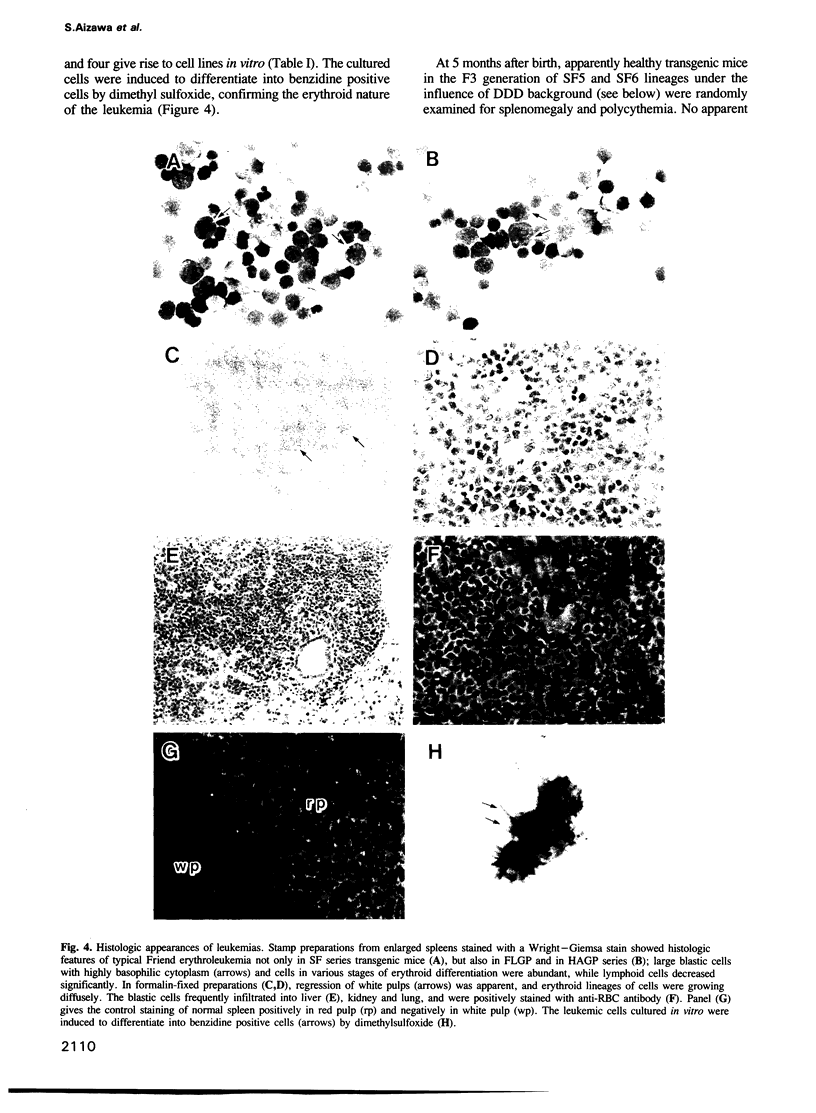
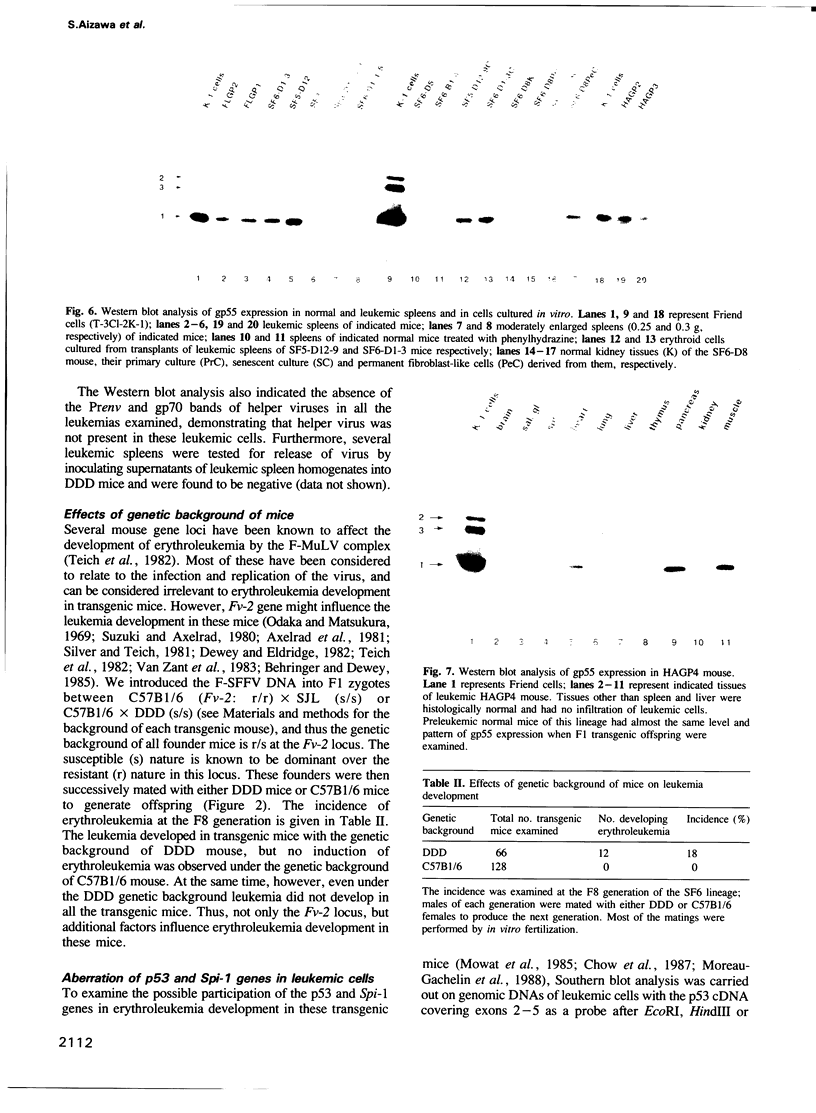
Abstract
A group of retroviruses carrying truncated viral genes has recently been suggested as the cause of new patterns of diseases. One such virus is the replication defective component of the Friend murine leukemia virus (F-MuLV) complex, called Friend spleen focus forming virus (F-SFFV). This virus induces erythroblastosis, and a virion envelope-related glycoprotein, gp55, encoded by F-SFFV has been suggested as the pathogenic gene. The role of the gp55 gene is, however, yet unclear in the apparently multistep erythroleukemogenesis. By separately producing transgenic mice harboring the whole F-SFFV DNA, the gp55 gene alone under the control of the retroviral long terminal repeat (LTR) and the gp55 gene under the control of cytoplasmic beta actin transcriptional regulatory unit, we show here that the gp55 gene is capable of inducing neoplastic proliferation of erythroid progenitor cells specifically in the absence of helper virus and other F-SFFV sequences. Under the control of the viral LTR the gp55 expression was detected only in leukemic tissues, but under the control of cytoplasmic beta-actin regulatory sequences, the gp55 was also expressed in a variety of normal tissues including preleukemic normal spleens. The development of erythroleukemia was suppressed under the genetic background of C57B1/6 mouse (resistant to F-MuLV; Fv-2rr), and required additional events even under the background of DDD mouse (susceptible to F-MuLV; Fv-2ss). The p53 and Spi-1 genes were frequently aberrant in transplanted tumors and cell lines derived from them, but were not in primary leukemic spleens.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amanuma H., Katori A., Obata M., Sagata N., Ikawa Y. Complete nucleotide sequence of the gene for the specific glycoprotein (gp55) of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3913–3917. doi: 10.1073/pnas.80.13.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amanuma H., Watanabe N., Nishi M., Ikawa Y. Requirement of the single base insertion at the 3' end of the env-related gene of Friend spleen focus-forming virus for pathogenic activity and its effect on localization of the glycoprotein product (gp55). J Virol. 1989 Nov;63(11):4824–4833. doi: 10.1128/jvi.63.11.4824-4833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai N., Nomura D., Yokota K., Wolf D., Brill E., Shohat O., Rotter V. Immunologically distinct p53 molecules generated by alternative splicing. Mol Cell Biol. 1986 Sep;6(9):3232–3239. doi: 10.1128/mcb.6.9.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrad A. A., Croizat H., Eskinazi D. A washable macromolecule from Fv2rr marrow negatively regulates DNA synthesis in erythropoietic progenitor cells BFU-E. Cell. 1981 Oct;26(2 Pt 2):233–244. doi: 10.1016/0092-8674(81)90306-8. [DOI] [PubMed] [Google Scholar]
- Aziz D. C., Hanna Z., Jolicoeur P. Severe immunodeficiency disease induced by a defective murine leukaemia virus. Nature. 1989 Apr 6;338(6215):505–508. doi: 10.1038/338505a0. [DOI] [PubMed] [Google Scholar]
- Behringer R. R., Dewey M. J. Cellular site and mode of Fv-2 gene action. Cell. 1985 Feb;40(2):441–447. doi: 10.1016/0092-8674(85)90158-8. [DOI] [PubMed] [Google Scholar]
- Ben David Y., Prideaux V. R., Chow V., Benchimol S., Bernstein A. Inactivation of the p53 oncogene by internal deletion or retroviral integration in erythroleukemic cell lines induced by Friend leukemia virus. Oncogene. 1988 Aug;3(2):179–185. [PubMed] [Google Scholar]
- Berger S. A., Sanderson N., Bernstein A., Hankins W. D. Induction of the early stages of Friend erythroleukemia with helper-free Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1985 Oct;82(20):6913–6917. doi: 10.1073/pnas.82.20.6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestwick R. K., Hankins W. D., Kabat D. Roles of helper and defective retroviral genomes in murine erythroleukemia: studies of spleen focus-forming virus in the absence of helper. J Virol. 1985 Dec;56(3):660–664. doi: 10.1128/jvi.56.3.660-664.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Hartley J. W., Rowe W. P., Hopkins N. Role for the 3' end of the genome in determining disease specificity of Friend and Moloney murine leukemia viruses. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4408–4411. doi: 10.1073/pnas.80.14.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatis P. A., Holland C. A., Silver J. E., Frederickson T. N., Hopkins N., Hartley J. W. A 3' end fragment encompassing the transcriptional enhancers of nondefective Friend virus confers erythroleukemogenicity on Moloney leukemia virus. J Virol. 1984 Oct;52(1):248–254. doi: 10.1128/jvi.52.1.248-254.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Morse H. C., 3rd, Makino M., Ruscetti S. K., Hartley J. W. Defective virus is associated with induction of murine retrovirus-induced immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1989 May;86(10):3862–3866. doi: 10.1073/pnas.86.10.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Stimpfling J. Host genetic control of recovery from Friend leukemia virus-induced splenomegaly: mapping of a gene within the major histocompatability complex. J Exp Med. 1974 Dec 1;140(6):1457–1467. doi: 10.1084/jem.140.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow V., Ben-David Y., Bernstein A., Benchimol S., Mowat M. Multistage Friend erythroleukemia: independent origin of tumor clones with normal or rearranged p53 cellular oncogenes. J Virol. 1987 Sep;61(9):2777–2781. doi: 10.1128/jvi.61.9.2777-2781.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. P., Mak T. W. Complete nucleotide sequence of an infectious clone of Friend spleen focus-forming provirus: gp55 is an envelope fusion glycoprotein. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5037–5041. doi: 10.1073/pnas.80.16.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey M. J., Eldridge P. W. Friend viral pathogenesis in C57BL/6 reversible DBA/2 allophenic mice. Exp Hematol. 1982 Oct;10(9):723–731. [PubMed] [Google Scholar]
- Eliyahu D., Goldfinger N., Pinhasi-Kimhi O., Shaulsky G., Skurnik Y., Arai N., Rotter V., Oren M. Meth A fibrosarcoma cells express two transforming mutant p53 species. Oncogene. 1988 Sep;3(3):313–321. [PubMed] [Google Scholar]
- Evans L. H., Morrey J. D. Tissue-specific replication of Friend and Moloney murine leukemia viruses in infected mice. J Virol. 1987 May;61(5):1350–1357. doi: 10.1128/jvi.61.5.1350-1357.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Fredrickson T. N., Yetter R. A., Makino M., Morse H. C., 3rd Retrovirus-induced murine acquired immunodeficiency syndrome: natural history of infection and differing susceptibility of inbred mouse strains. J Virol. 1989 Mar;63(3):1223–1231. doi: 10.1128/jvi.63.3.1223-1231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland C. A., Anklesaria P., Sakakeeny M. A., Greenberger J. S. Enhancer sequences of a retroviral vector determine expression of a gene in multipotent hematopoietic progenitors and committed erythroid cells. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8662–8666. doi: 10.1073/pnas.84.23.8662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa Y., Aida M., Inoue Y. Isolation and characterization of high and low differentiation-inducible Friend leukemia lines. Gan. 1976 Oct;67(5):767–770. [PubMed] [Google Scholar]
- Li J. P., Bestwick R. K., Spiro C., Kabat D. The membrane glycoprotein of Friend spleen focus-forming virus: evidence that the cell surface component is required for pathogenesis and that it binds to a receptor. J Virol. 1987 Sep;61(9):2782–2792. doi: 10.1128/jvi.61.9.2782-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Golemis E., Hartley J. W., Hopkins N. Disease specificity of nondefective Friend and Moloney murine leukemia viruses is controlled by a small number of nucleotides. J Virol. 1987 Mar;61(3):693–700. doi: 10.1128/jvi.61.3.693-700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linemeyer D. L., Menke J. G., Ruscetti S. K., Evans L. H., Scolnick E. M. Envelope gene sequences which encode the gp52 protein of spleen focus-forming virus are required for the induction of erythroid cell proliferation. J Virol. 1982 Jul;43(1):223–233. doi: 10.1128/jvi.43.1.223-233.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Gachelin F., D'Auriol L., Robert-Lezenes J., Galibert F., Tambourin P., Tavitian A. Analysis of integrated proviral DNA sequences with an octadecanucleotide probe designed for specific identification of spleen focus-forming virus in the mouse genome. J Virol. 1986 Jan;57(1):349–352. doi: 10.1128/jvi.57.1.349-352.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau-Gachelin F., Tavitian A., Tambourin P. Spi-1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988 Jan 21;331(6153):277–280. doi: 10.1038/331277a0. [DOI] [PubMed] [Google Scholar]
- Mowat M., Cheng A., Kimura N., Bernstein A., Benchimol S. Rearrangements of the cellular p53 gene in erythroleukaemic cells transformed by Friend virus. Nature. 1985 Apr 18;314(6012):633–636. doi: 10.1038/314633a0. [DOI] [PubMed] [Google Scholar]
- Munroe D. G., Rovinski B., Bernstein A., Benchimol S. Loss of a highly conserved domain on p53 as a result of gene deletion during Friend virus-induced erythroleukemia. Oncogene. 1988 Jun;2(6):621–624. [PubMed] [Google Scholar]
- Nigro J. M., Baker S. J., Preisinger A. C., Jessup J. M., Hostetter R., Cleary K., Bigner S. H., Davidson N., Baylin S., Devilee P. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989 Dec 7;342(6250):705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Odaka T. Inheritance of susceptibility to Friend mouse leukemia virus. VII. Establishment of a resistant strain. Int J Cancer. 1970 Jul 15;6(1):18–23. doi: 10.1002/ijc.2910060104. [DOI] [PubMed] [Google Scholar]
- Odaka T., Matsukura M. Inheritance of Susceptibility to Friend Mouse Leukemia Virus: VI. Reciprocal Alteration of Innate Resistance or Susceptibility by Bone Marrow Transplantation Between Congenic Strains. J Virol. 1969 Dec;4(6):837–843. doi: 10.1128/jvi.4.6.837-843.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornitz D. M., Hammer R. E., Messing A., Palmiter R. D., Brinster R. L. Pancreatic neoplasia induced by SV40 T-antigen expression in acinar cells of transgenic mice. Science. 1987 Oct 9;238(4824):188–193. doi: 10.1126/science.2821617. [DOI] [PubMed] [Google Scholar]
- Overbaugh J., Donahue P. R., Quackenbush S. L., Hoover E. A., Mullins J. I. Molecular cloning of a feline leukemia virus that induces fatal immunodeficiency disease in cats. Science. 1988 Feb 19;239(4842):906–910. doi: 10.1126/science.2893454. [DOI] [PubMed] [Google Scholar]
- Paquette Y., Hanna Z., Savard P., Brousseau R., Robitaille Y., Jolicoeur P. Retrovirus-induced murine motor neuron disease: mapping the determinant of spongiform degeneration within the envelope gene. Proc Natl Acad Sci U S A. 1989 May;86(10):3896–3900. doi: 10.1073/pnas.86.10.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovinski B., Munroe D., Peacock J., Mowat M., Bernstein A., Benchimol S. Deletion of 5'-coding sequences of the cellular p53 gene in mouse erythroleukemia: a novel mechanism of oncogene regulation. Mol Cell Biol. 1987 Feb;7(2):847–853. doi: 10.1128/mcb.7.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S. K., Scolnick E. M. Expression of a transformation-related protein (p53) in the malignant stage of Friend virus-induced diseases. J Virol. 1983 Jun;46(3):1022–1026. doi: 10.1128/jvi.46.3.1022-1026.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Wolff L. Spleen focus-forming virus: relationship of an altered envelope gene to the development of a rapid erythroleukemia. Curr Top Microbiol Immunol. 1984;112:21–44. doi: 10.1007/978-3-642-69677-0_2. [DOI] [PubMed] [Google Scholar]
- Ruta M., Bestwick R., Machida C., Kabat D. Loss of leukemogenicity caused by mutations in the membrane glycoprotein structural gene of Friend spleen focus-forming virus. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4704–4708. doi: 10.1073/pnas.80.15.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya T., Mak T. W. Isolation and induction of erythroleukemic cell lines with properties of erythroid progenitor burst-forming cell (BFU-E) and erythroid precursor cell (CFU-E). Proc Natl Acad Sci U S A. 1983 Jun;80(12):3721–3725. doi: 10.1073/pnas.80.12.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Teich N. Expression of resistance to Friend virus-stimulated erythropoiesis in bone marrow chimeras containing Fv-2rr and Fv-2ss bone marrow. J Exp Med. 1981 Jul 1;154(1):126–137. doi: 10.1084/jem.154.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitbon M., Sola B., Evans L., Nishio J., Hayes S. F., Nathanson K., Garon C. F., Chesebro B. Hemolytic anemia and erythroleukemia, two distinct pathogenic effects of Friend MuLV: mapping of the effects to different regions of the viral genome. Cell. 1986 Dec 26;47(6):851–859. doi: 10.1016/0092-8674(86)90800-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stephenson J. R., Axelrad A. A., McLeod D. L., Shreeve M. M. Induction of colonies of hemoglobin-synthesizing cells by erythropoietin in vitro. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1542–1546. doi: 10.1073/pnas.68.7.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y., Aizawa S., Hirai S., Inoue T., Furuta Y., Suzuki M., Hirohashi S., Ikawa Y. Driven by the same Ig enhancer and SV40 T promoter ras induced lung adenomatous tumors, myc induced pre-B cell lymphomas and SV40 large T gene a variety of tumors in transgenic mice. EMBO J. 1987 Dec 20;6(13):4055–4065. doi: 10.1002/j.1460-2075.1987.tb02751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda Y., Hirai S., Suzuki M., Ikawa Y., Aizawa S. Active ras and myc oncogenes can be compatible, but Sv40 large T antigen is specifically suppressed with normal differentiation of mouse embryonic stem cells. Exp Cell Res. 1988 Sep;178(1):98–113. doi: 10.1016/0014-4827(88)90382-5. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Axelrad A. A. Fv-2 locus controls the proportion of erythropoietic progenitor cells (BFU-E) synthesizing DNA in normal mice. Cell. 1980 Jan;19(1):225–236. doi: 10.1016/0092-8674(80)90404-3. [DOI] [PubMed] [Google Scholar]
- Szurek P. F., Yuen P. H., Jerzy R., Wong P. K. Identification of point mutations in the envelope gene of Moloney murine leukemia virus TB temperature-sensitive paralytogenic mutant ts1: molecular determinants for neurovirulence. J Virol. 1988 Jan;62(1):357–360. doi: 10.1128/jvi.62.1.357-360.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiesen H. J., Bösze Z., Henry L., Charnay P. A DNA element responsible for the different tissue specificities of Friend and Moloney retroviral enhancers. J Virol. 1988 Feb;62(2):614–618. doi: 10.1128/jvi.62.2.614-618.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Scolnick E. M. Rapid leukemia induced by cloned friend strain of replicating murine type-C virus. Association with induction of xenotropic-related RNA sequences contained in spleen focus-forming virus. Virology. 1978 Mar;85(1):17–27. doi: 10.1016/0042-6822(78)90408-7. [DOI] [PubMed] [Google Scholar]
- Van Zant G., Eldridge P. W., Behringer R. R., Dewey M. J. Genetic control of hematopoietic kinetics revealed by analyses of allophenic mice and stem cell suicide. Cell. 1983 Dec;35(3 Pt 2):639–645. doi: 10.1016/0092-8674(83)90096-x. [DOI] [PubMed] [Google Scholar]
- Wendling F., Moreau-Gachelin F., Tambourin P. Emergence of tumorigenic cells during the course of Friend virus leukemias. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3614–3618. doi: 10.1073/pnas.78.6.3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. Malignant transformation of erythroid cells in vivo by introduction of a nonreplicating retrovirus vector. Science. 1985 Jun 28;228(4707):1549–1552. doi: 10.1126/science.2990034. [DOI] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. The spleen focus-forming virus (SFFV) envelope gene, when introduced into mice in the absence of other SFFV genes, induces acute erythroleukemia. J Virol. 1988 Jun;62(6):2158–2163. doi: 10.1128/jvi.62.6.2158-2163.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. Tissue tropism of a leukemogenic murine retrovirus is determined by sequences outside of the long terminal repeats. Proc Natl Acad Sci U S A. 1986 May;83(10):3376–3380. doi: 10.1073/pnas.83.10.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Scolnick E., Ruscetti S. Envelope gene of the Friend spleen focus-forming virus: deletion and insertions in 3' gp70/p15E-encoding region have resulted in unique features in the primary structure of its protein product. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4718–4722. doi: 10.1073/pnas.80.15.4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Tambourin P., Ruscetti S. Induction of the autonomous stage of transformation in erythroid cells infected with SFFV: helper virus is not required. Virology. 1986 Jul 15;152(1):272–276. doi: 10.1016/0042-6822(86)90393-4. [DOI] [PubMed] [Google Scholar]
- Zakut-Houri R., Oren M., Bienz B., Lavie V., Hazum S., Givol D. A single gene and a pseudogene for the cellular tumour antigen p53. Nature. 1983 Dec 8;306(5943):594–597. doi: 10.1038/306594a0. [DOI] [PubMed] [Google Scholar]
- van Lohuizen M., Verbeek S., Krimpenfort P., Domen J., Saris C., Radaszkiewicz T., Berns A. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989 Feb 24;56(4):673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]