Abstract
In sub-Saharan Africa, infectious diseases and malnutrition constitute the main health problems in children, while adolescents and adults are increasingly facing cardio-metabolic conditions. Among adolescents as the largest population group in this region, we investigated the co-occurrence of infectious diseases, malnutrition and cardio-metabolic risk factors (CRFs), and evaluated demographic, socio-economic and medical risk factors for these entities. In a cross-sectional study among 188 adolescents in rural Ghana, malarial infection, common infectious diseases and Body Mass Index were assessed. We measured ferritin, C-reactive protein, retinol, fasting glucose and blood pressure. Socio-demographic data were documented. We analyzed the proportions (95% confidence interval, CI) and the co-occurrence of infectious diseases (malaria, other common diseases), malnutrition (underweight, stunting, iron deficiency, vitamin A deficiency [VAD]), and CRFs (overweight, obesity, impaired fasting glucose, hypertension). In logistic regression, odds ratios (OR) and 95% CIs were calculated for the associations with socio-demographic factors. In this Ghanaian population (age range, 14.4–15.5 years; males, 50%), the proportions were for infectious diseases 45% (95% CI: 38–52%), for malnutrition 50% (43–57%) and for CRFs 16% (11–21%). Infectious diseases and malnutrition frequently co-existed (28%; 21–34%). Specifically, VAD increased the odds of non-malarial infectious diseases 3-fold (95% CI: 1.03, 10.19). Overlap of CRFs with infectious diseases (6%; 2–9%) or with malnutrition (7%; 3–11%) was also present. Male gender and low socio-economic status increased the odds of infectious diseases and malnutrition, respectively. Malarial infection, chronic malnutrition and VAD remain the predominant health problems among these Ghanaian adolescents. Investigating the relationships with evolving CRFs is warranted.
Introduction
Infectious diseases and malnutrition still constitute major public health threats in sub-Saharan Africa. In 2015, communicable diseases, protein-energy malnutrition and micronutrient deficiencies ranked among the top 10 causes of disease burden in this region.[1] In Ghana, malaria remains ubiquitous and highly endemic with an annual incidence of 10,000 per 100,000 at risk,[2] and 26% of children aged 11–17 years are underweight.[3] Iron deficiency and vitamin A deficiency are among the most common micronutrient deficiencies in Ghana.[4]
At the same time, metabolic conditions, such as overweight, type 2 diabetes and hypertension are rapidly emerging in sub-Saharan Africa.[1] Among Ghanaian adolescents, the prevalence of overweight plus obesity is 3.2% among boys and 10.4% among girls according to age- and sex-specific cut-offs of Body Mass Index (BMI).[5] Type 2 diabetes occurs at 1.3% among young adults (20–29 years).[6] Moreover, one in five Ghanaians (aged 13–39 years) has hypertension defined by age- and sex-specific percentiles.[7]
The epidemiologic transition from infectious diseases and malnutrition to metabolic conditions due to increased life-expectancy and lower birth rates progresses slowly in sub-Saharan Africa.[8] As a consequence, these entities have been reported to co-occur at the country level, within households and even at the individual level. For instance, pooled data from rural West Africa revealed that 5% of women at childbearing age presented with symptoms of micronutrient deficiencies plus overweight, and 5% of mother-child pairs showed childhood stunting plus maternal overweight.[9] Today, two-thirds of Africa’s population is aged 10–24 years. This population group can enormously contribute to the well-being of African societies.[10] Yet, the health needs of young adults in Africa’s transitional phase have only insufficiently been examined.[11] For instance, factors for type 2 diabetes among Africans remain controversial,[12] and the extend of (mal-)nutrition-related susceptibility to infectious diseases among adolescents is well-described. Therefore, we aimed at investigating among adolescents in rural Ghana i) the proportions of common infectious diseases (malaria, diagnoses and symptoms compatible with another infectious disease), malnutrition (underweight, stunting, iron deficiency, vitamin A deficiency), and CRFs (overweight and obesity, impaired fasting glucose (IFG), hypertension), ii) the co-occurrence of these entities, and iii) demographic, socio-economic and medical risk factors for these entities.
Materials and methods
Study design and population
For this cross-sectional study, 201 adolescent boys and girls were consecutively recruited at the Presbyterian Mission Hospital in Agogo, southern Ghana between June and August 2015. Agogo Hospital is a 250-beds healthcare facility serving the Ashanti-Akim North District with a population of around 170,000.[13] Adolescents underwent a health check-up as part of a long-term follow-up on birth outcomes (manuscript in preparation), i.e., they did not present to hospital because of acute symptoms. Inclusion criteria were reaching the age of 15 years in the year of study conduct, informed written consent, absence of pregnancy, and no previous diagnosis of type 1 diabetes.
After an overnight-fast, venous blood was collected into EDTA for malaria diagnosis, for biomarkers of iron status and vitamin A metabolism, and for fasting plasma glucose (FPG). Axillary body temperature (°C), blood pressure (BP) and anthropometric measures were taken by trained study personnel. Socio-demographic data and medical history were documented in questionnaire-based interviews.
The study protocol was reviewed and approved by the Ethics Committee of the Kwame Nkrumah University of Science and Technology, Kumasi. Written informed consent was obtained from all caregivers and assent was given by all participants.
Physical examinations
All participants underwent a routine clinical examination by the study physician, and current diagnoses were documented. We measured axillary body temperature (°C; bosotherm flex, Bosch + Sohn, Germany) and anthropometric measures were taken in light clothes. Body weight was measured to the nearest 0.5 kg (Camry Person Scale, Model DT602, Hong Kong, China) and height was measured to the nearest 0.1 cm (Seca 213, Hamburg, Germany). Body Mass Index (BMI) was calculated as weight/(height)2 in kg/m2, and BMI-for-age z-scores (BAZ) and height-for-age z-scores (HAZ) were determined using the software package AnthroPlus (version 1.0.4, World Health Organization [WHO], Geneva, Switzerland). According to the WHO, overweight in adolescent age was defined as 1 ≤ BAZ < 2, obesity as BAZ ≥ 2, underweight (or thinness) as BAZ < -2, and stunting as HAZ < -2.
Systolic and diastolic BP were measured in triplicates every 3 minutes with an automated device (Tel-O-Graph BT, I.E.M. Stolberg, Germany) and appropriate cuffs in a separate room after a minimum of 5 minutes resting time. Mean systolic and mean diastolic BP were calculated using the last two measurements. Hypertension was defined as having a mean systolic or a mean diastolic BP >95th percentile of age-, sex- and height-specific reference data.[14]
Questionnaire-based interviews
Trained staff conducted questionnaire-based interviews (S1 Questionnaires) to document demographic data (age, sex, ethnic group, residence, place of school) and socio-economic status (SES). Even though the questionnaire had not been validated, it was successfully applied in the same geographic area in a case-control study for risk factors of type 2 diabetes and hypertension.[12] The presence of 11 household assets (electricity, pipe-borne water, radio, TV, fan, cupboard, fridge, bicycle, motorbike, car, cattle) was examined and a wealth-score was calculated as the proportion of present household assets. We recorded literacy of the child, parental education (none, primary, secondary, tertiary), parental occupation (intellectual, manual, other, unemployed), the number of people in the household, and the number of siblings. For medical history, current complaints and fever in the last 48h were documented.
Laboratory analyses
Laboratory analyses were performed within 4 hours after venous blood collection. Plasma was separated by centrifugation at 8000 rpm for 10 min. Full blood and plasma aliquots were transported to Germany on dry ice and stored at -80°C.
Malaria diagnosis
Malaria parasites were counted microscopically on Giemsa-stained thick blood films per 200 white blood cells. Following DNA extraction (QIAamp DNA blood mini kit, Qiagen, Hilden, Germany), semi-nested PCR assays were performed to ascertain Plasmodium infection and parasite species.[15] A malarial infection was present, if either microscopy or PCR result was positive. Clinical malaria was defined as positive microscopy for any Plasmodium species plus current fever (≥37.5°C) or a self-reported history of fever within the last 48h.
Biomarkers of malnutrition
For iron status, plasma concentrations of ferritin and C-reactive protein (CRP) were measured by immunoturbidimetry (Architect 16000, ABBOTT Laboratories, Chicago, USA). The inter-assay coefficients of variation were 0.85–2.15% for CRP and 9% for ferritin. Iron deficiency was defined as ferritin < 15 μg/L or as ferritin < 30 μg/L, if CRP was > 0.5 mg/L.[16]
For vitamin A metabolism, retinol concentrations were quantified by high-performance liquid chromatography (HPLC).[17] Vitamin A deficiency was defined according to WHO as a plasma retinol concentration < 0.7 μmol/L.[18]
Fasting plasma glucose
For FPG measurement, we used a portable device (Accu-Check Inform II, Roche Diagnostics, Germany). The inter-assay coefficient of variation was 2.9–4.1%. Impaired fasting glucose was defined according to American Diabetes Association criteria as 5.6 mmol/L ≤ FPG ≤ 6.9 mmol/L.[19]
Statistical analysis
Thirteen participants with missing or implausible values for age, sex, biomarkers or covariates were excluded from the analysis, resulting in a final analytical sample of 188. Infectious diseases were defined as a malarial infection or a diagnosed infectious disease (by study physician) or self-reported symptoms compatible with another infectious disease (e.g. cough, cold, fever); malnutrition comprised underweight, stunting, iron deficiency and vitamin A deficiency; and CRFs were defined as overweight or obesity, IFG or hypertension.
Given an α-level of 0.05, this study had a statistical power of 70% to detect a disease occurrence of 20% ± 7% (e.g. hypertension [7]). For all categorical variables, data are presented as percentage with 95% confidence interval (CI) as a measure of accuracy. Continuous variables are presented as median and interquartile range (IQR). Between-group comparisons were performed by Mann-Whitney-U test for continuous variables and by χ²-test for categorical variables. For the associations of demographic, socio-economic and medical factors with infectious diseases, malnutrition and CRFs, we used logistic regression to calculate odds ratios (OR) and their 95% CIs. Due to the small sample size of our study, we aimed at reducing the number of socio-economic variables for the risk factor analysis. Thus, we investigated the correlation structure of all SES variables using Spearman correlations. Variables with the strongest correlations were selected for further analysis. Therefore, the final regression model for the associations with infectious diseases, malnutrition and CRFs comprised age, sex, residence, maternal occupation, paternal occupation, the wealth score and all other entities. As a sensitivity analysis, we calculated logistic regression models with the same set of risk factors to investigate the relationships within the combined entities.
Statistical analyses were performed by IBM SPSS statistical software package version 23 (IBM, Armonk, NY, USA). The significance threshold was p < 0.05.
Results
Study population
The demographic and socio-economic characteristics of the study participants are shown in Table 1. The median age was 15.2 years (range: 14.4–15.5 years) and both sexes were equally represented. The majority of adolescents were of Akan ethnicity (93%) and two-thirds lived in Agogo. Most boys and girls attended school (98%) and were able to read and write (90%). These characteristics were similar between male and female participants. Secondary school education predominated among parents, most worked manually. The median number of people in the household was 11, and the median number of siblings was 4 (Table 1).
Table 1. Socio-demographic characteristics of 188 rural Ghanaian adolescents.
| Characteristic | Male (n = 94) | Female (n = 94) |
|---|---|---|
| Age in years | 15.2 (15.0–15.4) | 15.2 (14.9–15.5) |
| Ethnic group, Akan (%) | 88 | 97 |
| Residence, Agogo (%) | 70 | 71 |
| Place of school, Agogo | 53 | 64 |
| Wealth score | 0.45 (0.29–0.81) | 0.55 (0.28–0.82) |
| Literacy, illiterate (%) | 14 | 5 |
| Education of the father (%) | ||
| None | 3 | 4 |
| Primary | 13 | 11 |
| Secondary | 46 | 46 |
| Tertiary | 7 | 10 |
| Unknown | 31 | 30 |
| Education of the mother (%) | ||
| None | 5 | 3 |
| Primary | 16 | 21 |
| Secondary | 42 | 53 |
| Tertiary | 5 | 1 |
| Unknown | 32 | 21 |
| Occupation of the father (%) | ||
| Intellectual worker | 18 | 30 |
| Manual worker | 70 | 57 |
| Other worker | 7 | 11 |
| Unemployed | 4 | 2 |
| Occupation of the mother (%) | ||
| Intellectual worker | 7 | 7 |
| Manual worker | 87 | 85 |
| Other worker | 3 | 2 |
| Unemployed | 2 | 5 |
| Number of people in the household | 11 (2–19) | 11 (3–23) |
| Number of siblings | 4 (2–6) | 4 (2–6) |
Data are presented as median (interquartile range) for continuous variables and as percentage for categorical variables.
Proportions of infectious diseases, malnutrition and cardio-metabolic risk factors
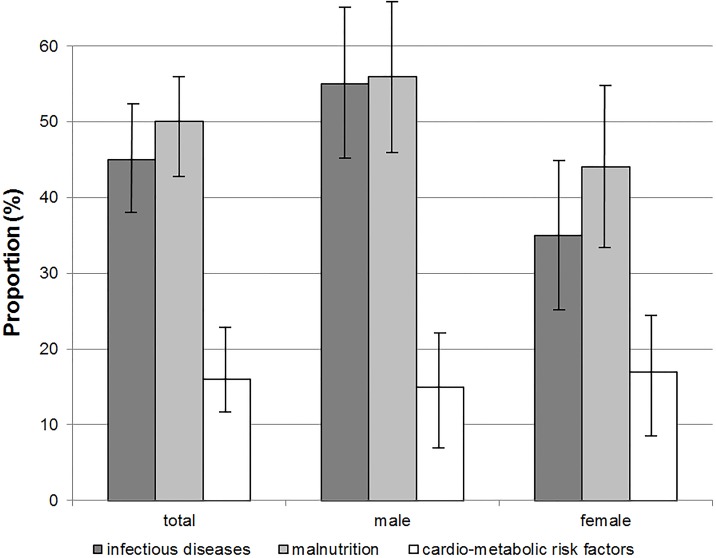
Fig 1 shows the proportions of infectious diseases, malnutrition and CRFs, while Table 2 presents the clinical and anthropometric characteristics. Among the adolescents, 45% (95% CI: 38–52%) had at least one infectious disease, half (95% CI: 43–57%) showed malnutrition, and 16% (95% CI: 11–21%) had at least one CRF. Infectious diseases and malnutrition were more common in boys than in girls, respectively, while overweight/obesity was more common in girls (Fig 1).
Fig 1. Proportions of infectious diseases, malnutrition and cardio-metabolic risk factors in 188 adolescents in rural Ghana.
Error bars indicate 95% confidence intervals. dark grey = infectious diseases, comprise malarial infection plus diagnoses of and symptoms compatible with another infectious disease; light grey = malnutrition, comprises underweight, stunting, iron deficiency and vitamin A deficiency; white = cardio-metabolic risk factors, comprise overweight, obesity, impaired fasting glucose and hypertension.
Table 2. Clinical and anthropometric characteristics of 188 rural Ghanaian adolescents.
| Characteristic | Total (n = 188) | Male (n = 94) | Female (n = 94) | p |
|---|---|---|---|---|
| Infectious diseases | ||||
| Malarial infection (%) | ||||
| by microscopy | 16 (11, 22) | 21 (13, 30) | 12 (5, 18) | 0.077 |
| by PCR | 40 (33, 47) | 49 (39, 59) | 31 (21, 40) | 0.011 |
| by microscopy or PCR | 41 (34, 48) | 51 (41, 61) | 31 (21, 40) | 0.005 |
| Geometric mean parasite density (/μL) | 160 (46–555) | 200 (85–469) | 98 (28–340) | 0.170 |
| Symptoms/diagnoses for another infectious disease (%) | 7 (4, 11) | 7 (2, 13) | 7 (2, 13) | 1.000 |
| History of fever within the last 48h (%) | 18 (12, 23) | 14 (7, 21) | 21 (13, 30) | 0.180 |
| Malnutrition | ||||
| Macronutrients: | ||||
| Body Mass Index (BMI; kg/m2) | 18.98 (15.93–22.03) | 18.70 (16.0–21.4) | 19.37 (16.95–22.79) | 0.002 |
| BMI-for-age z-score (BAZ) | -0.43 (-1.63–0.77) | -0.55 (-1.85–0.75) | -0.37 (-1.65–0.91) | 0.017 |
| Height-for-age z-score (HAZ) | -0.89 (-2.16–0.38) | -1.17 (-2.57–0.23) | -0.65 (-1.85–0.55) | 0.006 |
| Underweight (BAZ ≤ -2, %) | 7 (3, 11) | 10 (4, 16) | 4 (0, 8) | 0.151 |
| Stunting (HAZ ≤ -2, %) | 15 (10, 20) | 21 (13, 30) | 9 (3, 14) | 0.014 |
| Micronutrients: | ||||
| Ferritin (μg/L) | 57.4 (7.5–107.3) | 62.8 (15.1–110.5) | 51.6 (5.00–104.4) | 0.006 |
| Iron deficiency (ferritin < 15 μg/L or < 30 μg/L, if CRP > 0.5 mg/dL, %) | 4 (1, 7) | 1 (-1, 3) | 7 (2, 13) | 0.030 |
| Retinol (μmol/L) | 0.77 (0.49–1.05) | 0.75 (0.50–1.00) | 0.77 (0.50–1.05) | 0.231 |
| Vitamin A deficiency (retinol < 0.7 μmol/L, %) | 36 (29, 43) | 40 (30, 51) | 32 (22, 42) | 0.225 |
| Cardio-metabolic risk factors | ||||
| Overweight or obesity (BAZ ≥ 1) (%) | 7 (4, 11) | 4 (0, 8) | 11 (4, 17) | 0.096 |
| Fasting plasma glucose (mmol/L) | 4.3 (3.5–5.1) | 4.3 (3.4–5.2) | 4.2 (3.4–5.0) | 0.503 |
| Impaired fasting glucose (5.6–6.9 mmol/L) (%) | 1 (0, 3) | 1 (-1, 3) | 1 (-1, 3) | 1.000 |
| Mean systolic blood pressure (BP) (mmHg) | 110 (95–125) | 111 (98–124) | 109 (87–125) | 0.124 |
| Mean diastolic BP (mmHg) | 68 (56–80) | 68 (56–80) | 68 (56–80) | 0.942 |
| Hypertension (BP > 95th percentile or previously diagnosed, %) | 9 (4, 13) | 10 (4, 16) | 7 (2, 13) | 0.601 |
Data are presented as median (interquartile range) for continuous variables and as percentage (95% confidence interval) for categorical variables. Comparisons between males and females were made by Mann-Whitney-U test for continuous variables and by χ2-test for categorical variables.
For infectious diseases, 41% of the teenagers presented with malarial infection of generally low parasite density or detected by PCR only (Table 2). Plasmodium falciparum was the predominant parasite species (39%; P. ovale, 17%; P. malariae, 3%). Malarial infection was more frequent in boys than in girls (p = 0.005). Symptomatic malaria was observed in 2% of the juveniles. Current diagnoses or symptoms compatible with another infectious disease were seen in 7% of adolescents with no gender difference (Table 2). Recorded diagnoses were worm infestations, urinary tract infection, fluor genitalis, candidiasis, common cold, typhoid fever and chicken pox. Symptoms compatible with another infectious disease comprised cough, cold, white vaginal discharge and fever.
With respect to malnutrition, 7% of study participants were underweight and 15% were stunted. No gender-related differences were observed for underweight, but stunting was more common among boys. The median concentration of CRP was 0.63 mg/L (IQR: 0.10–2.11 mg/L), and this was similar between boys and girls (p = 0.32). Iron deficiency was seen in 4% and was more frequent among girls. For vitamin A deficiency, the overall proportion was 36%, with no differences between boys and girls (Table 2).
Regarding CRFs, 7% of adolescents were overweight or obese. This figure was higher in girls than in boys (11% vs. 4%; p = 0.096). FPG was normal in most teenagers (4.3 ± 0.6 mmol/L), but IFG was seen in one boy and one girl. The proportion of hypertension was 9% and this was similar between males and females.
Co-occurrence and risk factors of infectious diseases, malnutrition and cardio-metabolic risk factors
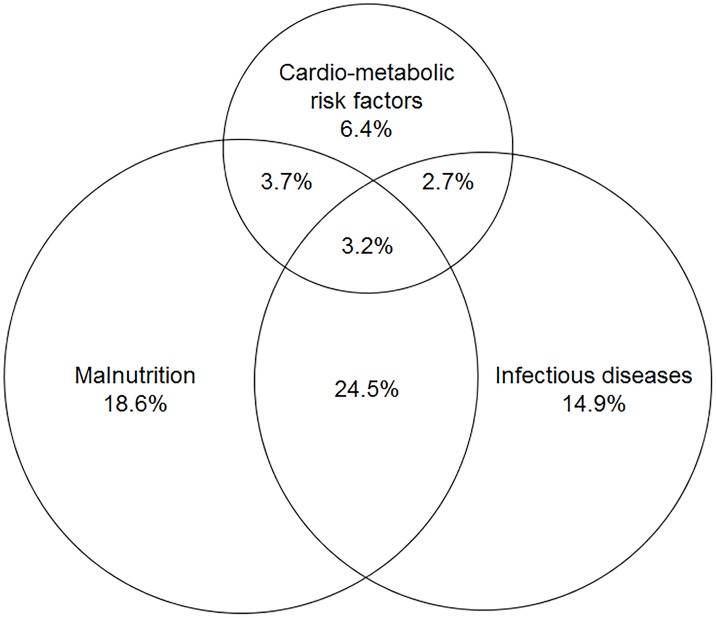
In Fig 2, we present the co-occurrence of infectious diseases, malnutrition and CRFs. Of all participants, roughly one-third (28%; 95% CI: 21–34%; n = 53) had an infectious disease and concomitant malnutrition. This was dominated by malarial infection plus vitamin A deficiency (n = 32/53). Further, the combination of CRFs with either infectious diseases or with malnutrition was discernible in 6% (95% CI: 2–9%; n = 11) and 7% (95% CI: 3–11%; n = 13), respectively. The former mainly comprised malarial infection plus hypertension (n = 8/11), while the latter was largely attributable to vitamin A deficiency plus overweight or obesity (n = 7/13).
Fig 2. Venn diagram for the co-occurrences of infectious diseases, malnutrition and cardio-metabolic risk factors in 188 adolescents in rural Ghana.
Data are presented as proportions of the total study population. Infectious diseases comprise malarial infection plus diagnoses of and symptoms compatible with another infectious disease; malnutrition comprises underweight, stunting, iron deficiency and vitamin A deficiency; cardio-metabolic risk factors comprise overweight, obesity, impaired fasting glucose and hypertension.
In Table 3, we present crude and multiple-adjusted associations of demographic, socio-economic and medical factors with infectious diseases, malnutrition and CRFs. In univariate analysis, female gender reduced the odds of infectious diseases, while manual paternal occupation (vs. intellectual occupation), a wealth score < median of 0.55 and malnutrition each more than doubled the odds of infectious diseases. In the multivariate model, the associations remained for sex, wealth score and malnutrition (Table 3). Conversely, infectious diseases conferred increased odds of malnutrition. Moreover, male gender and indicators of low SES (parental occupational status) tended to increase the odds of malnutrition in the multivariate model. Regarding CRFs, none of the risk factors was significantly associated. Yet, female gender and low parental occupational status nominally increased the odds of prevalent CRFs.
Table 3. Associations of demographic, socio-economic and medical factors with infectious diseases, malnutrition and CRFs.
| Risk factors | N | Infectious diseases | Malnutrition | Cardio-metabolic risk factors | |||
|---|---|---|---|---|---|---|---|
| Crude OR | Multivariate OR | Crude OR | Multivariate OR | Crude OR | Multivariate OR | ||
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||
| Age (per 1 month) | 0.93 (0.83, 1.04) | 0.91 (0.80, 1.04) | 0.93 (0.83, 1.04) | 0.95 (0.84, 1.07) | 0.91 (0.79, 1.05) | 0.91 (0.78, 1.05) | |
| Sex | |||||||
| Male | 94 | Reference | Reference | Reference | Reference | Reference | Reference |
| Female | 94 | 0.44 (0.24, 0.79) | 0.54 (0.28, 1.02) | 0.60 (0.34, 1.07) | 0.65 (0.35, 1.21) | 1.17 (0.54, 2.56) | 1.06 (0.46, 2.43) |
| Residence | |||||||
| Village | 55 | Reference | Reference | Reference | Reference | Reference | Reference |
| Agogo | 133 | 0.72 (0.39, 1.36) | 0.61 (0.30, 1.26) | 0.77 (0.41, 1.45) | 0.88 (0.44, 1.76) | 0.80 (0.35, 1.83) | 0.80 (0.33, 1.96) |
| Occupation of the father | |||||||
| Intellectual worker | 45 | Reference | Reference | Reference | Reference | Reference | Reference |
| Manual worker | 120 | 2.14 (1.05, 4.37) | 1.06 (0.45, 2.50) | 1.77 (0.88, 2.26) | 1.76 (0.77, 4.00) | 1.15 (0.42, 3.12) | 1.39 (0.45, 4.34) |
| Other worker | 17 | 1.40 (0.44, 4.41) | 0.60 (0.15, 2.36) | 1.33 (0.43, 4.10) | 1.33 (0.35, 5.04) | 2.71 (0.70, 10.47) | 2.57 (0.55, 12.12) |
| Unemployed | 6 | 0.40 (0.04, 3.74) | 0.23 (0.02, 2.53) | 1.50 (0.27, 8.28) | 1.77 (0.28, 11.18) | 1.30 (0.13, 13.13) | 1.75 (0.15, 19.84) |
| Occupation of the mother | |||||||
| Intellectual worker | 14 | Reference | Reference | Reference | Reference | Reference | Reference |
| Manual worker | 162 | 2.27 (0.68, 7.52) | 2.04 (0.54, 7.71) | 1.76 (0.56, 5.47) | 1.33 (0.39, 4.59) | 1.10 (0.23, 5.19) | 1.08 (0.20, 5.79) |
| Other worker | 5 | 3.75 (0.45, 31.62) | 3.32 (0.28, 39.41) | 7.20 (0.62, 83.34) | 5.47 (0.40, 74.69) | 4.00 (0.39, 41.23) | 2.89 (0.21, 39.34) |
| Unemployed | 7 | 0.42 (0.04, 4.66) | 0.22 (0.02, 3.13) | 4.50 (0.63, 32.30) | 5.39 (0.68, 42.51) | 1.00 (0.08, 13.37) | 1.00 (0.07, 14.92) |
| Wealth score | |||||||
| ≥ median (0.55) | 101 | Reference | Reference | Reference | Reference | Reference | Reference |
| < median (0.55) | 87 | 2.33 (1.29, 4.19) | 2.60 (1.30, 5.21) | 0.88 (0.50, 1.56) | 0.56 (0.29, 1.12) | 0.74 (0.33, 1.63) | 0.71 (0.29, 1.71) |
| Infectious disease | |||||||
| Negative | 103 | - | - | Reference | Reference | Reference | Reference |
| Positive | 85 | - | - | 2.29 (1.27, 4.12) | 2.26 (1.18, 4.33) | 0.66 (0.29, 1.47) | 0.68 (0.29, 1.64) |
| Malnutrition | |||||||
| Negative | 94 | Reference | Reference | - | - | Reference | Reference |
| Positive | 94 | 2.29 (1.27, 4.12) | 2.27 (1.18, 4.34) | - | - | 0.73 (0.33, 1.60) | 0.67 (0.29, 1.58) |
| Metabolic condition | |||||||
| Negative | 158 | Reference | Reference | Reference | Reference | - | - |
| Positive | 30 | 0.66 (0.29, 1.47) | 0.69 (0.28, 1.69) | 0.73 (0.33, 1.60) | 0.67 (0.29, 1.59) | - | - |
Odds ratios (OR) and 95% confidence intervals (CIs) were calculated by logistic regression; multivariate models include all other variables. Infectious diseases comprise malarial infection plus diagnoses of and symptoms compatible with another infectious disease; malnutrition comprises underweight, stunting, iron deficiency and vitamin A deficiency; cardio-metabolic risk factors comprise overweight, obesity, impaired fasting glucose and hypertension.
As a sensitivity analysis, we used the same set of demographic and socio-economic variables to calculate i) associations between malarial infection or other infectious diseases and nutrient deficiencies, ii) relationships between macro- and micronutrient deficiencies, and iii) interrelations between overweight/obesity and IFG or hypertension. Neither malarial infection nor other common infectious diseases were associated with underweight, stunting or iron deficiency. The presence of vitamin A deficiency increased the odds for common infectious diseases (OR: 3.23; 95% CI: 1.03, 10.19), but not for malarial infection. Also, anthropometric markers of protein-energy-malnutrition, i.e. underweight or stunting, had no effect on micronutrient deficiencies (iron, vitamin A). For IFG, the occurrence was too low (n = 2) to calculate regression models, while there was a lack of association between overweight/obesity and hypertension (OR: 0.79; 95% CI: 0.09, 6.72).
Discussion
Summary of main findings
In rural Ghana, we investigated the proportions and the co-occurrence of common infectious diseases, malnutrition and CRFs among 188 adolescents. Demographic, socio-economic and medical risk factors for the combined entities were assessed as well as associations of single diseases within these entities. Roughly, half of the study population had an infectious disease or was malnourished; 16% presented with a CRF. Infectious diseases and malnutrition were more common among boys, while CRFs tended to be more frequent among girls. Infectious diseases and malnutrition were strongly linked with each other (co-occurrence 28%). Particularly, vitamin A deficiency increased the risk of non-malarial infectious diseases more than 3-fold. Moreover, male gender and low household SES increased the odds of both, infectious diseases and malnutrition. The overlap of infectious diseases and malnutrition with CRFs was rather small (2 out of 25 teenagers), and no associations of demographic, socio-economic and medical factors with CRFs were observed.
Proportions of infectious diseases, malnutrition and cardio-metabolic risk factors
For malarial infection, there is a marked paucity of prevalence data from the adolescent population in Ghana. Compared to younger age groups in the country [20], we found a lower proportion of Plasmodium infections (41%) which were largely asymptomatic and of low or submicroscopic parasite density, arguing for a naturally acquired semi-immunity among these Ghanaian teenagers.[21] In general, the study findings may not be representative for Ghanaian adolescents, because of the limited sample size. Moreover, we focused on malaria and infectious diseases that are common and readily detectable. Thus, we might have underestimated the proportions of other common infectious diseases requiring diagnostic tests beyond routine physical examination, such as HIV/AIDS, tuberculosis and so-called neglected tropical diseases.[2]
The present estimates for malnutrition contribute uniquely to the scarce data of the teenage group in Ghana. Regarding macronutrient deficiencies, our findings suggest somewhat lower figures than expected for underweight (7%) [3], and similar proportions for stunting (15%).[22] At the same time, the male preponderance of macronutrient deficiencies has been frequently reported from SSA [3, 22] and is attributed to higher levels of physical activity due to manual labour among boys.[23] For micronutrient deficits, the degree of iron deficiency (4%) in the present study population was lower than previously reported [24], whereas the proportion of vitamin A deficiency (36%) was similar.[25]
For CRFs, the proportion (7%) and the female preponderance of overweight/obesity accord with previous reports from the region.[3, 12, 26] Likely, the differences in study design and in the degree of urbanization contribute to the comparatively low proportion of IFG in the present analysis.[27, 28] With regard to hypertension, the proportion of 9% was surprisingly high, compared to previous reports from urban Ghana (4%) [29], and given the percentile-based definition of hypertension (expected prevalence: 5%). While the available reference data stem from a large multi-ethnic survey [14], their application in sub-Saharan African settings is novel and may require independent verification.
Co-occurrence and risk factors of infectious diseases, malnutrition and metabolic conditions
The vicious circle of infectious diseases and malnutrition remains a major public health challenge in sub-Saharan Africa.[1] This seems to apply to adolescents in rural Ghana, too. Almost one-third of our study population presented with an infectious disease plus at least one form of nutritional deficits. Malarial infection and vitamin A deficiency were the predominant conditions (32/53). The association between clinical malaria and malnutrition has extensively been examined [30], and is seen also for asymptomatic infections among adolescents elsewhere in sub-Saharan Africa.[31] In our study, malarial infection and other common infectious diseases increased the odds of malnutrition 2.3-fold; and this was also observed vice versa. Moreover, low occupational status of the father and low wealth score increased the odds of infectious diseases and of malnutrition, in accordance with current findings from sub-Saharan Africa, linking poverty and disease.[1] More specifically, vitamin A deficiency was strongly associated with common infectious diseases (other than malaria), a finding that is commonly attributed to impaired mucosal epithelial regeneration and immune dysfunction.[32] Despite a considerable reduction of vitamin A deficiency-associated diseases in West Africa in the past 20 years, our results underscore that vitamin A deficiency remains the fourth leading cause of disease burden in this region.[33]
On the background of demographic and economic development, Ghana faces an epidemiologic transition from infectious diseases to metabolic conditions that appears to be delayed in rural areas and poorer social classes.[34, 35] Consequently, infectious diseases still predominate while metabolic conditions increase steadily. This “double burden of disease” has been recognized on the country level,[35] but only selective efforts were made to re-conceptualize healthy body ideals and to improve health literacy in Ghana.[36, 37] In the present study, we assessed the co-occurrence of infectious diseases and CRFs in the individual. This proportion of 6% was dominated by malarial infection plus hypertension. It appears unlikely that high blood pressure was an immediate consequence of malarial infection, as indicated by the lack of association in our study. Rather, malarial infection and malaria-related fever reduce systolic blood pressure [38], and our observations probably reflect paralleling diseases.
While the term “double burden of malnutrition” usually refers to the co-occurrence of underweight, stunting or micronutrient deficiencies plus overweight or obesity, the denominator for this constellation frequently varies. On the country level, the Double burden of malnutrition refers to considerable amounts of childhood stunting (27%) and maternal overweight (29%) in the Ghanaian population.[4] On the household level, the term describes families with at least one underweight, stunted or micronutrient deficient member plus at least one overweight or obese person.[39] For the individual level, the double burden of malnutrition addresses macro- and micronutrient deficiencies as comorbidities of adiposity in one person. The present study extends the latter concept to the co-occurrence of nutritional deficits plus overweight, obesity, IFG and hypertension. A similar analysis was conducted among urban adults aged 25–60 years in Burkina Faso, and revealed that one-quarter of the study population had at least one nutritional deficiency and one CRF (overweight or obesity or abdominal obesity, hypertension, hyperglycaemia or insulin resistance or diagnosed diabetes and dyslipidaemia).[40] Already at the age of 15 years, nutritient deficiencies plus CRFs were present in 7% of our study population, which was mainly attributed to vitamin A deficiency plus hypertension. Hypertension rates in Ghana are projected to increase dramatically, based on population growth and aging [41], while vitamin A deficiency still manifests in 2% of women at childbearing age.[42] Therefore, once the adolescents get older, the group of vitamin A deficient and hypertensive adults will definitely grow, challenging diagnosis and management of these entities.[43]
Strengths and limitations
So far, data on the co-occurrence of infectious diseases, malnutrition and CRFs are scarce for the population group that forms the basis of Africa’s future—adolescents.[10] Thus, our findings make an important contribution to the knowledge on the health of African populations under epidemiologic transition. Still, the present study was limited in sample size producing wide confidence intervals of the detected proportions. This calls for independent replications in larger surveys. In addition, we cannot comment on the characteristics of adolescents who did not follow the study invitation, and selection bias might have occurred. Also, the cross-sectional nature of our study bears the problem of recall bias for self-reported diagnoses and symptoms, and the potential of reverse causation for some risk factors. This may limit the interpretability of the associations between infectious diseases and malnutrition. Still, malarial infection, malnutrition and CRFs were objectively measured by well-trained study personnel. For instance, hypertension was defined based on the last two BP measurements performed by a validated, fully-automated device using sex-, age- and height-specific percentiles, to avoid misclassification through investigator-related BP increase (white-coat effect) or conventional BP cut-offs, respectively.
Conclusions
In conclusion, in this population of rural Ghanaian adolescents, asymptomatic malaria infection, chronic energy deficits and vitamin A deficiency still constitute major health threats. Already at this young age, obesity and hypertension evolve and even co-exist with infectious diseases and nutrient deficits on the individual level. Potential interrelations of malaria, malnutrition, and cardio-metabolic risk factors remain to be investigated for understanding disease trends and ultimately guide resource allocation for health care in sub-Saharan Africa.
Supporting information
(PDF)
Acknowledgments
The authors are grateful to the administration and staff of the Presbyterian Mission Hospital, Agogo, Ghana for supporting on-site data and sample collection.
Data Availability
The study comprises a relatively small sample of 188 individuals originating from a small village. The data contain information that can potentially reveal the participants’ identity. Most importantly, the consent form specifically states that data will be handled confidentially and that no third parties will have access to them. The institutional review board was the Committee on Human Research, Publication and Ethics, School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. While the review board can request access to the data, the actual datasets are stored (as indicated above) as password-protected computer files on the data storage server at the PI’s premise. Therefore, any data requests to the review board by third parties will be forwarded to the PI. The contact information is: Rev. Prof. John Appiah-Poku, Honorary Secretary, for Chairman (Email: chrp.knust@gmail.com or chrpe@knust.edu.gh).
Funding Statement
The authors received no specific funding for this work.
References
- 1.G.B.D. Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053): 1659–1724. doi: 10.1016/S0140-6736(16)31679-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murray CJ, Ortblad AF, Guinovart C, Lim SS, Wolock TM, Roberts DA, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2014;384(9947): 1005–70. doi: 10.1016/S0140-6736(14)60844-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manyanga T, El-Sayed H, Doku DT, Randall JR. The prevalence of underweight, overweight, obesity and associated risk factors among school-going adolescents in seven African countries. BMC Public Health 2014;14: 887 doi: 10.1186/1471-2458-14-887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ghana Statistical Service. Ghana Demographic and Health Survey 2014. Rockville, Maryland, USA: Ghana Health Service and ICF International, 2015. https://dhsprogram.com/pubs/pdf/FR307/FR307.pdf. Accessed 2016 December 20. [Google Scholar]
- 5.Peltzer K, Pengpid S. Overweight and obesity and associated factors among school-aged adolescents in Ghana and Uganda. Int J Environ Res Public Health 2011;8(10): 3859–70. doi: 10.3390/ijerph8103859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.International Diabetes Federation. IDF Diabetes Atlas, 7th edn Brussels, Belgium: International Diabetes Federation, 2015. http://www.diabetesatlas.org. Accessed 2017 January 10. [Google Scholar]
- 7.Duah FD, Werts N, Hutton-Rogers L, Amankwa D, Otupiri E. Prevalence and risk factors for hypertension in Adansi South, Ghana: A case for health promotion. SAGE Open 2013: 1–5. [Google Scholar]
- 8.Kuate Defoh B. Demographic, epidemiological, and health transitions: are they relevant to population health patterns in Africa? Global Health Action 2014;7: 22443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones AD, Acharya Y, Galway LP. Urbanicity gradients are associated with the household- and individual-level double burden of malnutrition in sub-Saharan Africa. J Nutr 2016;146(6): 1257–67. doi: 10.3945/jn.115.226654 [DOI] [PubMed] [Google Scholar]
- 10.Hervish A, Clifton D. Status report on adolescents and young people in sub-Saharan Africa: Opportunities and challenges. Johannesburg, South Africa: United Nations Population Fund (UNFPA), 2012. http://www.prb.org/pdf12/status-report-youth-subsaharan-Africa.pdf. Accessed 2016 November 15.
- 11.Oni T, Unwin N. Why the communicable/non-communicable disease dichotomy is problematic for public health control strategies: implications of multimorbidity for health systems in an era of health transition. International Health 2015;7(6): 390–399. doi: 10.1093/inthealth/ihv040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frank LK, Heraclides A, Danquah I, Bedu-Addo G, Mockenhaupt FP, Schulze MB. Measures of general and central obesity and risk of type 2 diabetes in a Ghanaian population. Trop Med Int Health 2013;18(2): 141–51. doi: 10.1111/tmi.12024 [DOI] [PubMed] [Google Scholar]
- 13.agogopresbyhospital.org [internet]. Agogo: Presbyterian Hospital Services; c2014 [cited 2017 Feb 08] http://www.agogopresbyhospital.org
- 14.US Department of Health and Human Services. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Bethesda, USA: National Institutes of Health, 2005. https://www.nhlbi.nih.gov/files/docs/resources/heart/hbp_ped.pdf. Accessed 2016 December 19.
- 15.Rubio JM, Post RJ, van Leeuwen WM, Henry MC, Lindegard G, Hommel M. Alternative polymerase chain reaction method to identify Plasmodium species in human blood samples: the semi-nested multiplex malaria PCR (SnM-PCR). Trans R Soc Trop Med Hyg 2002;96 Suppl 1: S199–204. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Geneva, Belgium: World Health Organization, 2004. http://www.who.int/vmnis/indicators/serum_ferritin.pdf. Accessed 2016 October 23. [Google Scholar]
- 17.Schweigert FJ, Steinhagen B, Raila J, Siemann A, Peet D, Buscher U. Concentrations of carotenoids, retinol and alpha-tocopherol in plasma and follicular fluid of women undergoing IVF. Hum Reprod 2003;18(6): 1259–64. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Global prevalence of vitamin A deficiency in populations at risk 1995–2005: WHO Global Database on Vitamin A Deficiency. Geneva, Belgium: World Health Organization, 2009. http://apps.who.int/iris/bitstream/10665/44110/1/9789241598019_eng.pdf. Accessed 2016 November 16. [Google Scholar]
- 19.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 2003;26(11): 3160–7. [DOI] [PubMed] [Google Scholar]
- 20.Ehrhardt S, Burchard GD, Mantel C, Cramer JP, Kaiser S, Kubo M, et al. Malaria, anemia, and malnutrition in african children—defining intervention priorities. J Infect Dis 2006;194(1): 108–14. doi: 10.1086/504688 [DOI] [PubMed] [Google Scholar]
- 21.Kurtis JD, Mtalib R, Onyango FK, Duffy PE. Human resistance to Plasmodium falciparum increases during puberty and is predicted by dehydroepiandrosterone sulfate levels. Infect Immun 2001;69(1): 123–8. doi: 10.1128/IAI.69.1.123-128.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senbanjo IO, Oshikoya KA, Odusanya OO, Njokanma OF. Prevalence of and risk factors for stunting among school children and adolescents in Abeokuta, southwest Nigeria. J Health Popul Nutr 2011;29(4): 364–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peltzer K. Leisure time physical activity and sedentary behavior and substance use among in-school adolescents in eight African countries. Int J Behav Med 2010;17(4): 271–278. doi: 10.1007/s12529-009-9073-1 [DOI] [PubMed] [Google Scholar]
- 24.Egbi G. Prevalence of vitamin A, zinc, iodine deficiency and anaemia among 2–10 year-old Ghanaian children. Afric J Food Agric Nut Devel 2012;12(2). [Google Scholar]
- 25.Abizari AR, Buxton C, Kwara L, Menah-Homiah J, Armar-Klemesu M, Brouwer ID. School feeding contributes to micronutrient adequacy of Ghanaian schoolchildren. Br J Nutr 2014;112(6): 1019–33. doi: 10.1017/S0007114514001585 [DOI] [PubMed] [Google Scholar]
- 26.Muthuri SK, Francis CE, Wachira LJ, Leblanc AG, Sampson M, Onywera VO, Tremblay MS. Evidence of an overweight/obesity transition among school-aged children and youth in sub-Saharan Africa: a systematic review. PLoS One 2014;9(3): e92846 doi: 10.1371/journal.pone.0092846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agbre-Yace ML, Oyenusi EE, Oduwole AO, Ake MD, Abodo JR. Prevalence of diabetes mellitus among children and adolescents in the district of Abidjan in Cote d'Ivoire: a population-based study. J Diabetes Metab Disord 2015;15: 38 doi: 10.1186/s40200-016-0261-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oluwayemi IO, Brink SJ, Oyenusi EE, Oduwole OA, Oluwayemi MA. Fasting blood glucose profile among secondary school adolescents in Ado-Ekiti, Nigeria. J Nutr Metab 2015;2015: 417859 doi: 10.1155/2015/417859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Afrifa-Anane E, Agyemang C, Codjoe SN, Ogedegbe G, de-Graft Aikins A. The association of physical activity, body mass index and the blood pressure levels among urban poor youth in Accra, Ghana. BMC Public Health 2015;15: 269 doi: 10.1186/s12889-015-1546-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira E, Alexandre MA, Salinas JL, de Siqueira AM, Benzecry SG, de Lacerda MV, Monteiro WM. Association between anthropometry-based nutritional status and malaria: a systematic review of observational studies. Malar J 2015;14: 346 doi: 10.1186/s12936-015-0870-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sifft KC, Geus D, Mukampunga C, Mugisha JC, Habarugira F, Fraundorfer K et al. Asymptomatic only at first sight: malaria infection among schoolchildren in highland Rwanda. Malar J 2016;15(1): 553 doi: 10.1186/s12936-016-1606-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stephensen CB. Vitamin A, infection and immune function. Annu Rev Nutr 2001;21: 167–192. doi: 10.1146/annurev.nutr.21.1.167 [DOI] [PubMed] [Google Scholar]
- 33.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859): 2224–60. doi: 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agyei-Mensah S, de-Graft Aikins A. Epidemiological transition and the double burden of disease in Accra, Ghana. J Urban Health 2010;87(5): 879–97. doi: 10.1007/s11524-010-9492-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smallman-Raynor M, Phillips D. Late stages of epidemiological transition: health status in the developed world. Health Place 1999;5(3): 209–22. [DOI] [PubMed] [Google Scholar]
- 36.Duda RB, Jumah NA, Hill AG, Seffah J, Britwum R. Interest in healthy living outweighs presumed cultural norms for obesity for Ghanaian women. Health Qual Life Outcomes 2006;4:44 doi: 10.1186/1477-7525-4-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babio N, Vicent P, López L, Benito A, Basulto J, Salas-Salvadó J. Adolescents' ability to select healthy food using two different front-of-pack food labels: a cross-over study. Pub Health Nutr 2014;17(6): 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gazzinelli RT, Kalantari P, Fitzgerald KA, Golenbock DT. Innate sensing of malaria parasites. Nat Rev Immunol 2014;14(11): 744–57. doi: 10.1038/nri3742 [DOI] [PubMed] [Google Scholar]
- 39.Dop MC, Pereira C, Mistura L, Martinez C, Cardoso E. Using Household Consumption and Expenditures Survey (HCES) data to assess dietary intake in relation to the nutrition transition: a case study from Cape Verde. Food Nutr Bull 2012;33(3 Suppl): S221–7. [DOI] [PubMed] [Google Scholar]
- 40.Zeba AN, Delisle HF, Renier G, Savadogo B, Baya B. The double burden of malnutrition and cardiometabolic risk widens the gender and socio-economic health gap: a study among adults in Burkina Faso (West Africa). Public Health Nutr 2012;15(12): 2210–9. doi: 10.1017/S1368980012000729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.World Health Organization. Global status report on noncommunicable diseases 2010: Description of the global burden of NCDs, their risk factors and determinants. Geneva, Belgium: World Health Organization, 2011. http://apps.who.int/iris/bitstream/10665/44579/1/9789240686458_eng.pdf. Accessed 2016 October 02.
- 42.Ghana Statistical Service. Ghana Demographic and Health Survey 2008. Calverton, Maryland, USA: Ghana Health Service, 2009. http://www.dhsprogram.com/pubs/pdf/FR221/FR221[13Aug2012].pdf. Accessed 2016 October 27. [Google Scholar]
- 43.Danquah I, Dobrucky CL, Frank LK, Henze A, Amoako YA, Bedu-Addo G, et al. Vitamin A: potential misclassification of vitamin A status among patients with type 2 diabetes and hypertension in urban Ghana. Am J Clin Nutr 2015;102(1): 207–14. doi: 10.3945/ajcn.114.101345 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
The study comprises a relatively small sample of 188 individuals originating from a small village. The data contain information that can potentially reveal the participants’ identity. Most importantly, the consent form specifically states that data will be handled confidentially and that no third parties will have access to them. The institutional review board was the Committee on Human Research, Publication and Ethics, School of Medical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana. While the review board can request access to the data, the actual datasets are stored (as indicated above) as password-protected computer files on the data storage server at the PI’s premise. Therefore, any data requests to the review board by third parties will be forwarded to the PI. The contact information is: Rev. Prof. John Appiah-Poku, Honorary Secretary, for Chairman (Email: chrp.knust@gmail.com or chrpe@knust.edu.gh).