancy.
Conclusion
BPSD occur on an almost regular basis as dementia evolves, regardless of the dementia type. BPSD are a heterogeneous group of symptoms and signs, but all of them may cause significant suffering in patients and caregivers. The causes of and risk factors for BPSD are multiple, even in a single patient, with interacting biological, psychological and social/environmental causes and vulnerability factors. Taking a detailed history and performing a sound clinical investigation including the patient and their family or care team are essential. In order to arrive at an individualized treatment plan, a therapeutic decision tree should be established taking into account the patient’s individual and their environmental risk profile. Psychosocial treatments are pivotal. Often, combining different non-pharmacological approaches precedes drug treatment that can be added if required. Consequently, an interventional algorithm is proposed to take care of patients suffering BPSD (Figure 2). Regular assessments of the treatment plan and any prescriptions must be carried out to detect signs of relapse and to stop any drug that has become inappropriate. Even with optimal management, BPSD will not disappear completely in some cases and will remain challenging for all involved parties.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Olivier Pierre Tible, Department of Psychiatry, Service Universitaire de Psychiatrie de l’Age Avancé (SUPAA), Lausanne University Hospital, CH-1008 Prilly, Switzerland.
Florian Riese, Department of Geriatric Psychiatry, University Hospital of Psychiatry, Zurich, Switzerland University Research Priority Programme ‘Dynamics of Healthy Aging’, University of Zurich, Zurich, Switzerland.
Egemen Savaskan, Department of Geriatric Psychiatry, University Hospital of Psychiatry, Zurich, Switzerland.
Armin von Gunten, Department of Psychiatry, Service Universitaire de Psychiatrie de l’Age Avancé (SUPAA), Lausanne University Hospital, Prilly, Switzerland.
References
- 1. International Psychogeriatric Association. The IPA complete guides to behavioral and psychological symptoms of dementia. Milwaukee, WI: International Psychogeriatric Association, 2010, www.ipa-online.org/publications/guides-to-bpsd (accessed 30 September 2010). [Google Scholar]
- 2. Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry 2008; 23: 170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devshi R, Shaw S, Elliott-King J, et al. Prevalence of behavioural and psychological symptoms of dementia in individuals with learning disabilities. Diagnostic 2015; 5: 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kales HC, Gitlin LN, Lyketsos CG. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. Similar to DICE (diagnose, investigate, create, evaluate). J Am Geriatr Soc 2014; 62: 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perez Romero A, Gonzalez Garrido S. The importance of behavioral and psychological symptoms in Alzheimer’s disease. Neurologia. DOI: 10.1016/j.nrl.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 6. Savaskan E, Bopp-Kistler I, Buerge M, et al. Therapy guidelines for the behavioural and psychological symptoms of dementia. Praxis 2014; 103: 135–148. [DOI] [PubMed] [Google Scholar]
- 7. Canevelli M, Adali N, Cantet C, et al. Impact of behavioral subsyndromes on cognitive decline in Alzheimer’s disease: data from the ICTUS study. J Neurol 2013; 260: 1859–1865. [DOI] [PubMed] [Google Scholar]
- 8. Feast A, Moniz-Cook E, Stoner C, et al. A systematic review of the relationship between behavioral and psychological symptoms (BPSD) and caregiver well-being. Int Psychogeriatr 2016; 28: 1761–1774. [DOI] [PubMed] [Google Scholar]
- 9. Nourhashémi F, Andrieu S, Sastres N, et al. Descriptive analysis of emergency hospital admissions of patients with Alzheimer disease. Alzheimer Dis Assoc Disord 2001; 15: 21–25. [DOI] [PubMed] [Google Scholar]
- 10. Toot S, Swinson T, Devine M, et al. Causes of nursing home placement for older people with dementia: a systematic review and meta-analysis. Int Psychogeriatr 2016; 3: 1–14. [DOI] [PubMed] [Google Scholar]
- 11. Yafee K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA 2002; 287: 2090–2097. [DOI] [PubMed] [Google Scholar]
- 12. Beeri MS, Werner P, Davidson M, et al. The cost of BPSD in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry 2002; 17: 403–408. [DOI] [PubMed] [Google Scholar]
- 13. Kraft E, Marti M, Werner S, et al. Cost of dementia in Switzerland. Swiss Med Wkly 2010; 140: w13093. [DOI] [PubMed] [Google Scholar]
- 14. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ 2015; 350: h369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gitlin LN, Marx KA, Stanley IH, et al. Assessing neuropsychiatric symptoms in people with dementia: a systematic review of measures. Int Psychogeriatr 2014; 26: 1805–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cummings JL, Mega M, Gray K, et al. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 2014; 44: 2308–2314. [DOI] [PubMed] [Google Scholar]
- 17. Reisberg B, Borenstein J, Salob SP, et al. Behavioral symptoms in Alzheimer’s disease: phenomenology and treatment. J Clin Psychiatry 1987; 48(Suppl.): 9–15. [PubMed] [Google Scholar]
- 18. Jeon YH, Sansoni J, Low LF, et al. Recommended measures for the assessment of behavioral disturbances associated with dementia. Am J Geriatr Psychiatry 2011; 19: 403–415. [DOI] [PubMed] [Google Scholar]
- 19. Aalten A, Verhey FR, Boziki M, et al. Consistency of neuropsychiatric syndromes across dementias: results from the European Alzheimer Disease Consortium. Part II. Dement Geriatr Cogn Disord 2008; 25: 1–8. [DOI] [PubMed] [Google Scholar]
- 20. Lyketsos CG, Lopez O, Jones B, et al. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. JAMA 2002; 288: 1475–1483. [DOI] [PubMed] [Google Scholar]
- 21. Mega MS, Cummings JL, Fiorello T, et al. The spectrum of behavioral changes in Alzheimer’s disease. Neurology 1996; 46: 130–135. [DOI] [PubMed] [Google Scholar]
- 22. Van der Linde RM, Dening T, Stephan BCM, et al. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. Br J Psychiatry 2016; 209: 366–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elflein HM, Rudy M, Lorenz K, et al. Charles Bonnet’s syndrome: not only a condition of the elderly. Graefes Arch Clin Exp Ophthalmol 2016; 254: 1637–1642. [DOI] [PubMed] [Google Scholar]
- 24. Cummings J, Mintzer J, Brodaty H, et al. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. Int Psychogeriatr 2015; 27: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gnanasekaran G. ‘Sundowning’ as a biological phenomenon: current understandings and future directions: an update. Aging Clin Exp Res 2016; 28: 383–392. [DOI] [PubMed] [Google Scholar]
- 26. Cohen-Mansfield J, Golander H, Ben-Israel J, et al. The meaning of delusions in dementia: a preliminary study. Psychiatry Res 2011; 189: 97–104. [DOI] [PubMed] [Google Scholar]
- 27. Cipriani G, Danti S, Vedovello M, et al. Understanding delusion in dementia: a review. Geriatr Gerontol Int 2014; 14: 32–39. [DOI] [PubMed] [Google Scholar]
- 28. Cohen-Mansfield J, Cohen R, Golander H, et al. The impact of psychotic symptoms on the persons with dementia experiencing them. Am J Geriatr Psychiatry 2016; 24: 213–220. [DOI] [PubMed] [Google Scholar]
- 29. Cipriani G, Vedovello M, Ulivi M, et al. Delusional misidentification syndromes and dementia: a border zone between neurology and psychiatry. Am J Alzheimers Dis Other Demen 2013; 28: 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lanctôt KL, Agüera-Ortiz L, Brodaty H, et al. Apathy associated with neurocognitive disorders: recent progress and future directions. Alzheimers Dement 2017; 13: 84–100. [DOI] [PubMed] [Google Scholar]
- 31. Robert PH, Clairet S, Benoit M, et al. The apathy inventory: assessment of apathy and awareness in Alzheimer’s disease, Parkinson’s disease and mild cognitive impairment. Int J Geriatr Psychiatry 2002; 17: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 32. Van der Linde RM, Matthews FE, Dening T, et al. Patterns and persistence of behavioural and psychological symptoms in those with cognitive impairment: the importance of apathy. Int J Geriatr Psychiatry. 32(3):306–315. DOI: 10.1002/gps.4464. [DOI] [PubMed] [Google Scholar]
- 33. Bubu OM, Brannick M, Mortimer J, et al. Sleep, cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. Sleep 2017; 40: zsw032. [DOI] [PubMed] [Google Scholar]
- 34. Ooms S, Ju YE. Treatment of sleep disorders in dementia. Curr Treat Options Neurol 2016; 18: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in dementia. Cochrane Database Syst Rev 2016; 16: CD009178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Heller J, Brcina N, Dogan I, et al. Brain imaging findings in idiopathic REM sleep behavior disorder (RBD): a systematic review on potential biomarkers for neurodegeneration. Sleep Med Rev. DOI: 10.1016/j.smrv.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 37. Cagnin A, Fragiacomo F, Camporese G, et al. Sleep–wake profile in dementia with Lewy bodies, Alzheimer’s disease and normal aging. J Alzheimer Dis 2016; 55: 1529–1536. [DOI] [PubMed] [Google Scholar]
- 38. McCarter SJ, St, Louis EK, Boeve BF. Sleep disturbances in frontotemporal dementia. Curr Neurol Neurosci Rep 2016; 16: 85. [DOI] [PubMed] [Google Scholar]
- 39. Mitchell AJ. Neuropsychiatry and behavioural neurology explained. Edinburgh: Saunders, 2004, p.536. [Google Scholar]
- 40. Bizdan M, Bidzan L, Pachalska M. Neuropsychiatric symptoms in patients with Alzheimer’s disease with a vascular component. Ann Agric Environ Med 2014; 21: 412–415. [DOI] [PubMed] [Google Scholar]
- 41. Kazui H, Yoshiyama K, Kanemoto H, et al. Differences of behavioral and psychological symptoms of dementia in disease severity in four major dementias. PLoS One 2016; 11: e0161092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bang J, Spina S, Miller BL. Frontotemporal dementia. Lancet 2015; 386: 1672–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Clerc MT, Von Gunten A. Frontotemporal lobar degeneration. In: Tanner P. (eds) Dementia: prevalence, risk factors and management strategies. Hauppauge (Headquarters), New York: Nova Science Publishers, 2014, pp.87–114. [Google Scholar]
- 44. Engelborghs S, Vloeberghs E, Le Bastard N, et al. The dopaminergic neurotransmitter system is associated with aggression and agitation in frontotemporal dementia. Neurochem Int 2008; 52: 1052–1060. [DOI] [PubMed] [Google Scholar]
- 45. Von Gunten A, Bouras C, Kövari E, et al. Neural substrates of cognitive and behavioral deficits in atypical Alzheimer’s disease. Brain Res Rev 2006; 51: 176–211. [DOI] [PubMed] [Google Scholar]
- 46. Huey ED, Lee S, Devanand DP. Brain regions involved in arousal and reward processing are associated with apathy in Alzheimer’s disease and frontotemporal dementia. J Alzheimer Dis 2016; 55: 551–558. [DOI] [PubMed] [Google Scholar]
- 47. Torso M, Serra L, Giulietti G, et al. Strategic lesions in the anterior thalamic radiation and apathy in early Alzheimer’s disease. PLoS One 2015; 10: e0124998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Makovac E, Serra L, Spano B, et al. Different patterns of correlation between grey and white matter integrity account for behavioral and psychological symptoms in Alzheimer’s disease. J Alzheimers Dis 2016; 50: 591–604. [DOI] [PubMed] [Google Scholar]
- 49. Tokuchi R, Hishikawa N, Sato K, et al. Age-dependent cognitive and affective differences in Alzheimer’s and Parkinson’s diseases in relation to MRI findings. J Neurol Sci 2016; 365: 3–8. [DOI] [PubMed] [Google Scholar]
- 50. Minger SL, Esiri MM, McDonald B, et al. Cholinergic deficits contribute to behavioral disturbance in patients with dementia. Neurology 2000; 55: 1460–1467. [DOI] [PubMed] [Google Scholar]
- 51. Lagarde J, Sarazin M, Chauviré V, et al. Cholinergic changes in aging and Alzheimer disease: an 18F-F-A 85380 exploratory PET study. Alzheimer Dis Assoc Disord. 31(1):8–12. DOI: 10.1097/WAD000000000163. [DOI] [PubMed] [Google Scholar]
- 52. Herrmann N, Lanctôt KL, Khan LR. The role of norepinephrine in the behavioral and psychological symptoms of dementia. J Neuropsychiatry Clin Neurosci 2004; 16: 261–276. [DOI] [PubMed] [Google Scholar]
- 53. David R, Koulibaly M, Benoit M, et al. Striatal dopamine transporter levels correlate with apathy in neurodegenerative diseases: a SPECT study with partial volume effect correction. Clin Neurol Neurosurg 2008; 110: 19–24. [DOI] [PubMed] [Google Scholar]
- 54. Mitchell RA, Herrmann N, Lanctôt KL. The role of dopamine in symptoms and treatment of apathy in Alzheimer’s disease. CNS Neurosci Ther 2011; 17: 411–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gould E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology 1999; 21(Suppl. 2): 46S–51S. [DOI] [PubMed] [Google Scholar]
- 56. Poletti S, Locatelli C, Falini A, et al. Adverse childhood experiences associate to reduced glutamate levels in the hippocampus of patients affected by mood disorders. Prog Neuropsychopharmacol Biol Psychiatry 2016; 71: 117–122. [DOI] [PubMed] [Google Scholar]
- 57. Ribeiro FM, Vieira LB, Pires RG, et al. Metabolic glutamate receptors and neurodegenerative diseases. Pharmacol Res 2016; 115: 179–191. [DOI] [PubMed] [Google Scholar]
- 58. Maidment ID, Haw C, Stubbs J, et al. Medication errors in older people with mental health problems: a review. Int J Geriatr Psychiatry 2008; 23: 564–573. [DOI] [PubMed] [Google Scholar]
- 59. Yesavage JA, Noda A, Hernandez B. Circadian clock gene polymorphisms and sleep–wake disturbance in Alzheimer disease. Am J Geriatr Psychiatry 2011; 19: 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mulin E, Zeitzer JM, Yesavage J, et al. Relationship between apathy and sleep disturbance in mild and moderate Alzheimer’s disease: an actigraphic study. J Alzheimers Dis 2011; 25: 85–91. [DOI] [PubMed] [Google Scholar]
- 61. Zheng Z, Wang J, Yi L, et al. Correlation between behavioural and psychological symptoms of Alzheimer type dementia and plasma homocysteine concentration. Biomed Res Int 2014: 383494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Flirski M, Sobow T, Kloszewska I. Behavioural genetics of Alzheimer’s disease: a comprehensive review. Arch Med Sci 2011; 7: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kolaczkowski M, Marcinkowska M, Bucki A, et al. Novel 5-HT6 receptor antagonists/D2 receptor partial agonists targeting behavioral and psychological symptoms of dementia. Eur J Med Chem 2015; 92: 221–235. [DOI] [PubMed] [Google Scholar]
- 64. Stefano P, Concetta C, Luigi D, et al. Role of neurodevelopment involved genes in psychiatric comorbidities and modulation of inflammatory processes in Alzheimer’s disease. J Neurol Sci 2016; 370: 162–166. [DOI] [PubMed] [Google Scholar]
- 65. Tible O. Présentation des équipes mobiles de Psychogériatrie, Brussels, Belgium, 7–9 September 2016. Société de Psychogériatrie de Langue Française, 32ème Congrès de Psychogériatrie de langue française, pp.25–26, www.splf-2016.org (accessed 9 September 2016). [Google Scholar]
- 66. Malara A, De Biase GA, Bettarini F, et al. Pain assessment in elderly with behavioral and psychological symptoms of dementia. J Alzheimers Dis 2016; 50: 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pocnet C, Rossier J, Antonietti JP, et al. Personality changes in patients with beginning Alzheimer disease. Can J Psychiatry 2011; 56: 408–417. [DOI] [PubMed] [Google Scholar]
- 68. Pocnet C, Rossier J, Antonietti JP, et al. Personality traits and behavioral and psychological symptoms in patients at an early stage of Alzheimer’s disease. Int J Geriatr Psychiatry 2013; 28: 276–283. [DOI] [PubMed] [Google Scholar]
- 69. Prior J, Abraham R, Nicholas H, et al. Are premorbid abnormal personality traits associated with behavioural and psychological symptoms in dementia? Int J Geriatr Psychiatry 2016; 31: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 70. Boyle LL, Lyness JM, Duberstein PR, et al. Trait neuroticism, depression, and cognitive function in older primary care patients. Am J Geriatr Psychiatry 2010; 18: 305–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Johansson L, Guo X, Duberstein PR, et al. Midlife personality and risk of Alzheimer disease and distress: a 38-year follow-up. Neurology 2014; 83: 1538–1544. [DOI] [PubMed] [Google Scholar]
- 72. Von Gunten A, Pocnet C, Rossier J. The impact of personality characteristics on the clinical expression in neurodegenerative disorders: a review. Brain Res Bull 2009; 80: 179–191. [DOI] [PubMed] [Google Scholar]
- 73. Hirshfeld-Becker DR, Micco J, Henin A, et al. Behavioral inhibition. Depress Anxiety 2008; 25: 357–367. [DOI] [PubMed] [Google Scholar]
- 74. Schwartz CE, Hirshfeld-Becker DR, Henin A, et al. Behavioral inhibition in childhood predicts smaller hippocampal volume in adolescent offspring of parents with panic disorder. Transl Psychiatry 2015; 21: e605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Van Rooij SJ, Kennis M, Vink M, et al. Childhood trauma and COMT genotype interact to increase hippocampal activation in resilient individuals. Front Psychiatry 2016; 14: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Bradley JM, Cafferty TP. Attachment among older adults: current issues and directions for future research. Attachment Hum Dev 2001; 3: 200–221. [DOI] [PubMed] [Google Scholar]
- 77. Browne CJ, Shlosberg E. Attachment theory, ageing and dementia: a review of the literature. Aging Ment Health 2006; 10: 134–142. [DOI] [PubMed] [Google Scholar]
- 78. Magai C, Cohen CI, Culver C, et al. Relation between premorbid personality and patterns of emotion expression in mid- to late-stage dementia. Int J Geriatr Psychiatry 1997; 12: 1092–1099. [DOI] [PubMed] [Google Scholar]
- 79. Fujii M, Butler JP, Sasaki H. Emotional function dementia patients. Psychogeriatrics 2014; 14: 202–209. [DOI] [PubMed] [Google Scholar]
- 80. Feast A, Orrell M, Russell I, et al. The contribution of caregiver psychosocial factors to distress associated with behavioral and psychological symptoms in dementia. Int J Geriatr Psychiatry 2017; 32: 76–85. [DOI] [PubMed] [Google Scholar]
- 81. Corbett A, Husebo B, Malcangio M, et al. Assessment and treatment of pain in people with dementia. Nat Rev Neurol 2012; 8: 264–274. [DOI] [PubMed] [Google Scholar]
- 82. Ouslander J, Bartesl S, Beck C, et al. Consensus statement on improving the quality of mental health care in US nursing homes: management of depression and behavioral symptoms associated with dementia. J Am Geriatr Soc 2003; 51: 1287–1298. [DOI] [PubMed] [Google Scholar]
- 83. S3-Leitlinie Demenzen. In: Deutsche Gesellschaft für Neurologie, HRSG; Leitlinien für Diagnostik und Therapie in der Neurologie, www.dgn.org/leitlinien (2016, accessed 27 January 2016).
- 84. Livingston G, Kelly L, Lewis-Holmes E, et al. Non-pharmacological interventions for agitation in dementia: systematic review of randomized controlled trials. Br J Psychiatry 2014; 205: 436–442. [DOI] [PubMed] [Google Scholar]
- 85. Abraha I, Rimland JM, Trotta FM, et al. Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-On Top series. BMJ Open 2017; 7: 3:e012759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Livingston G, Johnston K, Katona C, et al. Systematic review of psychological approaches to the management of neuropsychiatric symptoms of dementia. Am J Psychiatry 2005; 162: 1996–2021. [DOI] [PubMed] [Google Scholar]
- 87. Hepburn K, Lewis M, Tornatore J, et al. The Savvy Caregiver program: the demonstrated effectiveness of a transportable dementia caregiver programme. J Gerontol Nurs 2007; 33: 30–36. [DOI] [PubMed] [Google Scholar]
- 88. Copper C, Mukadam N, Katona C, et al. Systematic review of the effectiveness of non-pharmacological interventions to improve quality of life of people with dementia. Int Psychogeriatr 2012; 24: 856–870. [DOI] [PubMed] [Google Scholar]
- 89. Schoenmakers B, Buntinx F, DeLepeleire J. Supporting the dementia family caregiver: the effect of home care intervention on general well-being. Aging Ment Health 2010; 14: 44–56. [DOI] [PubMed] [Google Scholar]
- 90. Kolanowski A. An overview of the need-driven dementia-compromised behavior model. J Gerontol Nurs 1999; 25: 7–9. [DOI] [PubMed] [Google Scholar]
- 91. Kovach CR, Noonan PE, Schlidt AM, et al. The serial trial intervention: an innovative approach to meeting needs of individuals with dementia. J Gerontol Nurs 2006; 32: 18–25. [DOI] [PubMed] [Google Scholar]
- 92. McMinn B, Draper B. Vocally disruptive behaviour in dementia: development of an evidence based practice guideline. Aging Ment Health 2005; 9: 16–24. [DOI] [PubMed] [Google Scholar]
- 93. Hajjar RR, Kamel HK. Sexuality in the nursing home, part 1: attitudes and barriers to sexual expression. J Am Med Dir Assoc 2004; 5: 42–47. [DOI] [PubMed] [Google Scholar]
- 94. Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly patients with cognitive impairment and dementia: a meta-analysis. Arch Phys Med Rehabil 2004; 85: 1694–1704. [DOI] [PubMed] [Google Scholar]
- 95. Scholey KA, Woods BT. A series of brief cognitive therapy interventions with people experiencing both dementia and depression: a description of techniques and common themes. Clin Psychol Psychiatry 2003; 10: 175–185. [Google Scholar]
- 96. Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. Am J Psychiatry 2012; 169: 946–953. [DOI] [PubMed] [Google Scholar]
- 97. Sörensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist 2002; 42: 356–372. [DOI] [PubMed] [Google Scholar]
- 98. Trinh NH, Hoblyn J, Mohanty S, et al. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer’s disease: a meta-analysis. JAMA 2003; 289: 210–216. [DOI] [PubMed] [Google Scholar]
- 99. Howard RJ, Juszczak E, Ballard CG, et al. Donepezil for the treatment of agitation in Alzheimer’s disease. N Engl J Med 2007; 357: 1382–1392. [DOI] [PubMed] [Google Scholar]
- 100. Gauthier S, Loft H, Cummings J. Improvement in behavioural symptoms in patients with moderate to severe Alzheimer’s disease by memantine: a pooled data analysis. Int J Geriatr Psychiatry 2008; 23: 537–545. [DOI] [PubMed] [Google Scholar]
- 101. Wilcock GK, Ballard CG, Cooper JA, et al. Memantine for agitation/aggression and psychosis in moderately severe to severe Alzheimer’s disease: a pooled analysis of 3 studies. J Clin Psychiatry 2008; 69: 341–348. [DOI] [PubMed] [Google Scholar]
- 102. Von Gunten A. Schwabe Symposium on BPSD: prevalence, clinical importance and treatment options. Second Congress of the European Academy of Neurology, 28–31 May 2016, Copenhagen, Denmark, www.ean.org (accessed 31 May 2016). [Google Scholar]
- 103. Fox C, Crugel M, Maidment I, et al. Efficacy of memantine for agitation in Alzheimer’s dementia: a randomized double-blind placebo controlled trial. PLoS One 2012; 7: e35185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ballard C, Thomas A, Gerry S, et al. A double-blind randomized placebo-controlled withdrawal trial comparing memantine and antipsychotics for the long-term treatment of function and neuropsychiatric symptoms in people with Alzheimer’s disease (MAIN-AD). J Am Med Dir Assoc 2015; 16: 316–322. [DOI] [PubMed] [Google Scholar]
- 105. Von Gunten A, Schlaefke S, Überla K. Efficacy of Gingko Biloba extract EGb 761® in dementia with behavioural and psychological symptoms: a systematic review. World J Biol Psychiatry 2016; 17: 622–633. [DOI] [PubMed] [Google Scholar]
- 106. Lyketsos CG, Del Campo L, Steinberg M, et al. Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry 2003; 60: 737–746. [DOI] [PubMed] [Google Scholar]
- 107. Porsteinsson AP, Drye LT, Pollock BG, et al. Effect of citalopram on agitation in Alzheimer disease: the citAD randomized clinical trial. JAMA 2014; 311: 682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev 2006; 25: CD003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Maher AR, Maglione M, Bagley S, et al. Efficacy and comparative effectiveness of atypical antipsychotic medications for off-label uses in adults: a systematic review and meta-analysis. JAMA 2011; 306: 1359–1369. [DOI] [PubMed] [Google Scholar]
- 110. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry 2016; 173: 543–546. [DOI] [PubMed] [Google Scholar]
- 111. Tariot PN, Schneider L, Katz IR, et al. Quetiapine treatment of psychosis associated with dementia: a double-blind, randomized, placebo-controlled clinical trial. Am J Geriatr Psychiatry 2006; 14: 767–776. [DOI] [PubMed] [Google Scholar]
- 112. Kurlan R, Cummings J, Raman R, et al. Quetiapine for agitation or psychosis in patients with dementia and parkinsonism. Neurology 2007; 68: 1356–1363. [DOI] [PubMed] [Google Scholar]
- 113. Ballard CG, Gauthier S, Cummings JL, et al. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol 2009; 5: 245–255. [DOI] [PubMed] [Google Scholar]
- 114. Zhou QP, Jung L, Richards KC. The management of sleep and circadian disturbance in patients with dementia. Curr Neurol Neurosci Rep 2012; 12: 193–204. [DOI] [PubMed] [Google Scholar]