Abstract
Receptor activity-modifying protein 2 (Ramp2) is a single-pass transmembrane protein that heterodimerizes with several family B G-protein coupled receptors to alter their function. Ramp2 has been primarily characterized in association with calcitonin receptor-like receptor (Calcrl, CLR), forming the canonical receptor complex for the endocrine peptide adrenomedullin (Adm, AM). However, we previously demonstrated that Ramp2+/- female mice display a constellation of endocrine-related phenotypes that are distinct from those of Adm+/- and Calcrl+/- mice, implying that RAMP2 has physiological functions beyond its canonical complex. Here, we localize Ramp2 expression in the mouse placenta, finding that Ramp2 is robustly expressed in the fetal labyrinth layer, and then characterize the effects of loss of Ramp2 on placental development. Consistent with the expression pattern of Ramp2 in the placenta, Ramp2-/- placentas have a thinner labyrinth layer with significantly fewer trophoblast cells secondary to a reduction in trophoblast proliferation. We also find that absence of Ramp2 leads to failed spiral artery remodeling unaccompanied by changes in the uterine natural killer cell population. Furthermore, we assess changes in gene expression of other RAMP2-associated G-protein coupled receptors (GPCRs), concluding that Ramp2 loss decreases parathyroid hormone 1 receptor (Pthr1) expression and causes a blunted response to systemic parathyroid hormone (PTH) administration in mice. Ultimately, these studies provide in vivo evidence of a role for RAMP2 in placental development distinct from the RAMP2-CLR/AM signaling paradigm and identify additional pathways underlying the endocrine and fertility defects of the previously characterized Ramp2 heterozygous adult females.
Introduction
Receptor activity-modifying proteins (RAMPs) associate with G-protein coupled receptors (GPCRs) and enable the translocation of select GPCRs from the endoplasmic reticulum to the plasma membrane, ultimately affecting GPCR ligand bias and affinity, selectivity of downstream signaling pathways, and GPCR desensitization [1]. The pharmacologic tractability of GPCRs and, by extension, RAMPs emphasizes the importance of understanding these heterodimeric complexes [2, 3]. Since the majority of studies on RAMP-GPCR associations have focused on in vitro pharmacology, there is a need to assess the physiological and pathophysiological consequences of these interactions, particularly using genetically engineered mouse models [4].
RAMP2, the second of three mammalian RAMPs, has been well-characterized primarily for its association with calcitonin receptor-like receptor (Calcrl, CLR) as the canonical receptor for the endocrine peptide adrenomedullin (Adm, AM) [5–9]. Importantly, RAMP2 also binds other family B GPCRs: calcitonin receptor (Ctr), corticotrophin releasing hormone receptor 1 (Crhr1), glucagon receptor (Gcgr), parathyroid hormone receptor 1 (Pth1r), and vascoactive intestinal polypeptide receptor types 1 and 2 (Vpac1r and Vpac2r). Elucidation of the in vivo consequences of these diverse RAMP2-GPCR interactions is possible due to the availability of genetically engineered mouse models [1]. For example, global deletion of Ramp2 in mice causes embryonic lethality by e14.5 due to proliferative defects in lymphatic vascular development [10, 11]. Because these Ramp2 null phenotypes precisely mirror those observed in Adm and Calcrl knockout embryos, we and others have used this phenotypic conservation as in vivo validation substantiating the essential role of the canonical RAMP2-CLR interaction in vascular development. Interestingly, subsequent endothelial restoration of Ramp2 expression is sufficient to partially rescue the Ramp2-/- lethality but not without consequence, since surviving animals develop dilated cardiomyopathy associated with profound dysregulation Gcgr and Pth1r expression [12], indicating that genetic deletion of Ramp2 can impart physiological consequences that extend beyond canonical AM/CLR signaling. Furthermore, adult female mice heterozygous for Ramp2 demonstrate a constellation of endocrine abnormalities, including fetal growth restriction and hyperprolactinemia that are not phenotypically conserved in Adm+/- and Calcrl+/- female animals [13].
Here, we seek to expand our overall understanding of the numerous GPCR pathways impacted by RAMP2 by capitalizing on the mid-gestation Ramp2-/- placenta—which fortuitously provides a phenotypically-tractable endocrine tissue with homozygous loss of RAMP2 expression [10]. The maternal-fetal interface of the placenta, called the labyrinth layer in rodents, consists of a tightly interdigitated network of trophoblast-lined fetal and maternal blood sinuses that support gas, nutrient, and waste exchange. Maternal blood is delivered to the labyrinth via high capacitance, low resistance maternal vessels called spiral arteries, which undergo an immune cell-mediated remodeling during mid-gestation in order to meet the demands of the growing embryo. Failure of spiral arteries to remodel at mid-gestation is associated with preeclampsia, a common hypertensive disease of pregnancy, which can lead to both fetal growth restriction and intrauterine fetal demise [14]. We hypothesize that, given the canonical RAMP2-CLR/AM signaling paradigm, deletion of Ramp2 in the placenta will phenocopy Calcrl-/- and Adm-/- placentas and may further reveal additional phenotypes consistent with deletion of other RAMP2-associated GPCRs. Thus, a comprehensive characterization of the phenotypic effects of Ramp2 loss within the placenta may elucidate additional RAMP2-associated GPCRs that play critical roles in systemic endocrine physiology and disease.
Materials and methods
Animals
Mice with a deletion of Ramp2 were previously generated by our group and maintained as a heterozygote colony on an isogenic SvEv129/6-TC1 background [10]. Genotyping of the wild type and targeted Ramp2 genes was also described previously [10]. All animal experiments were approved by the University of North Carolina at Chapel Hill Institutional Animal Care and Use Committee (Protocol No. 15–209). Ramp+/- intercrosses were set up to generate Ramp2+/+ and Ramp2-/- littermate placentas, which were used in all studies. Embryonic and therefore placental viability were confirmed visually at the time of dissection prior to collection of placentas for phenotypic analysis. Visualization of the vaginal plug was considered embryonic day (e) 0.5. Prior to dissection, animals were euthanized by carbon dioxide inhalation followed by cervical dislocation.
PTH challenge
Five to seven week-old wild type and Ramp2+/- female mice were injected with a single bolus of 500 μgkg-1 hPTH, and blood was collected 30 minutes and two hours later. Serum phosphate levels were determined using Stanbio kits (Fisher Scientific).
Immunofluorescence
All tissues were fixed overnight in 4% paraformaldehyde, cryoprotected overnight in 30% sucrose, embedded in optical cutting temperature compound (Tissue-Tek), and serially sectioned at 8–10 μm. Sections were rehydrated in PBS, permeablized in 0.2% Triton-X100, blocked in 4% bovine serum albumin, and incubated overnight in primary antibody: rabbit polyclonal anti-cytokeratin (Dako, Z0622, AB_2650434); mouse monoclonal anti-BrdU (Invitrogen, 03–3900, AB_2532917); rat monoclonal anti-Ki-67 (Dako, M7249, AB_2250503); mouse monoclonal anti α-smooth muscle actin (Sigma, A2547, AB_476701); or mouse polyclonal parathyroid hormone receptor 1 (Novus, NBP1-40067, AB_2173351). After washing, sections were incubated in secondary antibody for 1–2 hours. Images were acquired on a Nikon E800 microscope with a Hammamatsu ORCA-ER charge-coupled device camera with MetaMorph software (Molecular Devices).
In situ hybridization
e13.5 placentas from wild type 129S6/SvEv intercrosses were collected and processed as described for immunofluorescence, with the exception that paraformaldehyde and sucrose solutions were prepared in diethylypyrocarbonate-treated PBS. Placentas were serially sectioned at 16 μm. Non-radioactive ISH was performed as previously described using sense and anti-sense RNA probes synthesized from plasmids containing cDNA sequences of mouse Ramp2 [15].
qRT-PCR
RNA was extracted using TRIzol (Invitrogen) per the manufacturer’s protocol. RNA was DNase treated and reverse transcribed using M-MLV Reverse Transcriptase (Invitrogen), and gene expression was measured using a MX3000 qPCR machine (Stratagene). TaqMan primer and probes sets (Applied Biosystems) were used to measure gene expression. mGapdh was used as an endogenous reference gene, and relative gene expression was calculated using the ΔΔCT method.
Western blotting
Placental lysates were isolated and then quantitated for protein concentration using a BCA Protein Assay Kit (Pierce). Protein was loaded on a 4–12% SDS-PAGE gel (Invitrogen) and then transferred to a nitrocellulose membrane. The membrane was blocked overnight at 4°C in 5% nonfat dry milk and then incubated overnight at 4° in primary antibodies: rabbit polyclonal anti-PTH1R (Covance, PRB-620B) and mouse monoclonal GAPDH (Novus, NB300-285, AB_2263090). Blots were washed three times in tris-buffered saline/0.1% Tween 20 and then incubated for two hours at room temperature in secondary antibodies. Membranes were then imaged on an Odyssey CLx (LI-COR).
Statistics
All data are presented as mean ± standard error of mean unless otherwise noted. All quantitative analyses were performed in GraphPad Prism 5 using unpaired two-tailed Student’s t-test. A p value less than 0.05 was considered statistically significant.
Results
Ramp2 is abundantly expressed in the labyrinth of the placenta
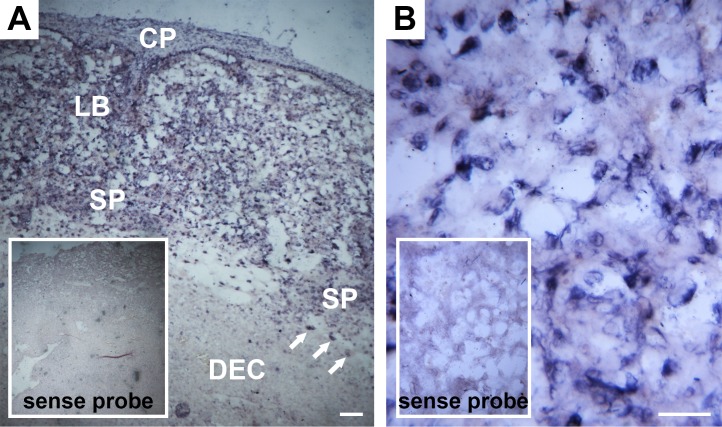
To characterize the expression and localization of Ramp2 in the mouse placenta, we performed in situ hybridization for Ramp2 in e13.5 wild type placentas. We observed expression of Ramp2 in the fetal compartments of the placenta but only diffuse, weak signal in the maternal decidua (Fig 1A). More specifically, Ramp2 expression was evident in the chorionic plate, labyrinth, spongiotrophoblast cells, and giant trophoblast cells, with the labyrinth layer demonstrating the most robust expression (Fig 1B).
Fig 1. In situ hybridization of Ramp2 in e13.5 placentas reveals robust expression in the labyrinth layer.
(A) In situ hybridization in both the fetal and maternal compartments of the wild type placenta (n = 4). Scale bar, 100 μm. (B) In situ hybridization in the labyrinth layer. Scale bar, 50 μm. Inset images in both panels show sense control probes. CP, chorionic plate; LB, labyrinth; SP, spongiotrophoblast cells; DEC, decidua. Arrows point to giant trophoblast cells.
Ramp2-/- placentas exhibit abnormal development of the labyrinth layer
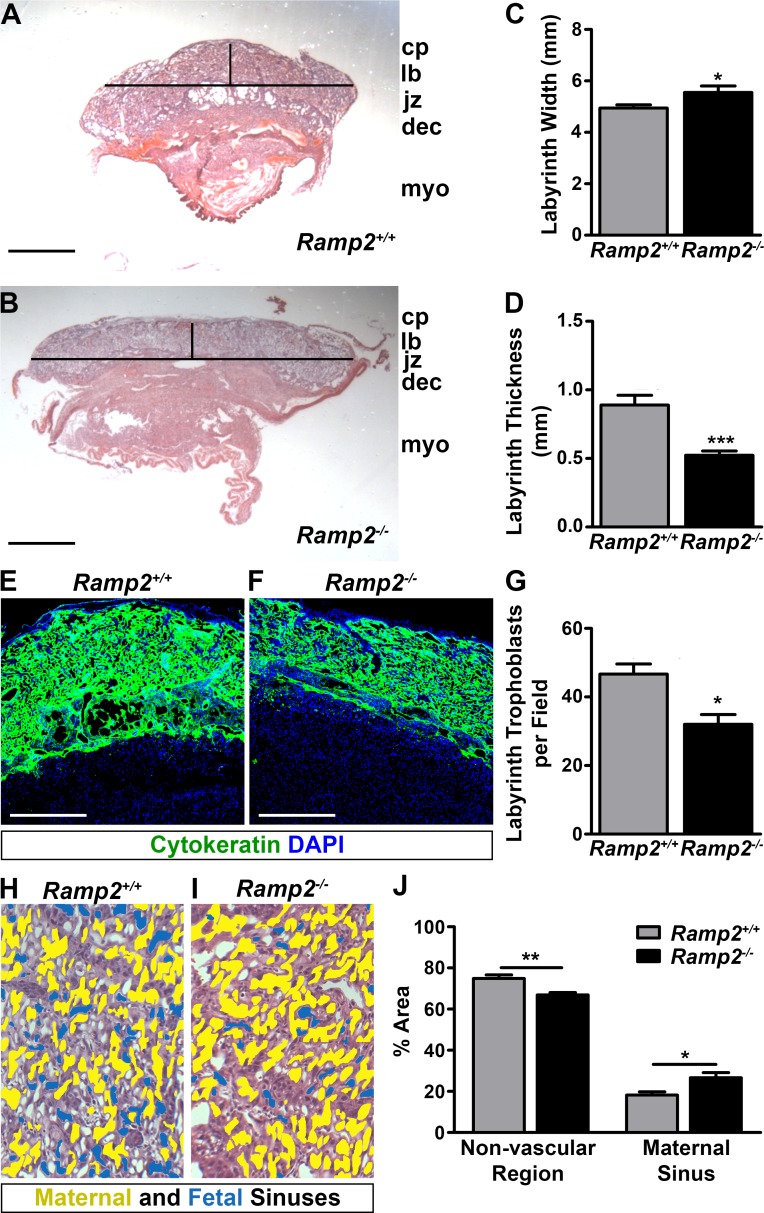
To evaluate the consequence of genetic loss of Ramp2 on placental development, we performed histological analyses of e13.5 wild type and Ramp2-/- littermate placentas obtained from Ramp2+/- intercrosses. All layers of the placentas of both genotypes were visible by hematoxylin and eosin staining (Fig 2A and 2B). However, quantitative, morphometric analysis of these sections revealed a significantly wider, thinner labyrinth layer in Ramp2-/- placentas (Fig 2C and 2D). We hypothesized that this thin labyrinth layer could be a consequence of fewer trophoblast cells. Therefore, we performed immunofluorescence on e13.5 placentas for the trophoblast marker cytokeratin, finding a reduction in the density of trophoblast cells in Ramp2-/- placentas compared to wild type controls (Fig 2E and 2F). We also quantified labyrinth trophoblast cell density from e13.5 wild type and Ramp2-/- placentas by histological identification. Indeed, Ramp2-/- placentas had significantly fewer trophoblasts in the labyrinth layer compared to wild type littermates (Fig 2G).
Fig 2. Ramp2 is essential for proper development of the labyrinth layer of the placenta.
(A and B), Hematoxylin and eosin staining of e13.5 wild type and Ramp2-/- littermate placentas. Horizontal and vertical lines are representative of axes measured in panels (C) and (D) Scale bars, 1 mm. (C and D), Quantification of width and thickness of wild type and Ramp2-/- labyrinth layers (n≥6 placentas per genotype). (E and F), Immunofluorescence for cytokeratin in wild type and Ramp2-/- placentas, showing trophoblast cells in the fetal compartment of the placenta. Scale bar, 500μm. (G) Quantitation of labyrinth trophoblast cells, identified by histology (n = 3 fields per animal, n≥3 placentas per genotype). (H and I) Representative images of wild type and Ramp2-/- placentas with pseudocolored maternal (yellow) and fetal (blue) sinuses. (J) Comparison of the total area of the non-vascular region and of the maternal sinuses in wild type and Ramp2-/- placentas (n≥2 fields per placenta from multiple placentas per genotype). cp, chorionic plate; lb, labyrinth; jz, junctional zone; dec, dedicua; myo, myometrium. *p<0.05, **p<0.01, ***p<0.001
We then evaluated the morphological consequences of labyrinth formation in Ramp2-/- placentas, distinguishing between maternal and fetal sinuses by the presence or absence of nucleated red blood cells, respectively. Consistent with decreased trophoblast cellularity in the labyrinth of Ramp2-/- placentas, the total area of non-vascular regions identified by this method was significantly less in Ramp2-/- placentas compared to wild type placentas (Fig 2H–2J). Concomitantly, the interdigitated footprint of maternal sinuses was significantly greater in Ramp2-/- placentas, indicating an improper balance between the fetal and maternal sinuses within the labyrinth layer. Altogether, these data point to a role for Ramp2 in the proper formation of the maternal-fetal interface within the labyrinth layer.
Ramp2 is required for proliferation of trophoblast cells
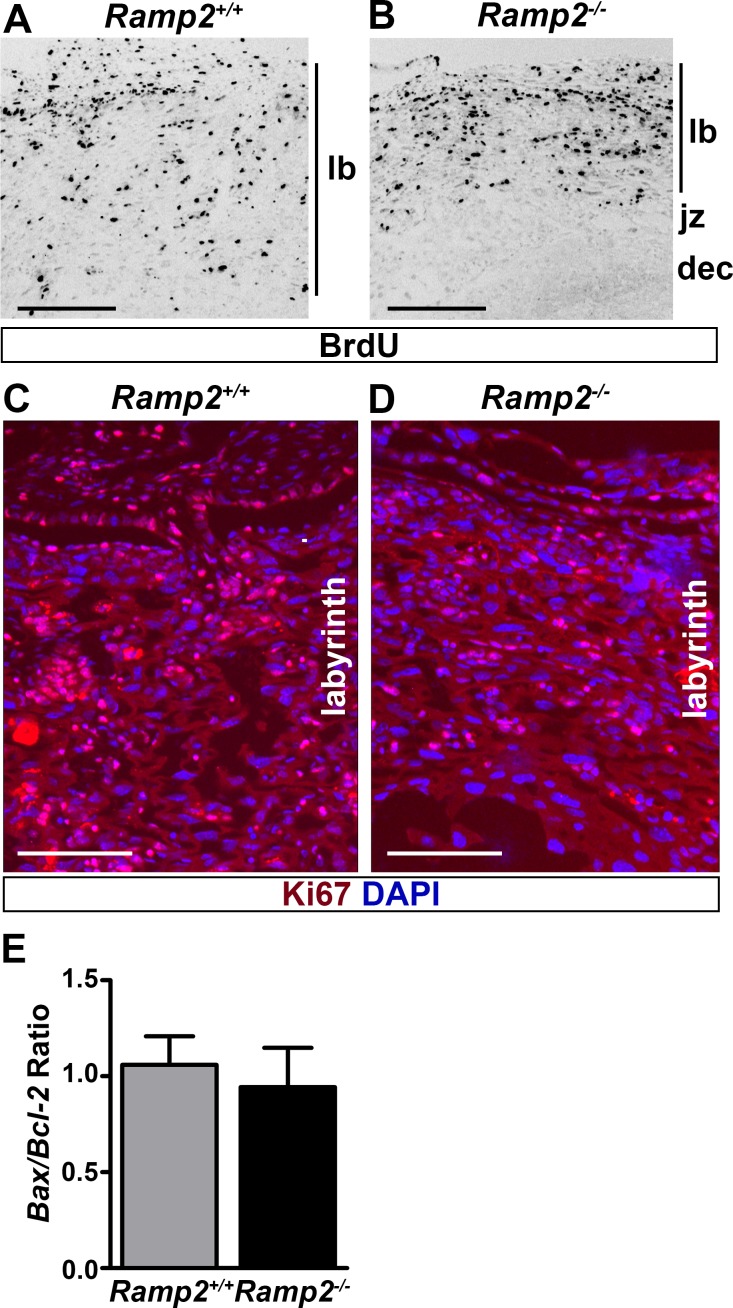
To determine if the decreased labyrinth thickness and trophoblast cell number were due to deficient trophoblast cell proliferation, we performed bromodeoxyuridine (BrdU) incorporation studies in pregnant Ramp2+/- mice bred with Ramp2+/- males on day 13.5 of gestation. BrdU staining revealed a marked reduction in cell proliferation in the labyrinth layer of Ramp2-/- placentas compared to controls (Fig 3A and 3B). Consistently, Ki67 staining also demonstrated reduced signal in the labyrinth of Ramp2-/- placentas compared to wild type littermate placentas, underscoring the important role of Ramp2 in proliferation of trophoblast cells of the labyrinth layer (Fig 3C and 3D). To determine whether an enhanced apoptotic rate contributes to the observed decrease in trophoblast cellularity, we performed qRT-PCR to quantitate the expression of pro-apoptotic Bax and anti-apoptotic Bcl2. The ratio of Bax to Bcl2 did not differ between the two genotypes, indicating that loss of Ramp2 does not promote apoptosis of trophoblast cells (Fig 3E).
Fig 3. Loss of Ramp2 leads to a proliferation defect in the labyrinth layer.
(A and B) Immunofluorescence for BrdU in e13.5 placentas. Scale bars, 500μm. (C and D) Immunofluorescence for Ki67 in the labyrinth of e13.5 placentas. Scale bars, 500 μm. (E) Ratio of Bax to Bcl2 mRNA expression normalized to Gapdh as measured by qRT-PCR (n≥5 placentas per genotype). lb, labyrinth; jz, junctional zone; dec, decidua.
Loss of Ramp2 results in failed spiral artery remodeling
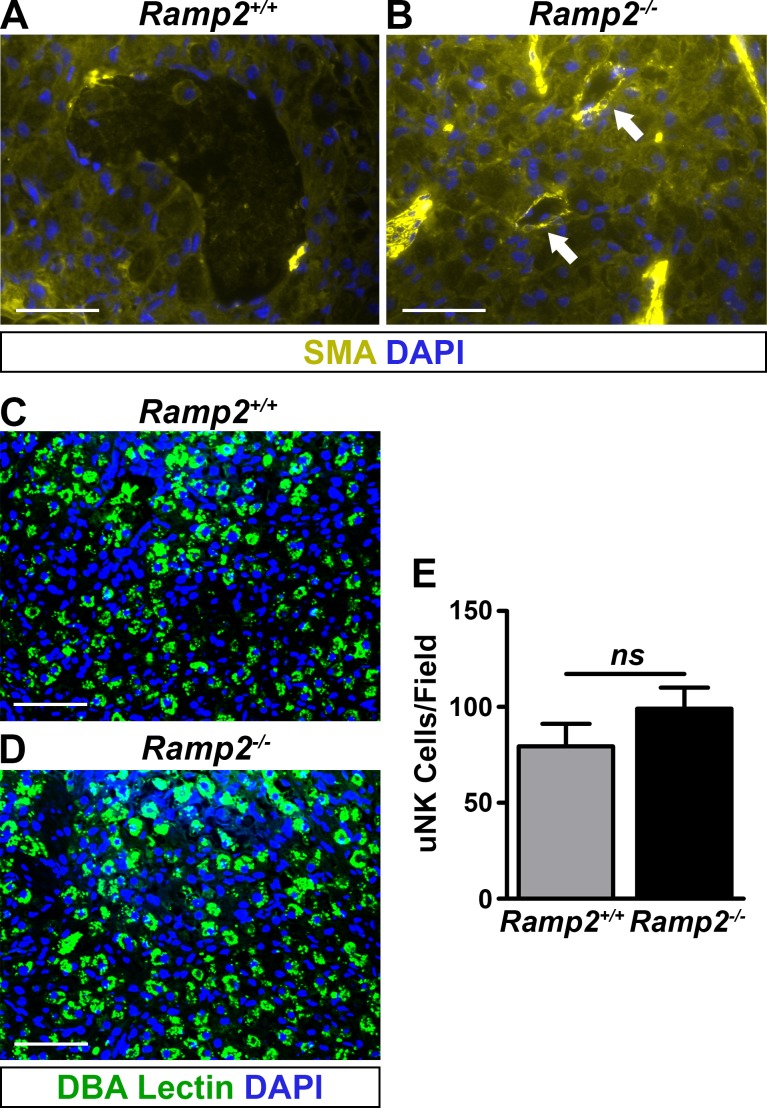
Maternal spiral artery remodeling, which consists of immune cell-mediated vascular smooth muscle cell loss and vessel dilation, is critical for maintaining appropriate placental function and promoting fetal health in both rodents and humans [16]. Therefore, we evaluated the extent of vascular smooth muscle coverage surrounding the maternal spiral arteries by performing immunofluorescence for smooth muscle actin on wild type and Ramp2-/- placentas. As expected, spiral arteries of wild type placentas progressively lost smooth muscle coverage until mid-gestation to accommodate the vascular demands of the growing embryo (Fig 4A). However, we observed persistent smooth muscle coverage of the spiral arteries of Ramp2-/- placentas (Fig 4B).
Fig 4. Ramp2 is essential for proper spiral artery remodeling.
(A and B), Immunofluorescence for smooth muscle actin in e13.5 littermate placentas (n≥6 placentas per genotype). Arrows point to retained smooth muscle actin expression in spiral arteries of Ramp2-/- placentas. Scale bars, 100 μm. (C and D), Immunofluorescence for DBA lectin, a marker of uNK cells, in e13.5 littermate placentas. Scale bars, 100μm. (E) Quantification of uNK cells from e13.5 placentas (n≥3 placentas per genotype). ns, not significant.
A specialized immune cell population in the placenta, uterine natural killer (uNK) cells, release factors that promote spiral artery remodeling [17–19]. We investigated the status of the uNK cell population in these placentas by performing immunofluorescence on e13.5 wild type and Ramp2-/- littermate placentas and found no difference in the size of the uNK cell population, suggesting that the failure to remodel maternal spiral arteries in Ramp2-/- placentas is inherent to the fetal genotype (Fig 4C–4E).
Effect of Ramp2 loss on gene expression of RAMP2-interacting GPCRs
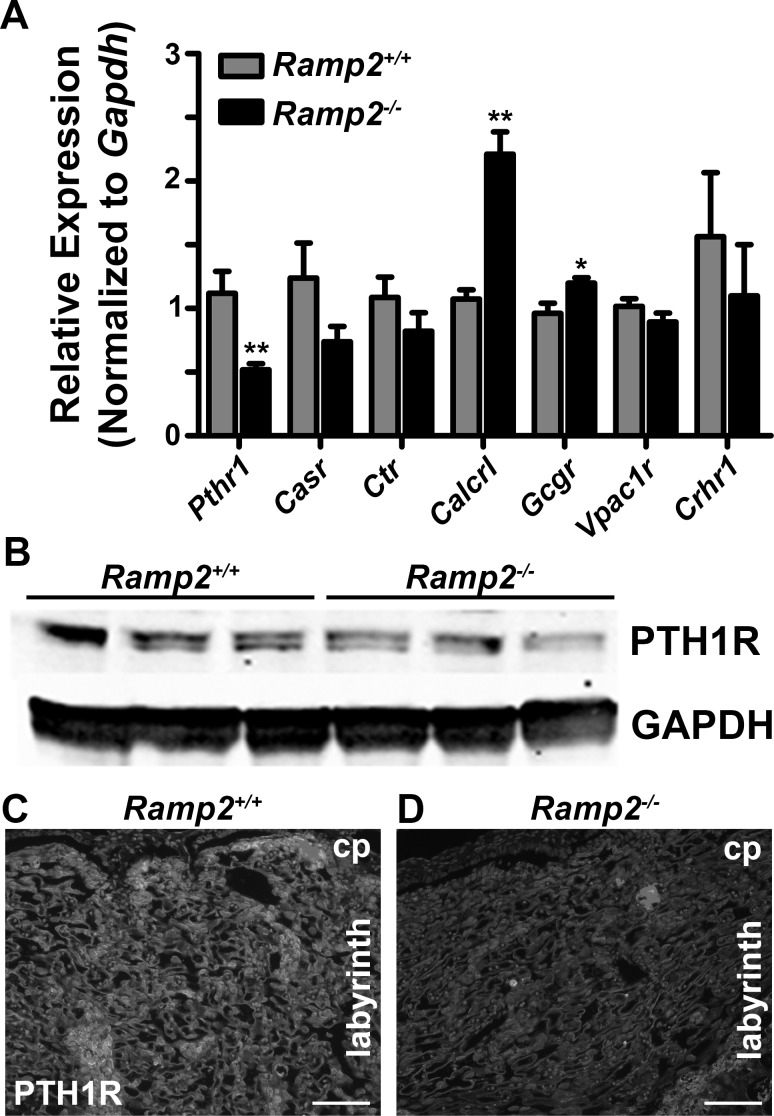
We next attempted to elucidate candidate GPCRs that may be involved in the Ramp2-/- placenta phenotype by performing qRT-PCR for a panel of known RAMP2-interacting GPCRs. We found a robust and significant increase in Calcrl gene expression, consistent with the retention of Calcrl-enriched smooth muscle cells surrounding the spiral arteries [20]. We also noted a modest yet significant increase in glucagon receptor. Most significantly, we found a 50% reduction in the expression of Pthr1, the receptor for parathyroid hormone (PTH) and parathyroid hormone-related protein (PTHrP). To determine whether the reduction of Pth1r mRNA correlated with reduced protein expression, we determined the levels of PTH1R in Ramp2-/- knockout placentas using both western blotting and immunofluorescence. Consistently, we observed a reduction in protein levels in placental lysates by immunoblotting (Fig 5B). Furthermore, immunofluorescence for PTH1R confirmed that this receptor is highly expressed in the labyrinth layer and that Ramp2-/- placentas had considerably less staining compared to wild type placentas (Fig 5C and 5D). Our results provide compelling evidence that loss of Ramp2 alters Pth1r gene and protein expression in the placenta and specifically within the labyrinth layer.
Fig 5. Loss of Ramp2 alters mRNA expression of RAMP2-associating GPCRs, including Pthr1.
(A) mRNA expression of RAMP2-interacting GPCRs from e13.5 wild type and Ramp2-/- placentas (n≥3 placentas per genotype). (B) PTH1R protein expression by immunoblot of e13.5 tissue lysates from wild type and Ramp2-/- placentas. (C and D), Immunofluorescence for PTH1R in the labyrinth of wild type and Ramp2-/- placentas. Scale bars, 100 μm. Pth1r, parathyroid hormone receptor 1; Casr, calcium-sensing receptor; Ctr, calcitonin receptor; Calcrl, calcitonin receptor-like receptor; Gcgr, glucagon receptor; Vpac1r, vasoactive intestinal peptide receptor 1; Crhr1, corticotrophin releasing hormone receptor 1. *p<0.01.
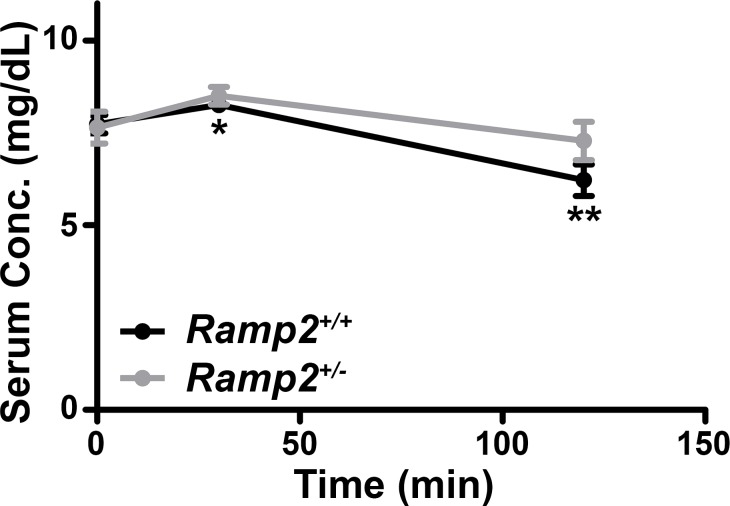
Haploinsufficiency for Ramp2 blunts PTH1R response to PTH
Given changes in Pthr1 expression in the context of Ramp2 loss, we hypothesized that adult mice haploinsufficient for Ramp2 may demonstrate a blunted physiological response to exogenous PTH administration. Indeed, exogenous PTH led to a significant reduction in serum phosphate levels in wild type mice, but this reduction did not occur in Ramp2+/- animals (Fig 6). Therefore, these data indicate that haploinsufficiency for Ramp2 results in blunted, systemic PTH1R signaling.
Fig 6. Ramp2+/- adult mice demonstrate a blunted physiological response to systemic PTH administration.
Serum concentrations of phosphate (solid lines) in wild type and Ramp2+/- animals following administration of a single bolus of PTH (n≥5 animals per genotype). *p<0.05, **p<0.01.
Discussion
In this study, we have localized Ramp2 within the mouse placenta, assessed the effect of Ramp2 loss on placental development and discovered associated changes in the expression of RAMP2-interacting GPCRs with physiological consequences in vivo. First, we found Ramp2 expressed primarily in the labyrinth of the e13.5 placenta. Interestingly, this expression pattern differs from the expression patterns of Calcrl and Adm at a similar developmental time points: at e13.5, Calcrl is diffusely expressed in the labyrinth but robustly expressed in endothelial cells lining spiral arteries, while Adm is primarily found in trophoblast giant cells and in the stroma of the decidua [20]. Furthermore, although Adm-/- and Calcrl-/- placentas consistently demonstrate decreased fetal vessel branching and other placental abnormalities, the structure of the labyrinth layer in these mouse models remains largely normal [20]. Therefore, these juxtaposed findings support the overall hypothesis that RAMP2 influences labyrinth development through association with another known or unknown GPCR.
We also found that Ramp2-/- placentas–similar to Adm-/- and Calcrl-/- placentas–exhibit persistent smooth muscle cell coverage of maternal spiral arteries–a hallmark sign of preeclampsia, a dangerous hypertensive disease of pregnancy [21]. Consistently, one human study found that Ramp2 mRNA expression in the umbilical artery and uterus of women was negatively correlated with pregnancy-induced hypertension [22]. Unlike Adm-/- and Calcrl-/- placentas, the size of the uNK cell population within the decidua was unchanged in Ramp2-/- placentas. Interactions between uNK cells and trophoblast cells help facilitate spiral artery remodeling during mid-gestation as spiral arteries become high capacitance, low resistance vessels to provide oxygen and nutrients to the developing fetus [14]. Although pregnant Ramp2+/- dams carrying Ramp2-/- embryos do not develop overt preeclampsia, likely due to embryonic lethality of Ramp2-/- embryos, our studies demonstrate that RAMP2 plays a role in spiral artery remodeling distinct from uNK cell recruitment and canonical AM signaling through RAMP2-CLR.
Given evidence for RAMP2-mediated effects on placental development independent of CLR, we analyzed changes in gene expression of all known RAMP2-associated GPCRs in Ramp2-/- placental lysates, finding that Pthr1 mRNA expression was approximately 50% lower by qRT-PCR compared to wild type littermate placentas. Elegant studies performed by Kovacs and colleagues previously identified roles for PTH1R ligands PTH and PTHrP in placental calcium transfer and trophoblast cell proliferation [23–26]. Consistent with these studies, we found that PTH1R expression was decreased in Ramp2-/- placentas by immunoblotting and by immunofluorescence. We further hypothesized that Ramp2+/- adult females would demonstrate a blunted response to systemic PTH administration. Indeed, systemic PTH caused a decrease in serum phosphate levels only in wild type females, not in Ramp2 heterozygous females. While the long-term physiologic consequences of this are unclear, the established therapeutic roles of PTH and PTHrP in diseases like osteoporosis and cancer underscore the importance of understanding consequences of the RAMP2-PTH1R interaction in various organ systems in vivo [27–30].
In summary, the present study capitalizes on the availability of genetically deficient Ramp2-/- tissue to provide insight into RAMP2-mediated effects on placental development and its possible role in the pathogenesis of preeclampsia, likely both in concert with and independent from canonical AM-CLR signaling. We further show that genetic loss of Ramp2 causes a significant reduction in PTH1R, with physiological consequences in both the placenta and broader systemic endocrine system. Although our current study focused on the cadre of currently identified RAMP2-associating GPCRs, it is likely that there are additional GPCRs to be identified, providing additional lenses through which in vivo data on RAMP2 genetic models could be interpreted.
Acknowledgments
The authors would like to acknowledge Helen Willcockson for technical assistance and expertise. The authors would also like to thank the Histology Research Core Facility and the In Situ Hybridization Core Facility, both at the University of North Carolina at Chapel Hill.
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by U.S. National Institutes of Health's Eunice Kennedy Shriver National Institute of Child Health and Human Development (www.nichd.nih.gov) R01 HD060860 (to KMC) and F30 HD085652 (to BCM) and by an American Heart Association (www.heart.org) Predoctoral Fellowship (to MK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Klein KR, Matson BC, Caron KM. The expanding repertoire of receptor activity modifying protein (RAMP) function. Crit Rev Biochem Mol Biol. 2016;51(1):65–71. doi: 10.3109/10409238.2015.1128875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunworth WP, Caron KM. G protein-coupled receptors as potential drug targets for lymphangiogenesis and lymphatic vascular diseases. Arterioscler Thromb Vasc Biol. 2009;29(5):650–6. doi: 10.1161/ATVBAHA.109.185066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sexton PM, Poyner DR, Simms J, Christopoulos A, Hay DL. RAMPs as drug targets. Adv Exp Med Biol. 2012;744:61–74. doi: 10.1007/978-1-4614-2364-5_6 [DOI] [PubMed] [Google Scholar]
- 4.Kadmiel M, Fritz-Six KL, Caron KM. Understanding RAMPs through genetically engineered mouse models. Adv Exp Med Biol. 2012;744:49–60. doi: 10.1007/978-1-4614-2364-5_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booe JM, Walker CS, Barwell J, Kuteyi G, Simms J, Jamaluddin MA, et al. Structural Basis for Receptor Activity-Modifying Protein-Dependent Selective Peptide Recognition by a G Protein-Coupled Receptor. Mol Cell. 2015;58(6):1040–52. doi: 10.1016/j.molcel.2015.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuwasako K, Kitamura K, Nagata S, Hikosaka T, Kato J. Function of the cytoplasmic tail of human calcitonin receptor-like receptor in complex with receptor activity-modifying protein 2. Biochem Biophys Res Commun. 2010;392(3):380–5. doi: 10.1016/j.bbrc.2010.01.030 [DOI] [PubMed] [Google Scholar]
- 7.Watkins HA, Chakravarthy M, Abhayawardana RS, Gingell JJ, Garelja M, Pardamwar M, et al. Receptor Activity-modifying Proteins 2 and 3 Generate Adrenomedullin Receptor Subtypes with Distinct Molecular Properties. J Biol Chem. 2016;291(22):11657–75. doi: 10.1074/jbc.M115.688218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watkins HA, Walker CS, Ly KN, Bailey RJ, Barwell J, Poyner DR, et al. Receptor activity-modifying protein-dependent effects of mutations in the calcitonin receptor-like receptor: implications for adrenomedullin and calcitonin gene-related peptide pharmacology. Br J Pharmacol. 2014;171(3):772–88. doi: 10.1111/bph.12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393(6683):333–9. doi: 10.1038/30666 [DOI] [PubMed] [Google Scholar]
- 10.Dackor R, Fritz-Six K, Smithies O, Caron K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J Biol Chem. 2007;282(25):18094–9. doi: 10.1074/jbc.M703544200 [DOI] [PubMed] [Google Scholar]
- 11.Fritz-Six KL, Dunworth WP, Li M, Caron KM. Adrenomedullin signaling is necessary for murine lymphatic vascular development. J Clin Invest. 2008;118(1):40–50. doi: 10.1172/JCI33302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kechele DO, Dunworth WP, Trincot CE, Wetzel-Strong SE, Li M, Ma H, et al. Endothelial Restoration of Receptor Activity-Modifying Protein 2 Is Sufficient to Rescue Lethality, but Survivors Develop Dilated Cardiomyopathy. Hypertension. 2016;68(3):667–77. doi: 10.1161/HYPERTENSIONAHA.116.07191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kadmiel M, Fritz-Six K, Pacharne S, Richards GO, Li M, Skerry TM, et al. Research resource: Haploinsufficiency of receptor activity-modifying protein-2 (RAMP2) causes reduced fertility, hyperprolactinemia, skeletal abnormalities, and endocrine dysfunction in mice. Mol Endocrinol. 2011;25(7):1244–53. doi: 10.1210/me.2010-0400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matson BC, Caron KM. Uterine natural killer cells as modulators of the maternal-fetal vasculature. Int J Dev Biol. 2014;58(2-3-4):199–204. [DOI] [PubMed] [Google Scholar]
- 15.Li M, Wu Y, Caron KM. Haploinsufficiency for Adrenomedullin Reduces Pinopodes and Diminishes Uterine Receptivity in Mice. Biol Reprod. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erlebacher A. Immunology of the maternal-fetal interface. Annu Rev Immunol. 2013;31:387–411. doi: 10.1146/annurev-immunol-032712-100003 [DOI] [PubMed] [Google Scholar]
- 17.Adamson SL, Lu Y, Whiteley KJ, Holmyard D, Hemberger M, Pfarrer C, et al. Interactions between trophoblast cells and the maternal and fetal circulation in the mouse placenta. Dev Biol. 2002;250(2):358–73. [DOI] [PubMed] [Google Scholar]
- 18.Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12(9):1065–74. doi: 10.1038/nm1452 [DOI] [PubMed] [Google Scholar]
- 19.Eastabrook G, Hu Y, von Dadelszen P. The role of decidual natural killer cells in normal placentation and in the pathogenesis of preeclampsia. J Obstet Gynaecol Can. 2008;30(6):467–76. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Schwerbrock NM, Lenhart PM, Fritz-Six KL, Kadmiel M, Christine KS, et al. Fetal-derived adrenomedullin mediates the innate immune milieu of the placenta. J Clin Invest. 2013;123(6):2408–20. doi: 10.1172/JCI67039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–80. doi: 10.1038/nrneph.2014.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makino Y, Shibata K, Makino I, Kangawa K, Kawarabayashi T. Alteration of the adrenomedullin receptor components gene expression associated with the blood pressure in pregnancy-induced hypertension. J Clin Endocrinol Metab. 2001;86(10):5079–82. doi: 10.1210/jcem.86.10.8099 [DOI] [PubMed] [Google Scholar]
- 23.Kovacs CS, Chafe LL, Fudge NJ, Friel JK, Manley NR. PTH regulates fetal blood calcium and skeletal mineralization independently of PTHrP. Endocrinology. 2001;142(11):4983–93. doi: 10.1210/endo.142.11.8509 [DOI] [PubMed] [Google Scholar]
- 24.Kovacs CS, Lanske B, Hunzelman JL, Guo J, Karaplis AC, Kronenberg HM. Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc Natl Acad Sci U S A. 1996;93(26):15233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmonds CS, Karsenty G, Karaplis AC, Kovacs CS. Parathyroid hormone regulates fetal-placental mineral homeostasis. J Bone Miner Res. 2010;25(3):594–605. doi: 10.1359/jbmr.090825 [DOI] [PubMed] [Google Scholar]
- 26.Tucci J, Hammond V, Senior PV, Gibson A, Beck F. The role of fetal parathyroid hormone-related protein in transplacental calcium transport. J Mol Endocrinol. 1996;17(2):159–64. [DOI] [PubMed] [Google Scholar]
- 27.Abdelgadir Adam M, Untch BR, Olson JA Jr. Parathyroid carcinoma: current understanding and new insights into gene expression and intraoperative parathyroid hormone kinetics. Oncologist. 2010;15(1):61–72. doi: 10.1634/theoncologist.2009-0185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kremer R, Li J, Camirand A, Karaplis AC. Parathyroid Hormone Related Protein (PTHrP) in Tumor Progression. Adv Exp Med Biol. 2011;720:145–60. doi: 10.1007/978-1-4614-0254-1_12 [DOI] [PubMed] [Google Scholar]
- 29.Mosekilde L, Torring O, Rejnmark L. Emerging anabolic treatments in osteoporosis. Curr Drug Saf. 2011;6(2):62–74. [DOI] [PubMed] [Google Scholar]
- 30.Toulis KA, Anastasilakis AD, Polyzos SA, Makras P. Targeting the osteoblast: approved and experimental anabolic agents for the treatment of osteoporosis. Hormones (Athens). 2011;10(3):174–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.