Abstract
African-Americans bear a disproportionate burden of the obesity epidemic, yet have historically been underrepresented in weight loss research. We conducted a narrative review of large (N > 75) randomized prospective clinical trials of standard behavioral treatment for weight loss that reported results in the past fifteen years (2001-2015) to: 1) determine the rates of inclusion and reported results for African-Americans, and 2) further identify strategies that may result in improved outcomes. Of the 23 trials reviewed, 69.6% of the studies met or exceeded population estimates for African-Americans in the United States. However, only 10 reported outcomes and/or considered race in the analytic approach. At 6 months, African-American participants consistently lost less weight than white participants. The use of culturally tailored intervention materials and monthly personal telephone calls were reported as factors that may have enhanced treatment response. Future behavioral weight loss trials should also increase reporting of outcomes by race.
Keywords: diet and exercise, narrative review, black, weight loss, standard behavioral treatment
Despite accumulating scientific evidence of the negative health risks and growing recognition among the lay public that obesity is a major risk factor for chronic diseases, the proportion of the population that is obese now exceeds 30% and shows no signs of abating, particularly among women and African Americans (AA) or blacks (Flegal, Carroll, Ogden, & Curtin, 2010). Indeed, the increasing prevalence of obesity over recent decades has been characterized as a global pandemic with profound public health implications (Swinburn et al., 2011). AAs, especially AA women, bear a disproportionate burden of the obesity epidemic in the United States. Data from the National Health and Nutrition Examination Survey (NHANES) 2011-2014 indicate that 76.3% of non-Hispanic AAs aged 20 and older were overweight (body mass index [BMI] 25.0-29.9) or obese (BMI >30) compared to 68.5% of White and 77.1% of Hispanic in the same age range (Ogden, Carroll, Kit, & Flegal, 2014). When the prevalence estimates were restricted to obesity (Class 1, 2 or 3), the over-representation of AA women became more pronounced; the prevalence of obesity in non-Hispanic AA women was significantly higher than rates for non-Hispanic whites, black men or Hispanics (56.9% vs. 35.5% in non-Hispanic white women, 45.7% in Hispanic women, 33.6% in white men, 37.5% in black men, and 39.0% in Hispanic men) (Ogden, Carroll, Fryar, & Flegal, 2015).
Inclusion of Ethnic Minorities in Trials of Standard Behavioral Treatment
Behavioral treatment of obesity has provided a well-developed framework for weight-loss interventions for over 25 years. Standard behavioral treatment (SBT), based on social cognitive theory, is currently the most efficacious non-medical treatment for moderate obesity(Digenio, Mancuso, Gerber, & Dvorak, 2009; Gold, Burke, Pintauro, Buzzell, & Harvey-Berino, 2007; Goodpaster et al., 2010; Rock et al., 2010; Wing, 2004). SBT takes a tripartite approach, and includes a focus on reduced energy intake, increased energy expenditure, and behavioral therapy. Goal-setting, self-monitoring, self-efficacy enhancement, and social support with guidance provided by behavioral counselors are the core behavioral strategies used in SBT (Gold et al., 2007; Perri et al., 2001; Turk et al., 2009; Wadden, Crerand, & Brock, 2005; Warziski, Sereika, Styn, Music, & Burke, 2008; Wing, 2004; Wing, Sinha, Considine, Lang, & Caro, 1996). With SBT, participants typically achieve a 5-10% of weight lossfrom baseline weight over 24 weeks (Burke & Wang, 2011; Franz et al., 2007), an amount thought to be significant for reducing cardiovascular risk(Blackburn, 1995, 1999).
Despite the high prevalence rates of overweight and obesity in African-Americans, two reviews of obesity interventions, conducted in the past fifteen years, have noted only a few studies which have adequate samples of minority participants (McTigue et al., 2003; Seo & Sa, 2008). Indeed, in a review of obesity screening and interventions that was published in 2003, McTigue et al. found that 10 of 18 randomized clinical trials of counseling and behavioral interventions for weight loss treatment published between 1995 and 2001 did not report the racial distribution of their sample. A more recent meta-analysis by Seo and Sa (2008) reviewing studies published between 1980 and 2006 aimed to “…examine the efficacy of psycho-behavioral intervention programs in US minority adults,” but a lack of available data regarding outcomes specific to race led the authors to change their strategy and focus on multiethnic samples (Seo & Sa, 2008).
Given the mandates by the National Institutes of Health (NIH), one might expect the inclusion of minorities and reporting of race in weight loss intervention research to have improved in the past decade. In 1994, the National Institutes of Health (NIH) mandated that minorities be included in clinical research (NIH, 1994) and required standardized reporting of race and ethnicity of research study participants since 2001(NIH, 2001). In addition to these mandates, members of the research community have urged their peers to increase the number of minority participants in their samples (Kumanyika, 2008).The extent to which there has been progress toward these goals in recent years with regard to weight loss intervention research is an important unanswered question.
The recruitment and inclusion of minorities, particularly AA women, is critically important in weight loss treatment not only because of the high prevalence of overweight and obesity in this population, but also due to the differential treatment response rates reported in the few studies that have been able to evaluate racial differences. For example, most recently, it was reported that in multi-centre NIH-funded clinical trials, AAs have lost less weight than whites at 6 months (AA: −1.6 to −7.5 kg; whites: −3.8 to −8.2 kg) (Wingo, Carson, & Ard, 2014). Additionally, in the Trials of Hypertension Prevention, Phase II (TOHP II) AA participants lost 1.8 kg less than white participants at both 6 and 18 months (Stevens et al., 2001) Similarly, in the Trial of Nonpharmacologic Interventions in the Elderly (TONE), Kumanyika et al. reported that AA participants lost about half as much weight at 6 months as white participants did (Kumanyika et al., 2002). Furthermore, Wing et al. reported that AA participants in the Diabetes Prevention Project were less than half as likely to achieve 7% weight loss as white participants.(Wing et al., 2004) Finally, in the Weight Loss Maintenance Trial (WLM), AA women lost the least amount of weight after 6 months – nearly 2 kg less than white women (Hollis et al., 2008).
The obesity epidemic in the United States cuts across all racial and ethnic groups, but AA women carry a particularly high burden of obesity. Over the past 15 years, many studies have attempted to identify strategies to improve initial weight loss success and weight loss maintenance. However, epidemiological reports suggest that progress has not been made in reducing the disparity of the prevalence of overweight and obesity between racial groups (Ogden, Carroll, Kit, & Flegal, 2012).
Purpose
A necessary step toward reducing the disparity would clearly be to increase minority representation in weight loss trials and to investigate possible racial differences in treatment response, and provide recommendations for improvement. We therefore sought to estimate the state of the literature over the past fifteen years (2001-2015) in the inclusion and reporting of AAs in randomized clinical trials that used SBT for weight loss. When adequate data were provided in the published reports, we attempted to identify factors that may have contributed to enhanced or reduced treatment response in AA participants.
Methods
This narrative review focused on interventions using SBT published between 2001 and 2015, and included several stages in the search strategy. First, inclusion criteria were established to guide the selection of articles using SBT during the computerized search of relevant databases. For this review, SBT was defined as treatment that included group treatment sessions focused on lifestyle change and a prescribed energy deficit through calorie restriction, increased physical activity or both (Burke & Wang, 2011). The following criteria were established: 1) Inclusion of at least one treatment arm that used SBT for weight loss; 2) Inclusion of weight change as a required outcome variable; 3) Sample size (N ≥ 75); 4) Interventions were at least 4 months (16 weeks) in length. The rationale for study size and duration was to avoid pilot studies and/or to make sure the study was large enough to stratify participants by race.
After establishing our inclusion criteria, an initial electronic literature search was conducted in Ovid MEDLINE and Ovid PsychINFO using the search terms (randomized clinical trial OR clinical trial OR randomized trial) AND (behavioral treatment OR behavior modification) AND (weight loss OR overweight OR obesity OR weight control OR weight maintenance) LIMITED TO English Language AND Publication years: 2001-2015. Within this initial search, a total of 73 publications were identified (Figure 1). However, several well- known trials, most notably the Obesity Reduction Black Intervention Trial (ORBIT), were missed using this strategy due to the inclusion of the term “behavioral” in the search strategy. Therefore the strategy was revised in a secondary search, which included the term “diet” instead of behavioral treatment or behavior modification. A total of 2101 publications were identified with the exclusion of terms relating to bariatric surgery and weight loss medications. We further limited studies of interest to those that focused on overweight or obese participants without major co-morbidities (e.g. diabetes, cancer, osteoarthritis). Studies could focus on participants with dyslipidemia and/or hypertension. Socioeconomic status of participants was not taken into account for this review.
Figure 1. Flow diagram of narrative review research methods.
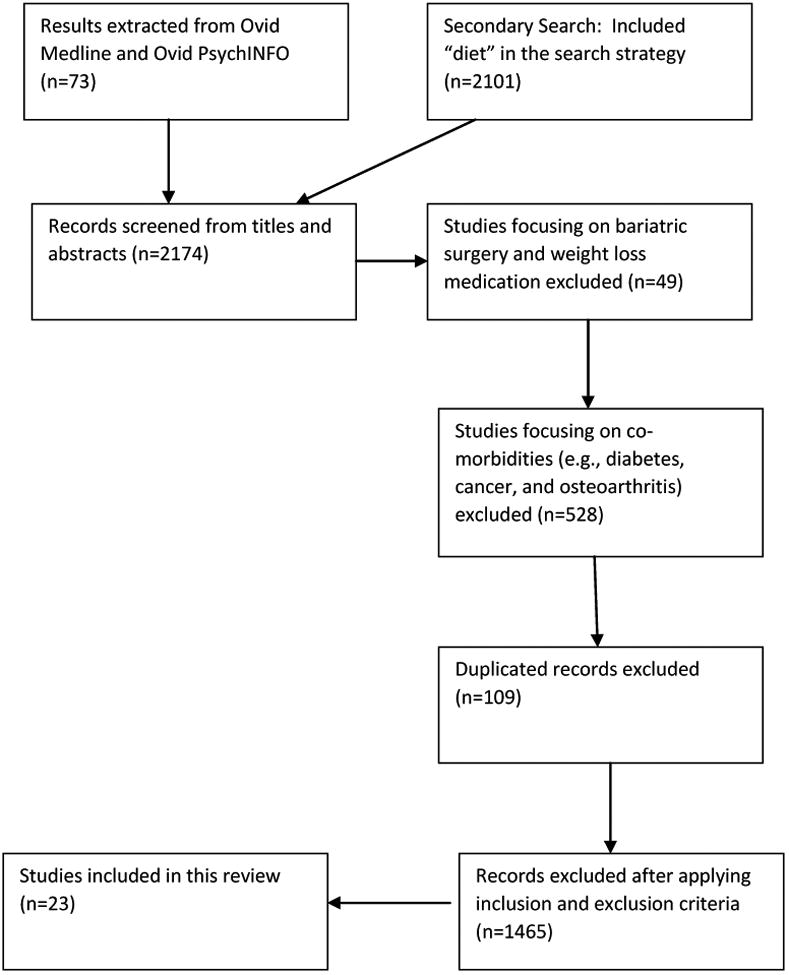
After reviewing the results from the primary and the secondary OVID Medline and OVID PsychINFO searches, we identified 23 clinical trials of SBT for weight loss with published results in the period 2001 -2015 that met our criteria. For each of the 23 studies, we extracted the following data (Table 1): (i) authors and year of publication; (ii) years the trial was conducted; (iii) number of participants; (iv) racial diversity; (v) length of intervention; (vi) key aspects of intervention; (vii) relevant findings. In some cases multiple published papers were used to acquire all of the required details (e.g. separate study design and study outcome papers or separate short term and long term results papers) for the summary table.
Table 1. Summary of randomized clinical trials for weight loss treatment with at least 75 participants and intervention lasting at least 4 months reporting results between 2001 and 2015, inclusive.
| Reference(s) | Trial Years | N | Baseline Characteristics (M± SD) | Racial Diversity | Length of intervention | Key aspects of intervention | Relevant findings |
|---|---|---|---|---|---|---|---|
|
Stevens et al., 2001 Lasser et al., 1995 |
1986-1998 | 1191 | Age: 43.3 ± 3.1 % Women: 34.3 BMI (kg/m2): 30.1 ± 3.3 Education (% college degree): 51.0 |
78.8% White; 17.5% Black | 36 mos. | TOHP II participants randomized to 1. Control group, 2. Weight loss group (WL). WL participants had group meetings weekly for 14 weeks then every other week for 3 mos. When bi-weekly meetings ended, participants were asked to contact the study bi-weekly and were encouraged to select 3 of 6 mini-modules (3 to 6 session enrichment courses) offered each year. | WL group lost more weight than control group. White participants lost 1.8 kg more than black participants at 6 and 18 mos. (p=.01, p=.03), but no difference was observed at 36 mos. Weight loss at 6 mos. was strongly related to attendance. Differential attendance by race was not reported. |
|
Ashley, St Jeor, Perumeuan-Chaney, et al., 2001 Ashley, St Jeor, Schrage, et al., 2001 |
NR* | 113 women | Age: 41.4 ± 4.7 % Women: 100.0 BMI (kg/m2): 30.0 ± 3.1 Education: NR |
NR | 12 mos. weight loss, 12 mos. maintenance | Participants randomized into 3 groups: 1. Dietitian-led group intervention (DLI); 2. DLI incorporating meal replacements (MR)(DLI+MR); and 3. Clinical office-based intervention with MR. DLI groups had 26 sessions of SBT: 3 mos. weekly, 3 mos. biweekly, 6 mos. monthly.MR groups advised to replace 2 of 3 main meals with MR shake or bar until 10% loss achieved. Once 10% loss achieved, replace 1 of 3 main meals unless regain occurs.In maintenance phase, all groups attended monthly seminars led by dietitian. | DLI+MR group lost significantly more weight than other two groups at 12 mos. and differences between groups persisted at 24 mos. Analysis by race not reported. |
| Perri et al., 2001 | NR | 103 | Age: 46.6 ± 8.8 % Women: 100.0 BMI (kg/m2): 35.8 ± 4.5 Education (yrs): 13.8 ± 1.8 |
NR | 5 mos. weight loss, 12 mos. maintenance | All received SBT for 5 mos. Randomized to receive no additional treatment (BT), Relapse Prevention Training (RPT) with bi-weekly sessions focused on “cognitive behavioral skills for anticipating, avoiding or coping with lapses, ” or Problem-Solving Therapy (PST) with bi-weekly sessions where participants reported on problems and, as a group, engaged in problem solving. | No significant weight change differences between RPT and BT, or RPT and PST. At month 17, PST had significantly greater total net loss. Analysis by race not reported. |
| McManus et al., 2001 | NR | 101 | Age: 44.0 ± 10.0 % Women: 90.0 BMI (kg/m2): 33.5 ± 4.0 Education: NR |
80% White 17% Black |
18 mos. | All participants received SBT with weekly meetings. Randomly assigned to:low fat diet (20% fat) or moderate fat diet (35% fat). | Moderate fat group lost 2.5 kg while low fat group gained 1.1 kg. Analysis by race not reported. |
| Yancy et al., 2004 | 2000-2001 | 120 | Age: 44.9 ± 9.5 % Women: 76.5 BMI (kg/m2): 34.3 ± 3.3 Education (% college degree): 59.5 |
76% White20% Black | 6 mos. | SBT with biweekly meetings for 3 mos. and monthly meetings for 3 mos.Participants randomly assigned to Atkins low-carbohydrate (low-carb) diet vs. standard low-fat diet. | Better weight loss in low carb group. Analysis by race not reported. |
| Jakicic et al., 2003 | 2000-2001 | 201 women | Age: 37.0 ± 5.7 % Women: 100 BMI (kg/m2): 32.6 ± 4.2 Education (% college degree): 27.0 |
81% White8% Black | 12 mos. | All received SBT. Weekly meetings for 24 weeks, biweekly meetings thereafter. Mos 7 to 12 received biweekly telephone call. Differed in exercise prescription - 4 exercise groups: 1. vigorous intensity/high duration, 2. moderate intensity/high duration, 3. moderate intensity/moderate duration, 4. vigorous intensity/moderate duration | No significant differences in weight loss. Analysis by race not reported. |
| Jeffery et al., 2003 | NR | 202 | Age: 42.2 ± 6.4 % Women: 58.0 BMI (kg/m2): 31.7 ± 2.6 Education (% college degree): 43.0 |
87% White | 18 mos. | All had SBT, meeting weekly for 1st 6 mos., bi-weekly for 2nd 6 mos., and monthly for final 6 mos. Randomly assigned to different physical activity goals: Standard Group: Energy Expenditure of 1000 kcal/week or approximately moderate walking for 30 min/day; High physical activity (HPA): Energy Expenditure of 2500 kcal/week or moderate walking for 75 min/day. | HPA group lost more weight from baseline to 18 mos. Analysis by race not reported. |
|
Samaha et al., 2003 Stern et al., 2004 |
2001-2002 | 132 | Age: 53.5 ± 9.0 % Women: 17.0 BMI (kg/m2): 42.2 ± 7.2 Education (% college degree): NR |
58% Black 38% White | 12 mos. | Intervention consisted of 2 hour sessions for 4 weeks, monthly 1-hr sessions for 5 months; Exercise not prescribed. Randomized to one of 2 diet groups: 1) Low Carb group - restrict carbs to 30 g/day or 2) less Low Fat Group - restrict fat to 30% or less of total calories, 500 calorie deficit per day | At 6 mos. weight loss greater for white participants than for black participants regardless of assignment. Low carb diet produced more weight loss overall, but not reported by race. High dropout rate overall (34%). |
| Burke et al., 2007 | 2002-2005 | 176 | Age: 44.1 ± 8.8 % Women: 86.9 BMI (kg/m2): 34.0 ± 4.1 Education (yrs.): 15.2 ± 2.5 |
71% White | 12 mos. with 6 mo follow-up | All participants received SBT for weight loss. Met weekly for 6 mos, biweekly for 3 mos, monthly for 3 mos and a follow-up assessment 6 mos after treatment ended. Participants were prescribed a standard low-fat diet or lacto-ovo-vegetarian diet. Participants were randomly assigned to either receive their preferred dietary assignment or to be randomly assigned to a diet group. | All groups lost weight. No effect of vegetarian diet or treatment preference on weight outcomes. Analysis by race not reported. |
|
Svetky et al., 2008 Hollis et al., 2008 Champagne et al., 2011 |
2003-2007 | 1685: Phase I (weight loss)1032: Phase II (maintenance) | Age: 54.8 ± 9.1 % Women: 67.3 BMI (kg/m2): 34.3 ± 4.8 Education (% ≥ college degree): 57.3 |
Phase I: 44% BlackPhase II: 37.6% Black | Phase I: 6 mos. Phase II: 30 mos. | Phase I: All participants received SBT in 20 weekly group sessions. Prescribed DASH Diet. Phase II: Randomized to maintenance therapy - 1. Self-directed control, 2. Interactive web site with encouragement to log on at least weekly, 3. Monthly 1:1 contact with interventionist |
Phase I: Men lost more weight than women, but within gender groups non-black participants lost more weight than black participants. Non-black participants attended more sessions, reported more physical activity, and kept more food records (p<.0001 for each test). Phase II: Those who had 1:1 contact with interventionist regained less weight than the other two groups. Statistically significant, but clinically modest. Results did not differ by race. |
| Foster et al., 2010 | 2003-2007 | 307 | Age: 45.6 ± 9.7 % Women: 67.5 BMI (kg/m2): 36.1 ± 3.5 Education: NR |
71% White 22% Black | 2 years | Low fat diet: energy intake limited to 1200 to 1800 kcal depending on gender; calories 55% carb, 30% fat, 15% protein; Low carb diet: followed Atkins guidelines, 1st 12 weeks carbs limited to 20 g/day of low glycemic index vegetables, gradually increased by 5 g/day after that. | Similar weight loss in both groups. Low carb diet improved HDL. Analysis by race not reported. |
| Jeffery et al., 2009 | 2005-2007 | 213 | Age: 48.8 ± 1.0 % Women: 53.1 BMI (kg/m2): 34.9 ± 0.3 Education (% > college degree): 71.3 |
67% White23% non-white | 18 mos. | Randomized to SBT or Maintenance Tailored Therapy (MTT). SBT met weekly 1st 6 mos., biweekly 2nd 6 mos., monthly final 6 mos. MTT had 6 8-week units with 4-week breaks between; each unit had a different focus | Weight loss greater for SBT in 1st 6 mos. No difference at 18 mos. Analysis by race not reported. |
| Sacks et al., 2009 | 2004-2007 | 811 | Age: 51.0 ± 9.0 % Women: 64.0 BMI (kg/m2): 33.0 ± 4.0 Education (% ≥ college degree): 68.0 |
79% White 16% Black |
24 mos. | All received SBT augmented with individual sessions. Groups met 3 of every 4 weeks for first 6 mos., 2 of every 4 weeks for remaining 2 years; individual sessions every 8 weeks. Randomly assigned to one of four dietary groups:1. a low-fat, average-protein diet (20% fat, 15% protein, and 65% carbohydrate),2. a low-fat, high-protein diet (20% fat, 25% protein, and 55% carbohydrate), 3. a high-fat, average-protein diet (40% fat, 15% protein, and 45% carbohydrate)4. a high-fat, high-protein diet (40% fat, 25% protein, and 35% carbohydrate) | No effect of diet type of weight change, craving, fullness, hunger, and diet satisfaction scores at 6 mos. and 2 years. Retention lower for black participants than white participants. Analysis by race not reported. |
|
Blumenthal et al., 2010 Epstein et al., 2012 |
2003-2008 | 144 | Age: 52.0 ± 10.0 % Women: 67.4 BMI (kg/m2): 33.1 ± 3.9 Education (% ≥ college degree): 46.0 |
60% White39% Black | 4 mos. | Randomized to 1 of 3 groups: 1. DASH Diet alone (DASH-A) participants were asked not to exercise or attempt to lose weight but to focus only on food choices, 2. DASH+ weight management (DASH-WM) participants received SBT and supervised exercise sessions, 3. Usual diet control participants were asked to not modify their behaviors. DASH-A and DASH-WM participants attended weekly small group sessions for 4 mos. | DASH-WM group lost significantly more weight than other groups (-8.7 kg vs.-0.3 kg (DASH-A) and 0.9 kg (control). Investigators did examine ethnicity in determining adherence; Overall, African-Americans were less adherent to the DASH diet compared to whites within the study. |
|
Fitzgibbon et al., 2008 Fitzgibbon et al., 2010 |
2005-2008 | 213 | Age: 46.0 ± 8.4 % Women: 100.0 BMI (kg/m2): 39.2 ± 5.7 Education (% college degree): 44.0 |
100% Black | 18 mos. (6 mos. weight loss + 12 mos. weight maintenance). | Participants randomized to control or culturally-tailored SBT intervention augmented with monthly motivational interviews (MI) via phone. SBT group met 2X per week for 12 mos. In mos. 13-15 met for a weekly exercise class + monthly MI session. No face-to-face meetings in mos. 16-18.Control group received monthly telephone call +newsletters covering health and safety topics weekly during the six month intervention and monthly during the 12 month maintenance phase | Participants in the SBT group lost 3.04 kg at 6 months, compared to a 0.22 kg weight gain in the control group. However, by 18 months, both groups gained weight, and there were no significant differences between the groups on weight change. |
| Burke et al., 2011 | 2006-2010 | 210 | Age: 46.8 ± 9.0 % Women: 84.8 BMI (kg/m2): 34.4 ± 4.6 Education (yrs.): 15.7 ± 3.0 |
79% White | 21 mos. (report limited to 6 mos. of treatment) | All participants received SBT for weight loss. Participants met weekly for 4 mos., biweekly for 12 mos., monthly for 6 mos. and had 1 maintenance-focused meeting in month 21. Mode of self-monitoring varied: 1. paper diary (PR), 2. electronic diary (PDA), 3. electronic diary with automated feedback program (PDA+FB) | No significant difference in weight loss between groups at 6 mos. More PDA+FB participants achieved at least 5% weight loss compared to PR. Analysis by race not reported. |
| Goodpaster et al., 2010 | 2007-2010 | 130 | Age: 46.8 ± 6.4 % Women: 88.6 BMI (kg/m2): 43.6 ± 5.4 Education (% college degree): NR |
37% Black | 12 mos. | Participants randomized into two groups, both receiving SBT for weight loss: 1. SBT + initial physical activity; 2. SBT + delayed physical activity (6 months). Participants had 3 group sessions and 1 telephone session for months 1-6. In months 7-12, participants had 2 group sessions, and 2 telephone contacts per month | Both groups lost a significant amount of weight by 6 months. The initial physical activity group lost significantly more weight in the first 6 months, compared to the delayed physical activity group. While AAs lost slightly less weight, overall, race had no significant impact on treatment outcomes between intervention groups. |
| Foster et al., 2012 | 2005-2007 | 123 | Age: 46.8 ± 12.4 % Women: 91.0 BMI (kg/m2): 34.0 ± 3.6 Education (% college degree): NR |
54% White 39% Black |
18 mos. | Participants randomized into two groups: 1. Almond-Enriched, low-calorie diet (AED); 2. Nut-free, low-calorie diet (NED). Both groups were prescribed a low-calorie diet (1200-1800kcal/d), and were encouraged to exercise 20-50 minutes 4 times per week. Both groups met weekly for 20 weeks, bi-weekly for another 20 weeks, and every 6 weeks for the remainder of the 18 mos. | Participants in the AED group loss significantly more weight than those in the NFD group at 6 months (-7.4 vs. -5.5 kg). There were no differences at 18 mos. Analysis by race not reported. |
| Foster-Schubert et al., 2012 | 2005-2009 | 439 | Age: 58.0 ± 5.0 % Women: 100.0 BMI (kg/m2): 30.9 ± 4.0 Education (% college degree): 65 |
85% White 8% Black |
12 mos. | Participants were randomized into 1 of 4 groups: 1. Calorie-reduced diet; 2. Aerobic Exercise; 3. Both interventions combined; 4. No-lifestyle change control. Participants received combination of individual and group contact for the duration of the study. | Compared to the control group, participants in diet (-8.5%), exercise (-2.4%), and the combined intervention group (-10.8%) achieved a significant weight loss at 12 months. Analysis by race not reported. |
| Jakicic et al., 2012 | 2008-2010 | 363 | Age: 42.2 ± 9.0 % Women: 83.0 BMI (kg/m2): 33.0 ± 3.6 Education (% college degree): 33.7 |
67% White 28% Black 5% Other | 18 mos. | Participants were randomized to two groups: 1. SBT; 2. Stepped-care weight loss intervention (STEP) (e.g. low-intensity weight loss intervention that increased if weight milestones are not achieved). All participants were prescribed a low-calorie diet, physical activity goals. The SBT group had weekly group meetings for months 1-6, bi-weekly for months 7-12, and monthly during 13-18 mos. The STEP group had several stages, beginning with a monthly group intervention and progressing to an individual session if needed. | At 18 months, percent weight change in the SBT group was -8.1% and -6.9% in the STEP. There were no between group differences in weight change. While race was considered in the analyses, outcomes by race were not reported. |
| Pinto et al., 2013 | 2008-2010 | 141 | Age: 49.7 ± 9.2 % Women: 90.0 BMI (kg/m2): 36.2 ± 5.5 Education: NR |
33% White 67% Non-White 55% Hispanic | 48 weeks (11 mos.) | Participants were randomly assigned to: 1) Behavioral weight loss (BWL); 2) Weight Watchers (WW); 3) 12 weeks of BWL, followed by 36 weeks of WW (CT). Those in the BWL group attended weekly meetings for 24 weeks, bi-weekly for 24 weeks. Those in the WW received free vouchers to attend WW meetings free for 48 weeks. | Significant weight loss was achieved by all groups by 12 weeks. Participants in the WW achieved greater weight loss (-6.0 kg) at 48 weeks, then compared to the CT group (-3.6 kg). Changes in the BWL group at 48 weeks were not significantly different from WW or CT (-5.4 kg). Analysis was not reported by race. |
| Nackers et al., 2013 | NR* | 125 women | Age: 52.0 ± 10.9 % Women: 100.0 BMI (kg/m2): 38.0 ± 3.9 Education (% ≥ college degree): 46.4 |
73.6% White 16.8% Black 6.15% Hispanic | 12 mos. | Participants were randomly assigned to: 1) Kcal goals of 1,000/day; 2) Kcal goal of 1,500/day. Both groups received SBT, and had 24 weekly meetings (months 0-6), and 6 monthly meetings from months 7-12. | For the first 6 months, participants in the 1,000 kcal/day group lost more weight (-10.03 kg vs. -6.23 kg). For months 7-12, however, the 1,000 kcal/day group experienced significant weight regain (+1.51 kg). Analysis was not reported by race. |
| Samuel-Hodge et al., 2013 | 2005-2007 | 189 | Age: 51.3 ± 10.9 % Women: 100.0 BMI (kg/m2): 37.1 ± 0.5 Education (yrs): 13.0 ± 0.2 |
53% Black 44% White 3% Other | 5 mos. | Participants randomized to two groups: 1) Special IV (SI) – 16 weekly group session with energy goal of no less than 1200 kcal/day, and 150 minutes/week of exercise; 2) Delayed IV (DI) – Wait-List Control – received 2 newsletters | Participants in the SI achieved a significant weight loss compared to the DI group (-3.1 kg vs. -0.4kg). Greater weight loss was associated with being non-AA (p=.005). |
NR = Not Reported
Results
Baseline Characteristics of Included Studies
Across the 23 studies included in our review, on average, the samples were predominately female (79.1%) and 47.2 ± 4.7 years of age. Among studies that reported the mean education level of their participants (n=12), 51% of participantshad received a college degree. Samples sizes ranged from 101-1191, and the length of the interventions ranged between 4-36 months.
Inclusion of AAs in the SBT Literature
All but two reports, which were published early in our timeframe, reported the racial distribution of the sample. Between 2000 and 2010, 12.9% to 13.6% of the United States (US) population self-reported as black or AA (Rastogi, Johnson, Hoeffel, & Drewery, 2011); of the 23 clinical trials identified, 14 trials exceeded the US population estimates. Rates of inclusion of AAs did not appear to be dependent on the size of the trial. Of the three trials who enrolled between 811-1200 participants, rates of inclusion for AA ranged from a low of 16.5% to 40-44% (Champagne et al., 2011; Hollis et al., 2008; Lasser et al., 1995; Sacks et al., 2009; Stevens et al., 2001; Svetkey et al., 2008). Of the nine trials who enrolled between 100-300 participants, rates of inclusion for AA ranged from 17-59% (Blumenthal et al., 2010; Foster et al., 2012; Goodpaster et al., 2010; Jakicic, Marcus, Gallagher, Napolitano, & Lang, 2003; McManus, Antinoro, & Sacks, 2001; Nackers et al., 2013; Samaha et al., 2003; Samuel-Hodge et al., 2013; Yancy, Olsen, Guyton, Bakst, & Westman, 2004). Finally, the rates of inclusion of AAs for the 2 trials recruiting 301-500 participants ranged from 8-22% (Foster-Schubert et al., 2012; Jakicic et al., 2012). Several of these trials did not report the racial distribution of sample, or only reported the distribution in terms of white and non-white (Ashley, St Jeor, Perumean-Chaney, Schrage, & Bovee, 2001; Burke et al., 2011; Burke et al., 2007; Jeffery et al., 2009; Jeffery, Wing, Sherwood, & Tate, 2003; Perri et al., 2001; Pinto, Fava, Hoffmann, & Wing, 2013). One trial included only AA women(Fitzgibbon et al., 2008, 2010).
Outcomes for African-American Participants
Only 10 studies reported outcomes by race and/or considered race in the analytic approach (Epstein et al., 2012; Fitzgibbon et al., 2010; Goodpaster et al., 2010; Hollis et al., 2008; Sacks et al., 2009; Samaha et al., 2003; Stern et al., 2004; Stevens et al., 2001; Jakicic et al., 2012; Samuel-Hodge et al., 2013). It is possible that the other studies chose not to report null results of analyses by race and/or were underpowered to examine racial differences. For studies that reported racial outcomes, results are presented below:
Weight Change
At 6 months, AA participants, on average, achieved a weight change in range of -2.1 kg to 5 kg, compared to -3.6 kg to 13 kg for white participants (Samaha et al., 2001; Hollis et al., 2008, Stevens et al., 2001; Fitzgibbon et al., 2008, 2010). The WLM trial reported higher than average weight change at 6 months in AAs (AA men (-5.4 kg ±7.7); AA women (-4.1 kg ± 2.9) (Hollis et al., 2008). Participants in the intervention arm of the ORBIT trial also achieved modest weight change success at 6 months (-3.04 kg), compared to a 0.22 kg weight gain in the control group. However, by 18 months, both groups gained weight, and there was no significant difference between the intervention and control group on weight change (IV: 1.01 kg; Control: 0.15 kg).
Attendance at Intervention Sessions
Rates of attendance varied across the interventions reporting outcomes by race. Within the WLM trial, 57% of AA men and 51% of AA women attended over 80% of sessions during the 6-month weight loss program (Hollis et al., 2008). Within the first 6 months of the ORBIT trial, participants attended an average of 53% of classes, with approximately 60% of participants attending at least half of all classes offered. During the maintenance phase (1 year duration), participants attended 27% of the classes, and 30% of participants attended at least half of the classes offered (Fitzgibbon et al., 2010).
Attrition
Several studies reported on the rates of attrition and study completion for AA participants within their trials. Samaha et al. (2001) noted the racial differences in study completion for a low-carbohydrate diet and a low-fat diet. Of the 77 AAs included in the study, 18.2% (n=14) dropped out of the low-carbohydrate arm and 26% (n=20) dropped out of the low-fat arm. When contrasted with white participants, another study reported that retention was lower for black participants (69% vs. 81% (white)) (Sacks, 2009). Among the ORBIT trial, that was exclusively African-American, at 6 months, 6.5% of participants in the intervention group vs. 7.5% of participants in the control group dropped out of the study (Fitzgibbon et al., 2010).
Dietary Adherence to Intervention
Several studies reported the adherence of AA participants. Within the ENCORE study, investigators reported that AAs were less adherent to the DASH diet compared to whites (4.68 [95% CI = 4.34, 5.03] v 5.83 [95% CI = 5.50, 6.11], p< .001) (Epstein et al., 2012). Moreover, in the WLM trial, AA men decreased their % of protein intake whereas other groups increased. Furthermore, AAshad less improvement in % of energy from fat, fruit and vegetable consumption, and energy from carbohydrates compared to non-AAs (Champagne et al., 2011). Less improvement in dietary adherence was also seen in the ORBIT trial, where at 18-months, there were no significant differences between groups in adjusted change in consumption of energy (kcal/day), fat (% kcal), fiber (g/1000 kcal), or vegetables (servings/day) (Fitzgibbon et al., 2010).
Factors contributing to Greater Inclusion of AA Participants
Several of the reviewed studies focused their recruitment efforts on the inclusion of AAs in large, urban metropolitan cities. The ORBIT Trial (Fitzgibbon et al., 2008, 2010) which was successful in recruiting 213 AAs to participate, used a variety of methods, including mass emails, face-to-face recruitment at local grocery stores, churches, and health clinics. Moreover, all recruitment was done within a 2-mile radius of the intervention site. The WLM trial, which was able to recruit 736 AA participants, used four clinical sites, and also relied on mass mailings of brochures, print media, coupons, and flyers (Hollis et al., 2008). Sacks et al., (2009) sent study information based upon information gathered from a list of registered voters and drivers. Additionally, in the ENCORE trial, where investigators enrolled AAs as approximately 40% of their sample, participants were recruited from community events and were referred from physicians (Blumenthal et al., 2010; Fitzgibbon et al., 2008). Another study, whose sample was 53% black, partnered with county health departments, and developed recruitment templates for sites; this template included a study brochure, flyer, study ad, public service announcement, and a letter for health department patients (Samuel-Hodge et al., 2013).
Factors Contributing to Treatment Response for AAs
Despite results that indicate poorer treatment outcomes for AAs, within trials that reported outcomes by race, investigators provided several recommendations and/or factors that may have contributed to greater treatment response among AA participants. While AA participants did not lose as much weight as white participants, within the WLM, AA's lost at least 4 kg of weight, and racial differences were less drastic (Hollis et al., 2008). The authors cited this may have been due to the extensive efforts to make the intervention culturally appropriate; including having AAs well-represented among investigators, interventionists, and other staff (Fitzgibbon et al., 2008; Hollis et al., 2008). Additionally, having a higher proportion of AAs in intervention group meeting may have made the environment more supportive (Hollis et al., 2008).
Moreover, due to the marked differences in dietary fat, carbohydrate, and fruit/vegetable consumption, it is also recommended that interventions provide culturally specific, tailored dietary recommendations (Fitzgibbon et al., 2008; Champagne et al., 2011). Furthermore, investigators also noted the behavior change skills, traditionally offered in standard behavioral interventions, may not be powerful enough to overcome an environment that presents barriers to weight loss, both in the ease of access to unhealthy foods and the limited options for physical activity (Fitzgibbon et al., 2010).
Several articles highlighted the specific influence of the intervention on the outcomes of AAs. Within the WLM, there were two maintenance arms designed to sustain weight loss that helped maintain the modest success achieved: personal contact and interactive technology. Within the personal contact arm, participants received monthly 10-15 minute telephone calls; among AA participants assigned to this arm, weight change at 30 months was observed at the following: women ( -2.2 ± 0.6 kg); men: (-4.9 ± 1.0 kg) (Svetkey et al., 2008). This responsiveness to personal contact has also been seen in the ORBIT trial, where participants in the control group who received monthly calls from a trial staff member following up on general health curriculum did not gain weight through the trial (Fitzgibbon et al., 2008). The interactive technology arm allowed participants to graph personal data, set goals and action plans, and have unlimited access to a web site designed to support weight loss. Among AA participantsassigned to this arm, the following weight change at 30 months was observed: men: (-3.0 ± 1.1 kg); women: (-1.3 ± 0.6 kg) (Svetkey et al., 2008).
Discussion
The purpose of this investigation was to examine the state of inclusion and the reporting of outcomes for AAs in standard behavioral weight loss programs over the last 15 years, and when possible, identify factors that may have resulted in their inclusion or enhanced treatment response. In 2003, when McTigueand colleagues published their review on randomized controlled trials of obesity treatment interventions between January 1994 and February 2003, only 27.7% of all trials reported the ethnic breakdown for their sample (McTigue et al., 2003). From the results of our review, it appears as if there has been some significant improvement; 69.6% of all trials reported the racial and/or ethnic demographics of their sample. This progress is quite encouraging.
While rates of inclusion have improved, or results also demonstrate that there is far more progress that needs to be made. It remains perplexing that most of the studies included in our review did not report outcomes by race – especially considering that African-Americans and Hispanics have the highest rates of obesity in the country (Ogden et al., 2015). Indeed, we must thoughtfully consider that our goals must not be to simply include more racial and ethnic minorities, but to consider the context of our research, and the extent to which we create communities of research where non-whites feel valued and thoughtfully considered in the design and conduct of our interventions (Quinn, Kass, & Thomas, 2013). Racial and ethnic disparities in obesity are quite prevalent; it is imperative for future research efforts to continue to develop interventions to address this unfortunate truth.
Evidence is quite clear that AAs do not lose as much weight as white participants when engaging in standard behavioral weight loss programs (Wingo et al., 2014; Hollis et al., 2008). However, we know far less about the methods that would enhance treatment response for this population. This review was able to examine the small body of existing knowledge and offer some suggestions on methods that may be helpful (e.g. face-to-face recruitment; inclusion of AA investigators and research staff). Partnering with other clinical sites appears to be a method that several investigators have used with positive results (Samuel-Hodge et al., 2013; Hollis et al., 2008). While not discussed in the studies reviewed, other investigators have highlighted the importance of including minority participants in the design of study process, and offering continuing diversity training for all staff (Kennedy et al., 2010). In addition, the joining of community resources may be particularly helpful in recruiting ethnic minority populations.
The role of personal contact and social support are factors deserving further exploration. It is noteworthy that two different studies in our review mentioned how personal contact positively influenced results (Fitzgibbon et al., 2010; Svetkey et al., 2008). Other investigations have attempted to leverage this among African-Americans by examining the benefit of enrolling with friends and family for support (Kumanyika et al., 2009). For participants who were randomized to the family arm, weight loss was reported at 5 to 6 kg at 6 months compared to the 3 to 4 kg in the control arm (Kumanykia et al., 2009). Moreover, in two behavioral interventions to prevent weight gain in primary care settings, investigators have included bi-weekly and/or monthly health coach calls in the intervention; both interventions have been able to produce significant weight gain prevention among their participants (Bennett et al., 2013; Herring et al., 2016).
With a literature that consistently summarizes less favorable outcomes of SBT on AA participants, future work may need to focus on examining the factors that impact the healthful eating and physical activity behaviors among this population. For instance, investigators have noted that AA women may engage in binge and/or compulsive overeating as a coping strategy for stress and trauma (Harrington, Crowther, Henrickson, & Mickelson, 2006; Harrington, Crowther, & Shipherd, 2010). A growing literature has described how any binge eating behavior is in fact more prevalent in AA women compared to white women (Marques et al., 2011). Moreover, these behaviors may lead to the development of Binge Eating Disorder, which is strongly associated with obesity (Hudson, Hiripi, Pope, & Kessler, 2007; Yanovski, 2003). Future interventions to address disordered eating behaviors are warranted and are being conducted by our research group (F31HL126425; PI: Goode).
Many questions regarding the relationships between cultural adaptations and treatment outcomes remain. Additional research with adequately powered studies to explore effects of race on SBT for weight loss is urgently needed to identify key components, and combinations of components of weight loss treatment that need to be adapted to result in more favorable outcomes. When possible, data from results of multiple trials could be merged to identify trends and generate hypotheses for larger studies focused on eliminating the disparity of the burden of obesity. Additionally, future research should focus on generating qualitative research focused on AAs who have undergone SBT for weight loss as experts, able to inform researchers on their needs with regard to weight loss and a healthy lifestyle. This may provide valuable insight for improving outcomes for treatment seeking overweight and obese AAs.
The results of this review provide several important implications for nursing professionals. First, due to the Patient Protection and Affordable Care Act (P.L.111-148), nurses and nurse practitioners have an increased opportunity to work on integrated health teams to engage AAs in weight promotion interventions. Working in these teams may provide nurses an opportunity to engage AAs and other ethnic minority populations who may be more likely to seek primary care, rather than engage in behavioral weight loss treatment. Additionally, due to the preliminary evidence that personal contact and social support may be particularly effective with AAs, nurses may be the optimal health professional to develop relevant interventions. Nurses and nurse practitioners may be uniquely qualified to provide one-on-one support within the healthcare setting, and leverage the relationship with their patients to increase the development of healthful behaviors.
There are some limitations in our review that deserve mention. First, this was not a systematic review of the literature, and thus, there exists the potential for bias in the selection of studies for inclusion. Additionally, due to the limited amount of information on outcomes by race, this study did not fully present the experience of SBT in participants who self-identify as African-American. Moreover, while all the included studies had at least one treatment arm of SBT, there were differences in the prescription of diet, physical activity, and group and/or individual sessions. This has the potential to limit the generalizability of study findings and outcomes by race. And finally, because we did not include studies that had interventions that were less than 4 months, we may have missed some pilot and/or feasibility studies that were presenting outcomes by race.
In summary, this review has examined the state of the literature on the inclusion and reporting of AAs within programs of SBT for weight loss, and factors that may have enhanced treatment response over the last 15 years. While rates of inclusion have improved, there is still a paucity of reporting on outcomes by race within these studies. There has been a considerable amount learned about the experience of AAs in weight loss programs (Fitzgibbon et al., 2012; Samuel-Hodge, Johnson, Braxton, & Lackey, 2014; Wingo et al., 2014). Future research should seek to expand our reach to explore and report outcomes on the effect of our interventions on AA and other ethnic minority populations who are most impacted by obesity.
References
- Ashley JM, St Jeor ST, Perumean-Chaney S, Schrage J, Bovee V. Meal replacements in weight intervention. Obesity Research. 2001;9(Suppl. 4):312S–320S. doi: 10.1038/oby.2001.136. [DOI] [PubMed] [Google Scholar]
- Ashley JM, St Jeor ST, Schrage JP, Perumean-Chaney SE, Gilbertson MC, McCall NL, Bovee V. Weight control in the physician's office. Archives of Internal Medicine. 2001;161(13):1599–1604. doi: 10.1001/archinte.161.13.1599. [DOI] [PubMed] [Google Scholar]
- Bennett GG, Foley P, Levine E, Whiteley J, Askew S, Steinberg DM, Puleo E. Behavioral treatment for weight gain prevention among black women in primary care practice: A randomized clinical trial. JAMA Internal Medicine. 2013;173(19):1770–1777. doi: 10.1001/jamainternmed.2013.9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn G. Effect of degree of weight loss on health benefits. Obesity Research. 1995;3(Suppl. 2):211s–216s. doi: 10.1002/j.1550-8528.1995.tb00466.x. [DOI] [PubMed] [Google Scholar]
- Blackburn G. Benefits of weight loss in the treatment of obesity. American Journal of Clinical Nutrition. 1999;69(3):347–349. doi: 10.1093/ajcn/69.3.347. [DOI] [PubMed] [Google Scholar]
- Blumenthal JA, Babyak MA, Hinderliter A, Watkins LL, Craighead L, Lin PH, Sherwood A. Effects of the DASH diet alone and in combination with exercise and weight loss on blood pressure and cardiovascular biomarkers in men and women with high blood pressure: The ENCORE study. Archives of Internal Medicine. 2010;170(2):126–135. doi: 10.1001/archinternmed.2009.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LE, Conroy MB, Sereika SM, Elci OU, Styn MA, Acharya SD, Glanz K. The effect of electronic self-monitoring on weight loss and dietary intake: A randomized behavioral weight loss trial. Obesity (Silver Spring) 2011;19(2):338–344. doi: 10.1038/oby.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke LE, Hudson AG, Warziski MT, Styn MA, Music E, Elci OU, Sereika SM. Effects of a vegetarian diet and treatment preference on biochemical and dietary variables in overweight and obese adults: a randomized clinical trial. American Journal of Clinical Nutrition. 2007;86(3):588–596. doi: 10.1093/ajcn/86.3.588. [DOI] [PubMed] [Google Scholar]
- Burke LE, Wang J. Treatment strategies for overweight and obesity. J Nurs Scholarsh. 2011;43(4):368–375. doi: 10.1111/j.1547-5069.2011.01424.x. [DOI] [PubMed] [Google Scholar]
- Champagne CM, Broyles ST, Moran LD, Cash KC, Levy EJ, Lin PH, Myers VH. Dietary intakes associated with successful weight loss and maintenance during the Weight Loss Maintenance trial. Journal of the American Dietetic Association. 2011;111(12):1826–1835. doi: 10.1016/j.jada.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digenio AG, Mancuso JP, Gerber RA, Dvorak RV. Comparison of methods for delivering a lifestyle modification program for obese patients: A randomized trial. Annals of Internal Medicine. 2009;150(4):255–262. doi: 10.7326/0003-4819-150-4-200902170-00006. [DOI] [PubMed] [Google Scholar]
- Epstein DE, Sherwood A, Smith PJ, Craighead L, Caccia C, Lin PH, Blumenthal JA. Determinants and consequences of adherence to the dietary approaches to stop hypertension diet in African-American and white adults with high blood pressure: Results from the ENCORE trial. Journal of the Academy of Nutrition and Dietetics. 2012;112(11):1763–1773. doi: 10.1016/j.jand.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon ML, Stolley MR, Schiffer L, Sharp LK, Singh V, Dyer A. Obesity reduction black intervention trial (ORBIT): 18-month results. Obesity (Silver Spring) 2010;18(12):2317–2325. doi: 10.1038/oby.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon ML, Stolley M, Schiffer L, Sharp L, Singh V, Van Horn L, Dyer A. Obesity Reduction Black Intervention Trial (ORBIT): Design and baseline characteristics. Journal of Womens Health (Larchmt) 2008;17(7):1099–1110. doi: 10.1089/jwh.2007.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbon ML, Tussing-Humphreys LM, Porter JS, Martin IK, Odoms-Young A, Sharp LK. Weight loss and African-American women: A systematic review of the behavioural weight loss intervention literature. Obesity Reviews. 2012;13(3):193–213. doi: 10.1111/j.1467-789X.2011.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US Adults, 1999-2008. JAMA. 2010;303(3):235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Foster-Schubert KE, Alfano CM, Duggan CR, Xiao L, Campbell KL, Kong A, McTiernan A. Effect of diet and exercise, alone or combined, on weight and body composition in overweight-to-obese postmenopausal women. Obesity (Silver Spring) 2012;20(8):1628–1638. doi: 10.1038/oby.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster GD, Shantz KL, Vander Veur SS, Oliver TL, Lent MR, Virus A, Gilden-Tsai A. A randomized trial of the effects of an almond-enriched, hypocaloric diet in the treatment of obesity. American Journal of Clinical Nutrition. 2012;96(2):249–254. doi: 10.3945/ajcn.112.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz MJ, VanWormer JJ, Crain AL, Boucher JL, Histon T, Caplan W, Pronk NP. Weight-loss outcomes: A systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. Journal of the American Dietetic Association. 2007;107(10):1755–1767. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Gold BC, Burke S, Pintauro S, Buzzell P, Harvey-Berino J. Weight loss on the web: A pilot study comparing a structured behavioral intervention to a commercial program. Obesity. 2007;15(1):155–164. doi: 10.1038/oby.2007.520. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, DeLany JP, Otto AD, Kuller L, Vockley J, South-Paul JE, Jakicic JM. Effects of diet and physical activity interventions on weight loss and cardiometabolic risk factors in severely obese adults: A randomized trial. Journal of the American Medical Association. 2010;304(16):1795–1802. doi: 10.1001/JAMA.2010.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington EF, Crowther JH, Henrickson HC, Mickelson KD. The relationships among trauma, stress, ethnicity, and binge eating. Cultural Diversity & Ethnic Minority Psychology. 2006;12(2):212–229. doi: 10.1037/1099-9809.12.2.212. [DOI] [PubMed] [Google Scholar]
- Harrington EF, Crowther JH, Shipherd JC. Trauma, binge eating, and the “strong Black woman”. Journal of Consulting and Clinical Psychology. 2010;78(4):469–479. doi: 10.1037/a0019174. [DOI] [PubMed] [Google Scholar]
- Herring SJ, Cruice JF, Bennett GG, Rose MZ, Davey A, Foster GD. Preventing excessive gestational weight gain among African American women: A randomized clinical trial. Obesity (Silver Spring) 2016;24(1):30–36. doi: 10.1002/oby.21240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard KD Weight Loss Maintenance Trial Research Group. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. American Journal of Preventive Medicine. 2008;35(2):118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson JI, Hiripi E, Pope HG, Jr, Kessler RC. The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication. Biological Psychiatry. 2007;61(3):348–358. doi: 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakicic JM, Marcus BH, Gallagher KI, Napolitano M, Lang W. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: A randomized trial. JAMA. 2003;290(10):1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- Jakicic JM, Tate DF, Lang W, Davis KK, Polzien K, Rickman AD, Finkelstein EA. Effect of a stepped-care intervention approach on weight loss in adults: A randomized clinical trial. JAMA. 2012;307(24):2617–2626. doi: 10.1001/jama.2012.6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, Levy RL, Langer SL, Welsh EM, Flood AP, Jaeb MA, Finch EA. A comparison of maintenance-tailored therapy (MTT) and standard behavior therapy (SBT) for the treatment of obesity. Preventive Medicine. 2009;49(5):384–389. doi: 10.1016/j.ypmed.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery RW, Wing RR, Sherwood NE, Tate DF. Physical activity and weight loss: does prescribing higher physical activity goals improve outcome? American Journal of Clinical Nutrition. 2003;78(4):684–689. doi: 10.1093/ajcn/78.4.684. [DOI] [PubMed] [Google Scholar]
- Kennedy BM, Kumanyika S, Ard JD, Reams P, Johnson CA, Karanja N, Harsha DW. Overall and minority-focused recruitment strategies in the PREMIER multicenter trial of lifestyle interventions for blood pressure control. Contemporary Clinical Trials. 2010;31(1):49–54. doi: 10.1016/j.cct.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumanyika S. Ethnic minorities and weight control research priorities: Where are we now and where do we need to be? Preventive Medicine. 2008;47:583–586. doi: 10.1016/j.ypmed.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Kumanyika SK, Wadden TA, Shults J, Fassbender JE, Brown SD, Bowman MA, Wu X. Trial of family and friend support for weight loss in African American adults. Archives of Internal Medicine. 2009;169(19):1795–1804. doi: 10.1001/archinternmed.2009.337. [DOI] [PubMed] [Google Scholar]
- Kumanyika SK, Espeland MA, Bahnson JL, Bottom JB, Charleston JB, Folmar S Tone Cooperative ResearchGroup. Ethnic comparison of weight loss in the Trial of Nonpharmacologic Interventions in the Elderly. Obesity Research. 2002;10(2):96–106. doi: 10.1038/oby.2002.16. [DOI] [PubMed] [Google Scholar]
- Lasser VI, Raczynski JM, Stevens VJ, Mattfeldt-Beman MK, Kumanyika S, Evans M Trials of Hypertension Prevention (TOHP) Collaborative Research Group. Trials of Hypertension Prevention, Phase II. Structure and content of the weight loss and dietary sodium reduction interventions. Annals of Epidemiology. 1995;5(2):156–164. doi: 10.1016/1047-2797(94)00060-7. [DOI] [PubMed] [Google Scholar]
- Marques L, Alegria M, Becker AE, Chen CN, Fang A, Chosak A, Diniz JB. Comparative prevalence, correlates of impairment, and service utilization for eating disorders across US ethnic groups: Implications for reducing ethnic disparities in health care access for eating disorders. International Journal of Eating Disorders. 2011;44(5):412–420. doi: 10.1002/eat.20787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManus K, Antinoro L, Sacks F. A randomized controlled trial of a moderate-fat, low-energy diet compared with a low fat, low-energy diet for weight loss in overweight adults. International Journal of Obesity & Related Metabolic Disorders. 2001;25(10):1503–1511. doi: 10.1038/sj.ijo.0801796. [DOI] [PubMed] [Google Scholar]
- McTigue KM, Harris R, Hemphill B, Lux L, Sutton S, Bunton AJ, Lohr KN. Systematic evidence review. Screening and interventions for obesity in adults: Summary of the evidence for the U.S. Preventive Services Task Force. Annals of Internal Medicine. 2003;139(11):933–949. doi: 10.7326/0003-4819-139-11-200312020-00013. [DOI] [PubMed] [Google Scholar]
- Nackers LM, Middleton KR, Dubyak PJ, Daniels MJ, Anton SD, Perri MG. Effects of prescribing 1,000 versus 1,500 kilocalories per day in the behavioral treatment of obesity: a randomized trial. Obesity (Silver Spring) 2013;21(12):2481–2487. doi: 10.1002/oby.20439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health. HNI guidelines on the inclusion of women and minorities as subjects in clinical research. P.T. 34. Bethesda, MD: Author; 1994. [Google Scholar]
- National Institutes of Health. NIH policy on reporting race and ethnicity data: subjects in clinical research. Bethesda, MD: Author; 2001. [Google Scholar]
- Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011-2014. NCHS Data Brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity in the United States, 2009-2010. NCHS Data Brief. 2012;(82):1–8. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patient Protection and Affordable Care Act, P.L. 111-148, 124 Stat. 2010;119 [Google Scholar]
- Perri MG, Nezu AM, McKelvey WF, Shermer RL, Renjilian DA, Viegener BJ. Relapse prevention training and problem-solving therapy in the long-term management of obesity. Journal of Consulting and Clinical Psychology. 2001;69(4):722–726. [PubMed] [Google Scholar]
- Pinto AM, Fava JL, Hoffmann DA, Wing RR. Combining behavioral weight loss treatment and a commercial program: a randomized clinical trial. Obesity (Silver Spring) 2013;21(4):673–680. doi: 10.1002/oby.20044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn SC, Kass NE, Thomas SB. Building trust for engagement of minorities in human subjects research: Is the glass half full, half empty, or the wrong size? American Journal of Public Health. 2013;103(12):2119–2121. doi: 10.2105/AJPH.2013.301685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi S, Johnson TD, Hoeffel EM, Drewery MP. The Black Population: 2010 Census Briefs. 2011 Retrieved from http://www.census.gov/prod/cen2010/briefs/c2010br-06.pdf.
- Rock CL, Flatt SW, Sherwood NE, Karanja N, Pakiz B, Thomson CA. Effect of a free prepared meal and incentivized weight loss program on weight loss and weight loss maintenance in obese and overweight women: A randomized controlled trial. Journal of the American Medical Association. 2010;304(16):1803–1810. doi: 10.1001/jama.2010.1503. [DOI] [PubMed] [Google Scholar]
- Sacks FM, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, Williamson DA. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. New England Journal of Medicine. 2009;360(9):859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Stern L. A low-carbohydrate as compared with a low-fat diet in severe obesity. New England Journal of Medicine. 2003;348(21):2074–2081. doi: 10.1056/NEJMoa022637. [DOI] [PubMed] [Google Scholar]
- Samuel-Hodge CD, Garcia BA, Johnston LF, Gizlice Z, Ni A, Cai J, Keyserling TC. Translation of a behavioral weight loss intervention for mid-life, low-income women in local health departments. Obesity (Silver Spring) 2013;21(9):1764–1773. doi: 10.1002/oby.20317. [DOI] [PubMed] [Google Scholar]
- Samuel-Hodge CD, Johnson CM, Braxton DF, Lackey M. Effectiveness of diabetes prevention program translations among African Americans. Obesity Reviews. 2014;15(Suppl 4):107–124. doi: 10.1111/obr.12211. [DOI] [PubMed] [Google Scholar]
- Seo DC, Sa J. A meta-analysis of psycho-behavioral obesity interventions among US multiethnic and minority adults. Preventive Medicine. 2008;47(6):573–582. doi: 10.1016/j.ypmed.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Stern L, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J, Samaha FF. The effects of low-carbohydrate versus conventional weight loss diets in severely obese adults: One-year follow-up of a randomized trial. Annals of Internal Medicine. 2004;140(10):778–785. doi: 10.7326/0003-4819-140-10-200405180-00007. [DOI] [PubMed] [Google Scholar]
- Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, Cohen J. Trials for the hypertension prevention research. long-term weight loss and changes in blood pressure: Results of the Trials of Hypertension Prevention, phase II. Annals of International Medicine. 2001;134(1):1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- Svetkey LP, Stevens VJ, Brantley PJ, Appel LJ, Hollis JF, Loria CM, Aicher K. Comparison of strategies for sustaining weight loss: The weight loss maintenance randomized controlled trial. JAMA. 2008;299(10):1139–1148. doi: 10.1001/jama.299.10.1139. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: Shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: A review. Journal of Cardiovascular Nursing. 2009;24(1):58–80. doi: 10.1097/01.JCN.0000317471.58048.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadden ThomasA, Crerand CaniceE, Brock Johanna. Behavioral treatment of obesity. Psychiatric Clinics of North America. 2005;28(1):151–170. doi: 10.1016/j.psc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Warziski M, Sereika S, Styn MA, Music E, Burke LE. Changes in self-efficacy and dietary adherence: The impact on weight loss in the PREFER study. Journal of Behavioral Medicine. 2008;31(1):81–92. doi: 10.1007/s10865-007-9135-2. [DOI] [PubMed] [Google Scholar]
- Wing RR. Behavioral approaches to the treatment of obesity. In: Bray GA, Bourchard C, James WPT, editors. Handbook of obesity: Clinical applications. 2nd. New York NY: Marcel Dekker; 2004. pp. 147–167. [Google Scholar]
- Wing RR, Sinha MK, Considine RV, Lang W, Caro JF. Relationship between weight loss maintenance and changes in serum leptin levels. Hormone & Metabolic Research. 1996;28(12):698–703. doi: 10.1055/s-2007-979881. [DOI] [PubMed] [Google Scholar]
- Wing RR, Hamman RF, Bray GA, Delahanty L, Edelstein SL, Hill JO Diabetes Prevention Program Research Group. Achieving weight and activity goals among diabetes prevention program lifestyle participants. Obesity Research. 2004;12(9):1426–1434. doi: 10.1038/oby.2004.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo BC, Carson TL, Ard J. Differences in weight loss and health outcomes among African Americans and whites in multicentre trials. Obesity Reviews. 2014;15(Suppl 4):46–61. doi: 10.1111/obr.12212. [DOI] [PubMed] [Google Scholar]
- Yancy WS, Jr, Olsen MK, Guyton JR, Bakst RP, Westman EC. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: A randomized, controlled trial. Annals of Internal Medicine. 2004;140(10):769–777. doi: 10.7326/0003-4819-140-10-200405180-00006. [DOI] [PubMed] [Google Scholar]
- Yanovski SZ. Binge eating disorder and obesity in 2003: could treating an eating disorder have a positive effect on the obesity epidemic? International Journal of Eating Disorders. 2003;34(Suppl):S117–S120. doi: 10.1002/eat.10211. [DOI] [PubMed] [Google Scholar]


