Abstract
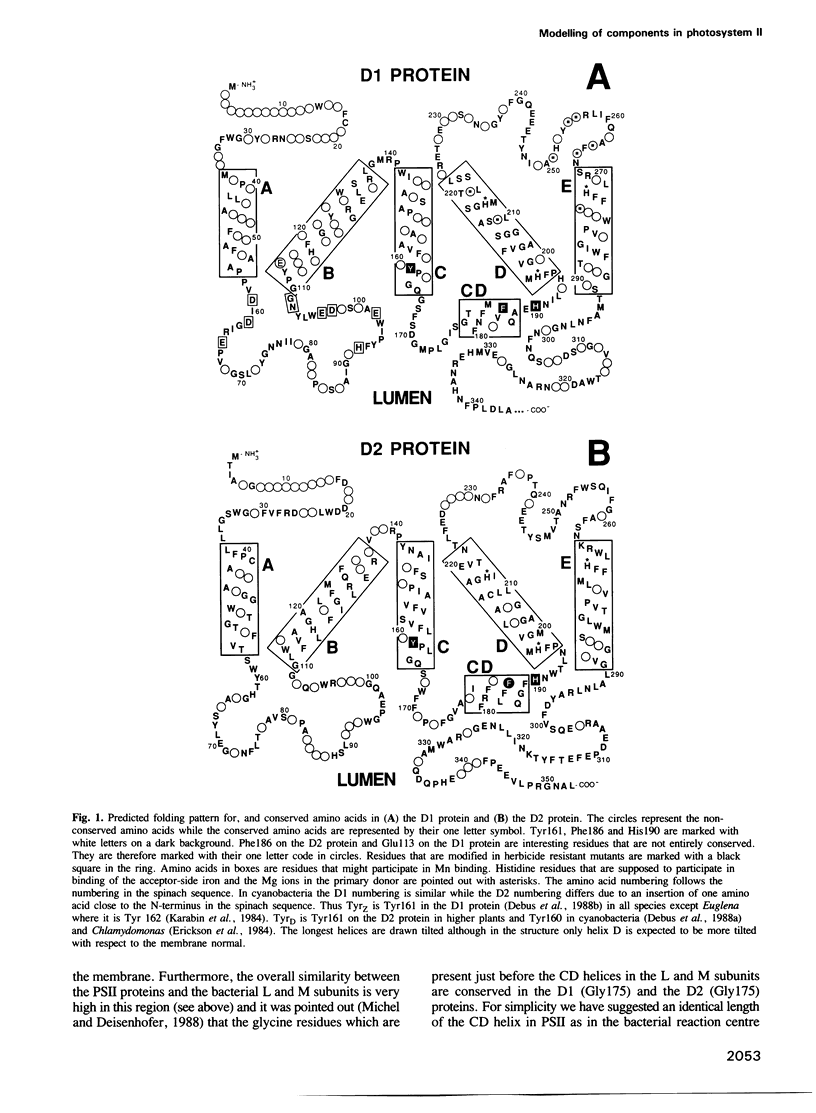
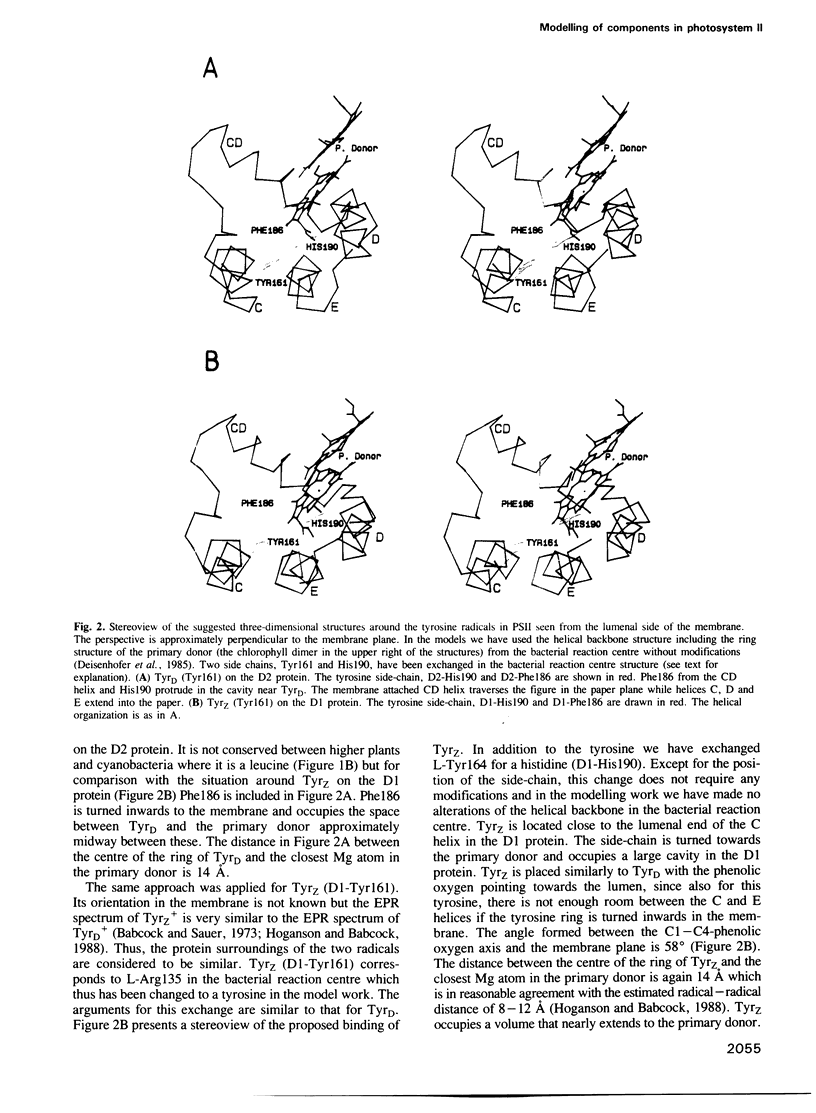
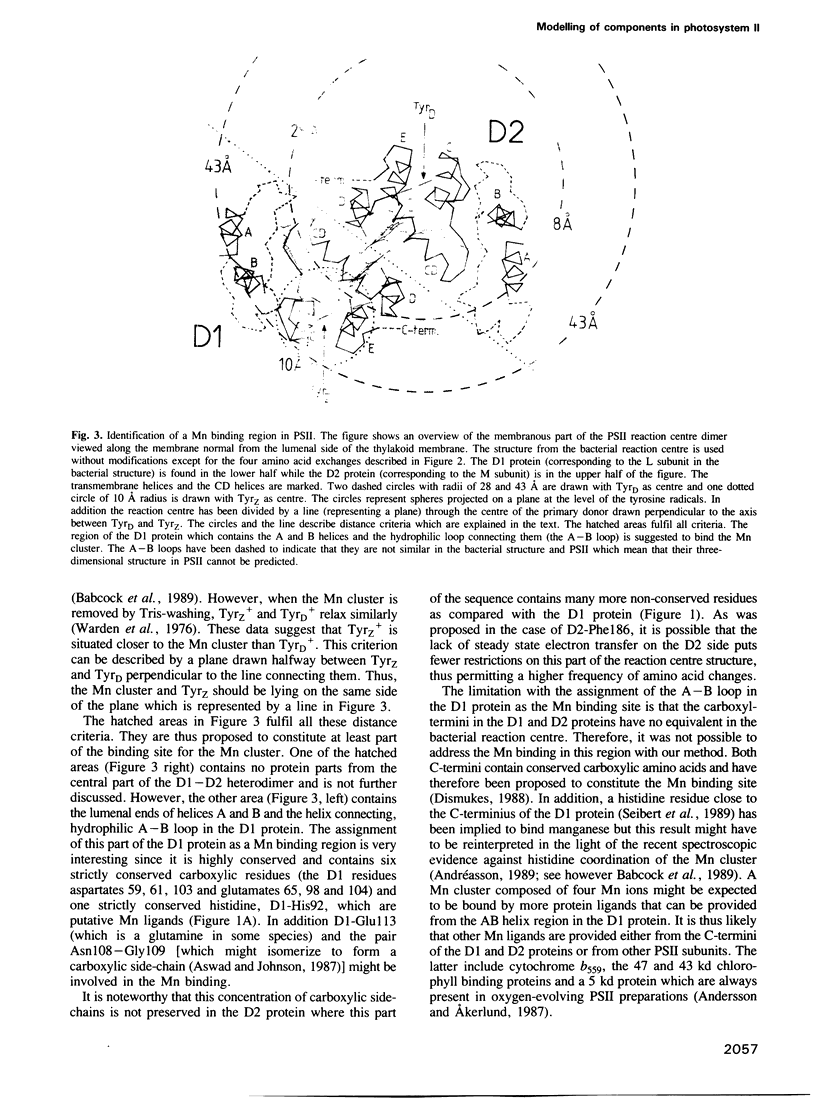
Thirty-one and eleven sequences for the photosystem II reaction centre proteins D1 and D2 respectively, were compared to identify conserved single amino acid residues and regions in the sequences. Both proteins are highly conserved. One important difference is that the lumenal parts of the D1 protein are more conserved than the corresponding parts in the D2 protein. The three-dimensional structures around the electron donors tyrosineZ and tyrosineD on the oxidizing side of photosystem II have been predicted by computer modelling using the photosynthetic reaction centre from purple bacteria as a framework. In the model the tyrosines occupy two cavities close to the lumenal surface of the membrane. They are symmetrically arranged around the primary donor P680 and the distances between the centre of the tyrosines and the closest Mg ion in P680 are around 14 A. Both tyrosineZ and tyrosineD are suggested to form a hydrogen bond with histidine 190 from the loop connecting helices C and D in the D1 and D2 proteins, respectively. The Mn cluster in the oxygen evolving complex has been localized by using known and estimated distances from the tyrosine radicals. It is suggested that a binding region for the Mn cluster is constituted by the lumenal ends of helices A and B and the loop connecting them in the D1 protein. This part of the D1 protein contains a large number of strictly conserved carboxylic acid residues and histidines which could participate in the Mn binding. There is little probability that the Mn cluster binds on the lumenal surface of the D2 protein.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldrich J., Cherney B., Merlin E., Christopherson L. A., Williams C. Sequence of the chloroplast-encoded psbA gene for the QB polypeptide of petunia. Nucleic Acids Res. 1986 Dec 9;14(23):9536–9536. doi: 10.1093/nar/14.23.9536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich J., Cherney B., Merlin E. Sequence of the chloroplast-encoded psbA gene for the QB polypeptide of alfalfa. Nucleic Acids Res. 1986 Dec 9;14(23):9537–9537. doi: 10.1093/nar/14.23.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock G. T., Barry B. A., Debus R. J., Hoganson C. W., Atamian M., McIntosh L., Sithole I., Yocum C. F. Water oxidation in photosystem II: from radical chemistry to multielectron chemistry. Biochemistry. 1989 Dec 12;28(25):9557–9565. doi: 10.1021/bi00451a001. [DOI] [PubMed] [Google Scholar]
- Babcock G. T., Sauer K. Electron paramagnetic resonance signal II in spinach chloroplasts. I. Kinetic analysis for untreated chloroplasts. Biochim Biophys Acta. 1973 Dec 14;325(3):483–503. doi: 10.1016/0005-2728(73)90209-0. [DOI] [PubMed] [Google Scholar]
- Barry B. A., Babcock G. T. Tyrosine radicals are involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7099–7103. doi: 10.1073/pnas.84.20.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship R. E., Babcock G. T., Warden J. T., Sauer K. Observation of a new EPR transient in chloroplasts that may reflect the electron donor to photosystem II at room temperature. FEBS Lett. 1975 Mar 1;51(1):287–293. doi: 10.1016/0014-5793(75)80909-4. [DOI] [PubMed] [Google Scholar]
- Bukharov A. A., Kolosov V. L., Klezovich O. N., Zolotarev A. S. Nucleotide sequence of rye chloroplast DNA fragment, comprising psbD, psbC and trnS genes. Nucleic Acids Res. 1989 Jan 25;17(2):798–798. doi: 10.1093/nar/17.2.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conjeaud H., Mathis P. Electron Transfer in the Photosynthetic Membrane: Influence of PH and Surface Potential on the P-680 Reduction Kinetics. Biophys J. 1986 Jun;49(6):1215–1221. doi: 10.1016/S0006-3495(86)83750-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Babcock G. T., McIntosh L. Site-directed mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen-evolving system. Proc Natl Acad Sci U S A. 1988 Jan;85(2):427–430. doi: 10.1073/pnas.85.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debus R. J., Barry B. A., Sithole I., Babcock G. T., McIntosh L. Directed mutagenesis indicates that the donor to P+680 in photosystem II is tyrosine-161 of the D1 polypeptide. Biochemistry. 1988 Dec 27;27(26):9071–9074. doi: 10.1021/bi00426a001. [DOI] [PubMed] [Google Scholar]
- Deisenhofer J., Michel H. Nobel lecture. The photosynthetic reaction centre from the purple bacterium Rhodopseudomonas viridis. EMBO J. 1989 Aug;8(8):2149–2170. doi: 10.1002/j.1460-2075.1989.tb08338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov V. A., Andreeva A. V., Reverdatto S. V., Chakhmakhcheva O. G. Nucleotide sequence of the barley chloroplast psbD gene for the D2 protein of photosystem II. Nucleic Acids Res. 1988 Jun 24;16(12):5686–5686. doi: 10.1093/nar/16.12.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimov V. A., Andreeva A. V., Reverdatto S. V., Jung R., Chakhmakhcheva O. G. Nucleotide sequence of the barley chloroplast psbA gene for the QB protein of photosystem II. Nucleic Acids Res. 1988 Jun 24;16(12):5685–5685. doi: 10.1093/nar/16.12.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Malnoë P., Girard-Bascou J., Pierre Y., Bennoun P., Rochaix J. D. Lack of the D2 protein in a Chlamydomonas reinhardtii psbD mutant affects photosystem II stability and D1 expression. EMBO J. 1986 Aug;5(8):1745–1754. doi: 10.1002/j.1460-2075.1986.tb04422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. M., Rahire M., Rochaix J. D. Chlamydomonas reinhardii gene for the 32 000 mol. wt. protein of photosystem II contains four large introns and is located entirely within the chloroplast inverted repeat. EMBO J. 1984 Dec 1;3(12):2753–2762. doi: 10.1002/j.1460-2075.1984.tb02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Brusslan J., Haselkorn R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986 Nov;5(11):2789–2798. doi: 10.1002/j.1460-2075.1986.tb04569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden S. S., Stearns G. W. Nucleotide sequence and transcript analysis of three photosystem II genes from the cyanobacterium Synechococcus sp. PCC7942. Gene. 1988 Jul 15;67(1):85–96. doi: 10.1016/0378-1119(88)90011-x. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Edelman M., Hallick R. B. Chloroplast-coded atrazine resistance in Solanum nigrum: psbA loci from susceptible and resistant biotypes are isogenic except for a single codon change. Nucleic Acids Res. 1984 Dec 21;12(24):9489–9496. doi: 10.1093/nar/12.24.9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg J., McIntosh L. Molecular Basis of Herbicide Resistance in Amaranthus hybridus. Science. 1983 Dec 23;222(4630):1346–1349. doi: 10.1126/science.222.4630.1346. [DOI] [PubMed] [Google Scholar]
- Holschuh K., Bottomley W., Whitfeld P. R. Structure of the spinach chloroplast genes for the D2 and 44 kd reaction-centre proteins of photosystem II and for tRNASer (UGA). Nucleic Acids Res. 1984 Dec 11;12(23):8819–8834. doi: 10.1093/nar/12.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes J. B., Brudvig G. W. Location and magnetic relaxation properties of the stable tyrosine radical in photosystem II. Biochemistry. 1989 Feb 7;28(3):1116–1125. doi: 10.1021/bi00429a028. [DOI] [PubMed] [Google Scholar]
- Janssen I., Jakowitsch J., Michalowski C. B., Bohnert H. J., Löffelhardt W. Evolutionary relationship of psbA genes from cyanobacteria, cyanelles and plastids. Curr Genet. 1989 May;15(5):335–340. doi: 10.1007/BF00419913. [DOI] [PubMed] [Google Scholar]
- Jones T. A. Diffraction methods for biological macromolecules. Interactive computer graphics: FRODO. Methods Enzymol. 1985;115:157–171. doi: 10.1016/0076-6879(85)15014-7. [DOI] [PubMed] [Google Scholar]
- Karabin G. D., Farley M., Hallick R. B. Chloroplast gene for Mr 32000 polypeptide of photosystem II in Euglena gracilis is interrupted by four introns with conserved boundary sequences. Nucleic Acids Res. 1984 Jul 25;12(14):5801–5812. doi: 10.1093/nar/12.14.5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosov V. L., Bukharov A. A., Zolotarev A. S. Nucleotide sequence of the rye chloroplast psbA gene, encoding D1 protein of photosystem II. Nucleic Acids Res. 1989 Feb 25;17(4):1759–1759. doi: 10.1093/nar/17.4.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link G., Langridge U. Structure of the chloroplast gene for the precursor of the Mr 32,000 photosystem II protein from mustard (Sinapis alba L.). Nucleic Acids Res. 1984 Jan 25;12(2):945–958. doi: 10.1093/nar/12.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz J. G., Nixon P. J., Rögner M., Brudvig G. W., Diner B. A. Directed alteration of the D1 polypeptide of photosystem II: evidence that tyrosine-161 is the redox component, Z, connecting the oxygen-evolving complex to the primary electron donor, P680. Biochemistry. 1989 Aug 22;28(17):6960–6969. doi: 10.1021/bi00443a028. [DOI] [PubMed] [Google Scholar]
- Michel H., Weyer K. A., Gruenberg H., Dunger I., Oesterhelt D., Lottspeich F. The 'light' and 'medium' subunits of the photosynthetic reaction centre from Rhodopseudomonas viridis: isolation of the genes, nucleotide and amino acid sequence. EMBO J. 1986 Jun;5(6):1149–1158. doi: 10.1002/j.1460-2075.1986.tb04340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morden C. W., Golden S. S. psbA genes indicate common ancestry of prochlorophytes and chloroplasts. Nature. 1989 Jan 26;337(6205):382–385. doi: 10.1038/337382a0. [DOI] [PubMed] [Google Scholar]
- Mulligan B., Schultes N., Chen L., Bogorad L. Nucleotide sequence of a multiple-copy gene for the B protein of photosystem II of a cyanobacterium. Proc Natl Acad Sci U S A. 1984 May;81(9):2693–2697. doi: 10.1073/pnas.81.9.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Páy A., Smith M. A., Nagy F., Márton L. Sequence of the psbA gene from wild type and triazin-resistant Nicotiana plumbaginifolia. Nucleic Acids Res. 1988 Aug 25;16(16):8176–8176. doi: 10.1093/nar/16.16.8176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravnikar P. D., Debus R., Sevrinck J., Saetaert P., McIntosh L. Nucleotide sequence of a second psbA gene from the unicellular cyanobacterium Synechocystis 6803. Nucleic Acids Res. 1989 May 25;17(10):3991–3991. doi: 10.1093/nar/17.10.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford A. W. Photosystem II, the water-splitting enzyme. Trends Biochem Sci. 1989 Jun;14(6):227–232. doi: 10.1016/0968-0004(89)90032-7. [DOI] [PubMed] [Google Scholar]
- Sayre R. T., Andersson B., Bogorad L. The topology of a membrane protein: the orientation of the 32 kd Qb-binding chloroplast thylakoid membrane protein. Cell. 1986 Nov 21;47(4):601–608. doi: 10.1016/0092-8674(86)90624-0. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielmann A., Stutz E. Nucleotide sequence of soybean chloroplast DNA regions which contain the psb A and trn H genes and cover the ends of the large single copy region and one end of the inverted repeats. Nucleic Acids Res. 1983 Oct 25;11(20):7157–7167. doi: 10.1093/nar/11.20.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turmel M., Boulanger J., Lemieux C. Two group I introns with long internal open reading frames in the chloroplast psbA gene of Chlamydomonas moewusii. Nucleic Acids Res. 1989 May 25;17(10):3875–3887. doi: 10.1093/nar/17.10.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermass W. F., Rutherford A. W., Hansson O. Site-directed mutagenesis in photosystem II of the cyanobacterium Synechocystis sp. PCC 6803: Donor D is a tyrosine residue in the D2 protein. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8477–8481. doi: 10.1073/pnas.85.22.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden J. T., Blankenship R. E., Sauer K. A flash photolysis ESR study of photosystem II signal IIvf, the physiological donor to P-680+. Biochim Biophys Acta. 1976 Mar 12;423(3):462–478. doi: 10.1016/0005-2728(76)90201-2. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Bohnert H. J., Whitfeld P. R., Bottomley W. Nucleotide sequence of the gene for the M(r) 32,000 thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyi predicts a totally conserved primary translation product of M(r) 38,950. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7699–7703. doi: 10.1073/pnas.79.24.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]