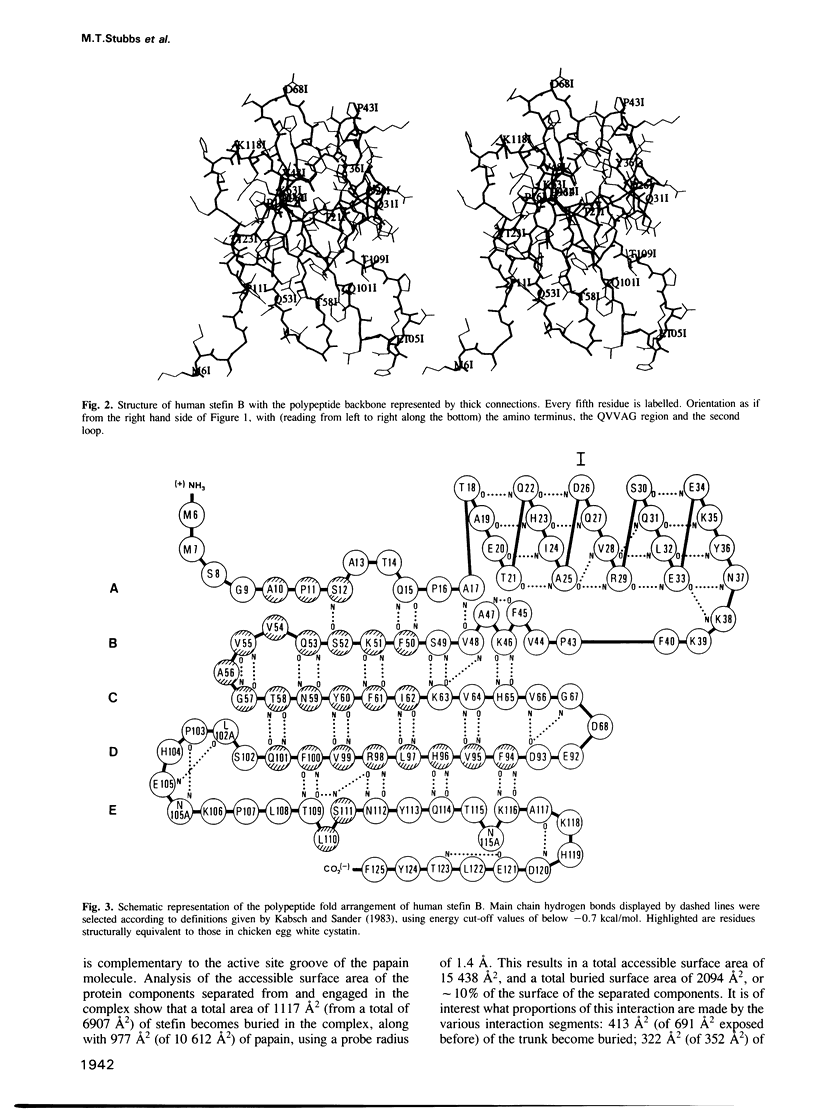
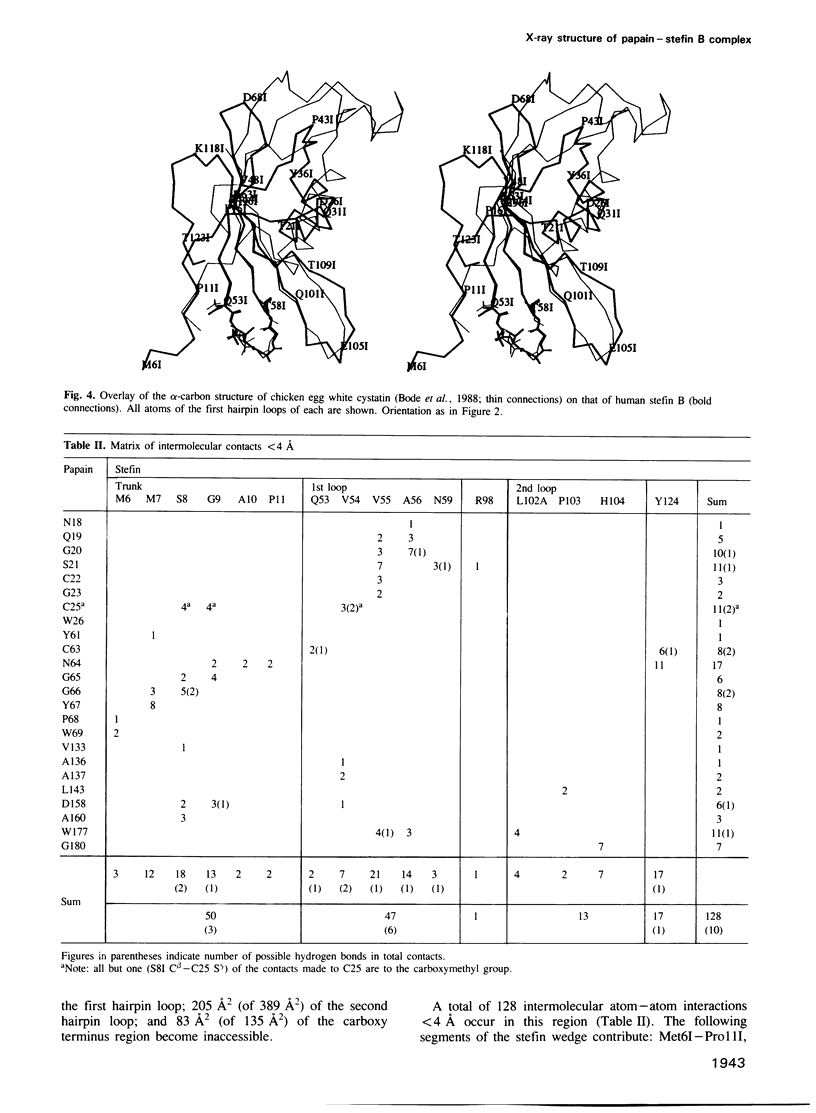
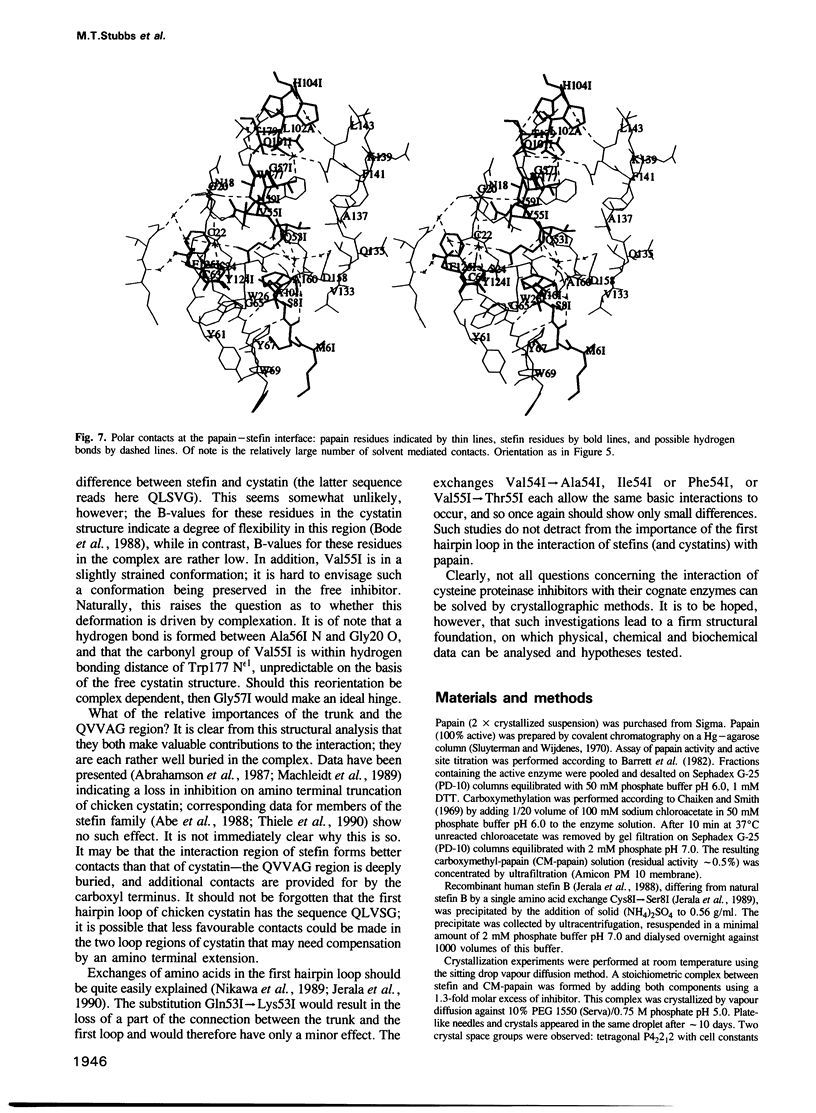
Abstract
A stoichiometric complex of human stefin B and carboxymethylated papain has been crystallized in a trigonal crystal form. Data to 2.37 A resolution were collected using the area detector diffractometer FAST. The crystal structure of the complex has been solved by Patterson search techniques using papain as search model. Starting from the structure of chicken cystatin, the stefin structure was elucidated through cycles of model building and crystallographic refinement. The current crystallographic R factor is 0.19. Like cystatin, the stefin molecule consists of a five stranded beta-sheet wrapped around a five turn alpha-helix, but with an additional carboxy terminal strand running along the convex side of the sheet. Topological equivalence of stefin and cystatin reveal the previous sequence alignment to be incorrect in part, through deletion of the intermediate helix. The conserved residues form a tripartite wedge, which slots into the papain active site as proposed through consideration of the tertiary structures of the individual components (Bode et al., 1988). The main interactions are provided by the amino terminal 'trunk' (occupying the 'unprimed' subsites of the enzyme), and by the first hairpin loop, containing the highly conserved QVVAG sequence, with minor contributions from the second hairpin loop. The carboxyl terminus of stefin provides an additional interaction region with respect to cystatin. The interaction is dominated by hydrophobic contacts. Inhibition by the cysteine proteinase inhibitors is fundamentally different to that observed for the serine proteinase inhibitors.
Full text
PDF
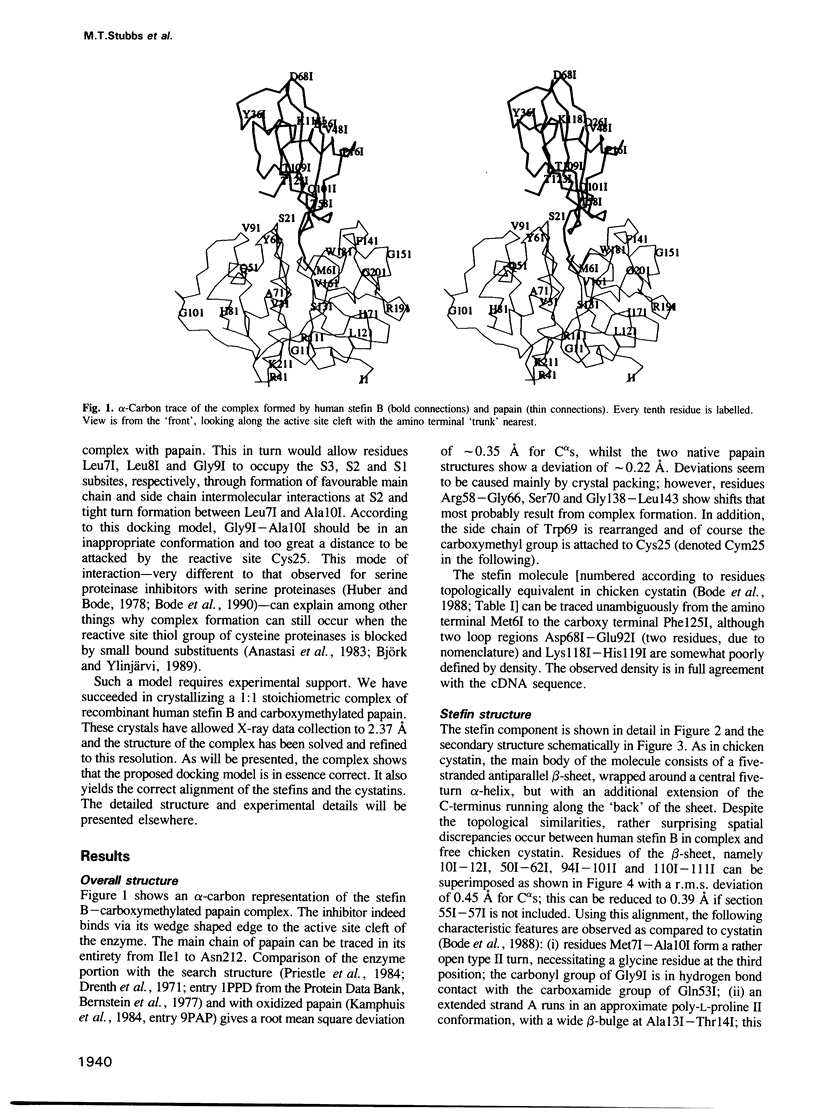
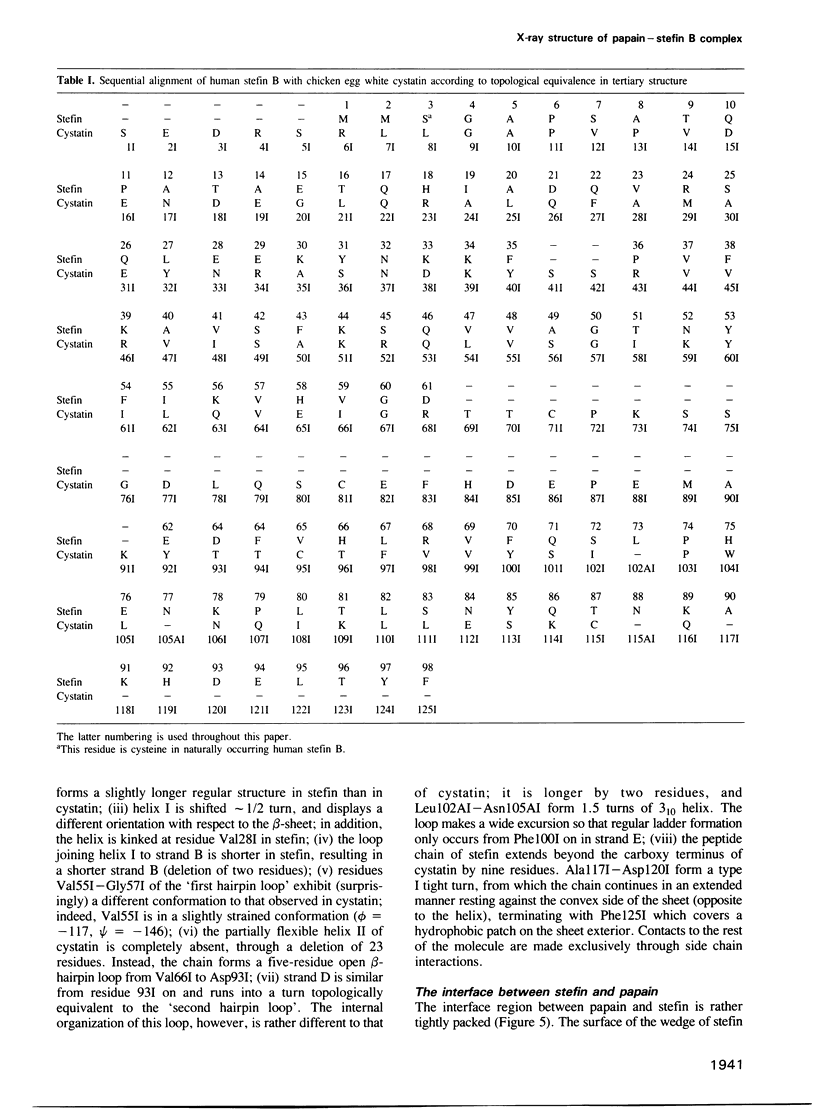



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe K., Emori Y., Kondo H., Arai S., Suzuki K. The NH2-terminal 21 amino acid residues are not essential for the papain-inhibitory activity of oryzacystatin, a member of the cystatin superfamily. Expression of oryzacystatin cDNA and its truncated fragments in Escherichia coli. J Biol Chem. 1988 Jun 5;263(16):7655–7659. [PubMed] [Google Scholar]
- Abrahamson M., Ritonja A., Brown M. A., Grubb A., Machleidt W., Barrett A. J. Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J Biol Chem. 1987 Jul 15;262(20):9688–9694. [PubMed] [Google Scholar]
- Anastasi A., Brown M. A., Kembhavi A. A., Nicklin M. J., Sayers C. A., Sunter D. C., Barrett A. J. Cystatin, a protein inhibitor of cysteine proteinases. Improved purification from egg white, characterization, and detection in chicken serum. Biochem J. 1983 Apr 1;211(1):129–138. doi: 10.1042/bj2110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Björk I., Ylinenjärvi K. Interaction of chicken cystatin with inactivated papains. Biochem J. 1989 May 15;260(1):61–68. doi: 10.1042/bj2600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode W., Engh R., Musil D., Thiele U., Huber R., Karshikov A., Brzin J., Kos J., Turk V. The 2.0 A X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J. 1988 Aug;7(8):2593–2599. doi: 10.1002/j.1460-2075.1988.tb03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiken I. M., Smith E. L. Reaction of the sulfhydryl group of papain with chloroacetic acid. J Biol Chem. 1969 Oct 10;244(19):5095–5099. [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Wolthers B. G. The structure of papain. Adv Protein Chem. 1971;25:79–115. doi: 10.1016/s0065-3233(08)60279-x. [DOI] [PubMed] [Google Scholar]
- Green G. D., Kembhavi A. A., Davies M. E., Barrett A. J. Cystatin-like cysteine proteinase inhibitors from human liver. Biochem J. 1984 Mar 15;218(3):939–946. doi: 10.1042/bj2180939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerala R., Trstenjak M., Lenarcic B., Turk V. Cloning a synthetic gene for human stefin B and its expression in E. coli. FEBS Lett. 1988 Oct 24;239(1):41–44. doi: 10.1016/0014-5793(88)80541-6. [DOI] [PubMed] [Google Scholar]
- Järvinen M., Rinne A. Human spleen cysteineproteinase inhibitor. Purification, fractionation into isoelectric variants and some properties of the variants. Biochim Biophys Acta. 1982 Nov 9;708(2):210–217. [PubMed] [Google Scholar]
- Kabsch W., Sander C. Dictionary of protein secondary structure: pattern recognition of hydrogen-bonded and geometrical features. Biopolymers. 1983 Dec;22(12):2577–2637. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- Kamphuis I. G., Kalk K. H., Swarte M. B., Drenth J. Structure of papain refined at 1.65 A resolution. J Mol Biol. 1984 Oct 25;179(2):233–256. doi: 10.1016/0022-2836(84)90467-4. [DOI] [PubMed] [Google Scholar]
- Lenney J. F., Tolan J. R., Sugai W. J., Lee A. G. Thermostable endogenous inhibitors of cathepsins B and H. Eur J Biochem. 1979 Nov 1;101(1):153–161. doi: 10.1111/j.1432-1033.1979.tb04227.x. [DOI] [PubMed] [Google Scholar]
- Machleidt W., Thiele U., Laber B., Assfalg-Machleidt I., Esterl A., Wiegand G., Kos J., Turk V., Bode W. Mechanism of inhibition of papain by chicken egg white cystatin. Inhibition constants of N-terminally truncated forms and cyanogen bromide fragments of the inhibitor. FEBS Lett. 1989 Jan 30;243(2):234–238. doi: 10.1016/0014-5793(89)80135-8. [DOI] [PubMed] [Google Scholar]
- Nikawa T., Towatari T., Ike Y., Katunuma N. Studies on the reactive site of the cystatin superfamily using recombinant cystatin A mutants. Evidence that the QVVAG region is not essential for cysteine proteinase inhibitory activities. FEBS Lett. 1989 Sep 25;255(2):309–314. doi: 10.1016/0014-5793(89)81112-3. [DOI] [PubMed] [Google Scholar]
- Ohkubo I., Kurachi K., Takasawa T., Shiokawa H., Sasaki M. Isolation of a human cDNA for alpha 2-thiol proteinase inhibitor and its identity with low molecular weight kininogen. Biochemistry. 1984 Nov 20;23(24):5691–5697. doi: 10.1021/bi00319a005. [DOI] [PubMed] [Google Scholar]
- Ritonja A., Machleidt W., Barrett A. J. Amino acid sequence of the intracellular cysteine proteinase inhibitor cystatin B from human liver. Biochem Biophys Res Commun. 1985 Sep 30;131(3):1187–1192. doi: 10.1016/0006-291x(85)90216-5. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. An agarose mercurial column for the separation of mercaptopapain and nonmercaptopapain. Biochim Biophys Acta. 1970 Mar 31;200(3):593–595. doi: 10.1016/0005-2795(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Teno N., Tsuboi S., Itoh N., Okamoto H., Okada Y. Significant effects of Z-Gln-Val-Val-OME, common sequences of thiol proteinase inhibitors on thiol proteinases. Biochem Biophys Res Commun. 1987 Mar 13;143(2):749–752. doi: 10.1016/0006-291x(87)91417-3. [DOI] [PubMed] [Google Scholar]
- Thiele U., Auerswald E. A., Gebhard W., Assfalg-Machleidt I., Popović T., Machleidt W. Inhibitorily active recombinant human stefin B. Gene synthesis, expression and isolation of an inhibitory active MS-2 pol-stefin B fusion protein and preparation of Des[Met1,2(2)]stefin B. Biol Chem Hoppe Seyler. 1988 Oct;369(10):1167–1178. doi: 10.1515/bchm3.1988.369.2.1167. [DOI] [PubMed] [Google Scholar]