Abstract
Microscopic colitis (MC) is an inflammatory condition of the large bowel that is associated with chronic, nonbloody diarrhea. Colonoscopy usually demonstrates normal mucosa, while tissue biopsy reveals intraepithelial lymphocytes or a subepithelial collagen band. Although no specific antibody has been discovered, MC is associated with several autoimmune disorders such as celiac disease, Hashimoto’s thyroiditis, and rheumatoid arthritis. There are only a small number of case reports documenting possible hereditary MC cases, but up to 12% of patients with MC have a family history of inflammatory bowel disease. Other associations include proton pump inhibitor use, cigarette smoking, HLA-DQ2/86, and possibly some gastrointestinal infections.
Introduction
Microscopic colitis (MC) causes chronic diarrhea, abdominal cramping, nausea, anxiety, and weight loss. Epidemiologic studies demonstrate that MC is a more common cause of diarrhea than previously shown, affecting approximately 5 of every 100,000 people per year, with a mean age at diagnosis of 65 years and a female preponderance.1,2 Several medications, autoimmune diseases, infections, and toxins have been associated with MC. Physical examination and colonoscopy are typically unremarkable.3 The diagnosis of MC can only be made on the basis of abnormalities seen on colonic biopsies. Fecal microbiota transplant (FMT), on the other hand, is the reconstitution of normal colonic flora by a stool transplant from a healthy donor to an individual infected with Clostridium difficile.
Case Report
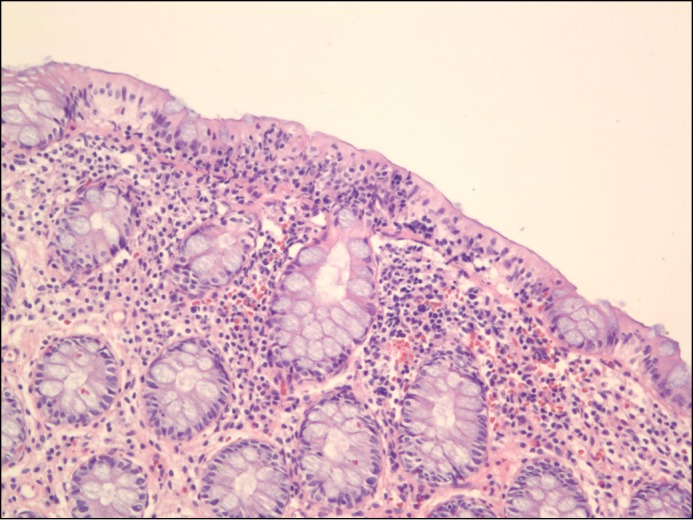
A 39-year-old woman with a past medical history significant for depression and recurrent Clostridium difficile infection presented with 2 weeks of profuse, watery diarrhea. She presented with similar symptoms 6 months prior, at which time she was found to have a positive stool C. difficile antigen. Over the next 10 weeks she was started on a succession of treatments (ie, metronidazole, followed by oral vancomycin, and then fidaxomicin) without symptom resolution. Biopsies from a colonoscopy were unremarkable (Figure 1). She underwent a FMT 4 weeks later and experienced complete symptom resolution during the initial 6 weeks after FMT. Her symptoms then returned, and she was again hospitalized.
Figure 1.
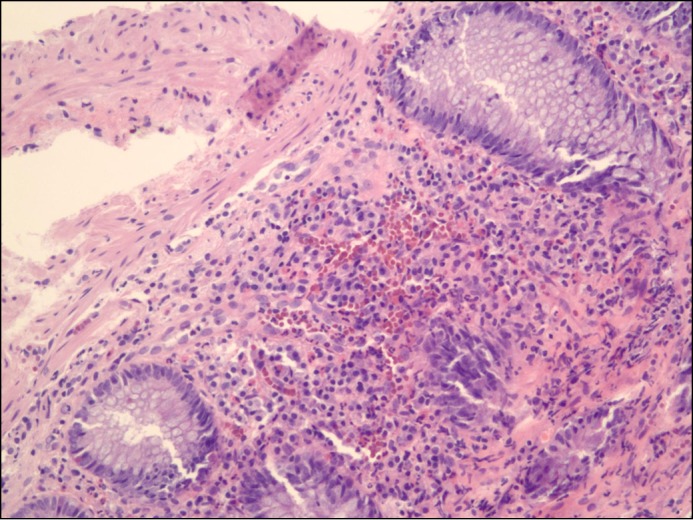
Normal colonic mucosa prior to FMT.
The patient described a gradual onset of profuse, watery diarrhea over 2 weeks that peaked at 10 bowel movements per day. She denied nausea, vomiting, fever, myalgias, dizziness, hematochezia, changes to her diet, recent travel, drug or tobacco use, and sick contacts. Physical exam was significant for an anxious-appearing, thin woman with mild left and right lower abdominal tenderness. Initial laboratory data, including basic metabolic profile, complete blood count, liver function test, thyroid-stimulating hormone, C-reactive protein, and fecal calprotectin were all within normal limits. Additional tests, including anti-Saccharomices cerevisiae and a celiac disease panel, were negative. Infectious workup for C. difficile, human immunodeficiency virus, Escherichia coli, salmonella, shigella, campylobacter, hepatitis panel, and fecal leukocytes was negative. Abdominal imaging, including ultrasound and computed tomography scan, was negative. Biopsies from a repeat colonoscopy revealed increased intraepithelial lymphocytes in the colonic epithelial layer and increased numbers of subepithelial chronic inflammatory cells consistent with lymphocytic colitis (LC) (Figure 2). The patient was promptly started on budesonide therapy, which resulted in a significant reduction in symptoms. She was subsequently discharged 2 days later and was able to return to work the next week.
Figure 2.
Abnormal colonic mucosa consistent with lymphocytic colitis showing >20 intraepithelial lymphocytes per 100 surface epithelial cells.
Discussion
MC is an inflammatory condition of the large bowel that is associated with chronic, nonbloody diarrhea with a grossly normal-appearing colonoscopy, and it is diagnosed by tissue biopsy. MC can be further classified into 2 distinct classes: LC and collagenous colitis (CC). The histopathological criteria for CC include a thickened subepithelial collagen layer of at least 10 µm, inflammation in the lamina propria with lymphocytes and plasma cells, and epithelial damage. The criteria for LC is a density of at least 20 intraepithelial lymphocytes per 100 surface epithelial cells, epithelial damage, and a subepithelial collagen layer of less than 10 µm.4 While the etiology remains obscure, the most common theories suggest that MC results from immune system activation in the colonic mucosa after exposure to antigenic factors, including toxins, infections, and medications. Commonly reported associations include autoimmune-based disorders such as celiac disease, thyroid disease, and rheumatoid arthritis, although no specific antibody has yet been associated with MC. There are a small number of case reports documenting cases of hereditary MC, but up to 12% of patients with MC have a family history of inflammatory bowel disease.5 Other potential associations include the use of proton pump inhibitors, cigarette smoking, and HLA-DQ2/8, and some case reports document newly diagnosed MC after gastrointestinal infection with C. difficile, Yersinia species, and Campylobacter species.6-8 The diagnosis of MC often occurs after an extensive workup for alternative causes of diarrhea. Withdrawal of toxic agents and drugs is required when MC began as a consequence of its use. While antidiarrheal agents are typically used, budesonide is the only drug proven to be effective in randomized, placebo-controlled trials, and it is currently the standard treatment for MC.
We describe a previously healthy 39-year-old woman presenting with new microscopic colitis after FMT for recurrent C. difficile colitis. We postulate that gut dysbiosis caused by the incorporation of a foreign microbiome resulted in a dysregulated immune response. We postulate that this caused chemotaxis of lymphocytes to the affected area as well as a spike in bacterial metabolite production.9 While FMT is rapidly being incorporated into clinical practice, much is still unknown regarding the stool donor protocol as well as complications of the therapy. The literature for reported complications of FMT include bloating, diarrhea, constipation, perforation, and bacteremia, as well as a few reports documenting adverse reactions including cancers.10 Other studies have described histologic findings consistent with MC on colonoscopic biopsies taken at the time of FMT, suggesting its pathogenesis may be associated with C. difficile rather than FMT.11 Biopsies from our patient, however, were negative for MC prior to initiation of FMT. We hope to bring attention to a potential gap in knowledge regarding adverse effects of FMT, and to suggest pre-FMT screening for MC during donor analysis.
Disclosures
Author contributions: MJ Fasullo wrote the manuscript and is the article guarantor. Y. Al-Azzawi and J. Abergel wrote and edited the manuscript.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
References
- 1.Bohr J, Tysk C, Eriksson S, Abrahamsson H, Järnerot G. Collagenous colitis: A retrospective study of clinical presentation and treatment in 163 patients. Gut. 1996;39(6):846.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tangri V, Chande N. Microscopic colitis: An update. J Clin Gastroenterol. 2009;43:293–6. [DOI] [PubMed] [Google Scholar]
- 3.Bohr J, Tysk C, Eriksson S, Järnerot G. Collagenous colitis in Orebro, Sweden: An epidemiological study 1984–1993. Gut. 1995;37(3):394.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazenby AJ, Yardley JH, Giardiello FM, Jessurun J, Bayless TM. Lymphocytic ("microscopic") colitis: A comparative histopathologic study with particular reference to collagenous colitis. Hum Pathol. 1989;20(1):18–28. [DOI] [PubMed] [Google Scholar]
- 5.Järnerot G, Hertervig E, Grännö C, et al. Familial occurrence of microscopic colitis: A report on five families. Scand J Gastroenterol. 2001;36(9):959–62. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox GM, Mattia AR. Microscopic colitis associated with omeprazole and esomeprazole exposure. J Clin Gastroenterol. 2009;43:551–3. [DOI] [PubMed] [Google Scholar]
- 7.Fernández-Bañares F, Esteve M, Farré C, et al. Predisposing HLA-DQ2 and HLA-DQ8 haplotypes of coeliac disease and associated enteropathy in microscopic colitis. Eur J Gastroenterol Hepatol. 2005;17(12):1333–8. [DOI] [PubMed] [Google Scholar]
- 8.Ingle SB, Adgaonkar BD, Hinge CR. Microscopic colitis: Common cause of unexplained nonbloody diarrhea. World J Gastrointest Pathophysiol. 2014;5:48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tariq R, Smyrk T, Pardi DS, Tremaine WJ, Khanna S. New-onset microscopic colitis in an ulcerative colitis patient after fecal microbiota transplantation. Am J Gastroenterol. 2016;111(5):751–2. [DOI] [PubMed] [Google Scholar]
- 10.Rao K, Safdar N. Fecal microbiota transplantation for the treatment of Clostridium difficile infection. J Hosp Med. 2016;11(1):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal M, Aroniadis OC, Brandt LJ, et al. The long-term efficacy and safety of fecal microbiota transplant for recurrent, severe, and complicated clostridium difficile infection in 146 elderly individuals. J Clin Gastroenterol. 2016;50(5):403–7. [DOI] [PubMed] [Google Scholar]


