Abstract
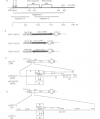
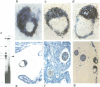
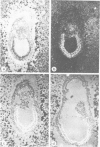
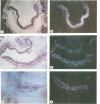
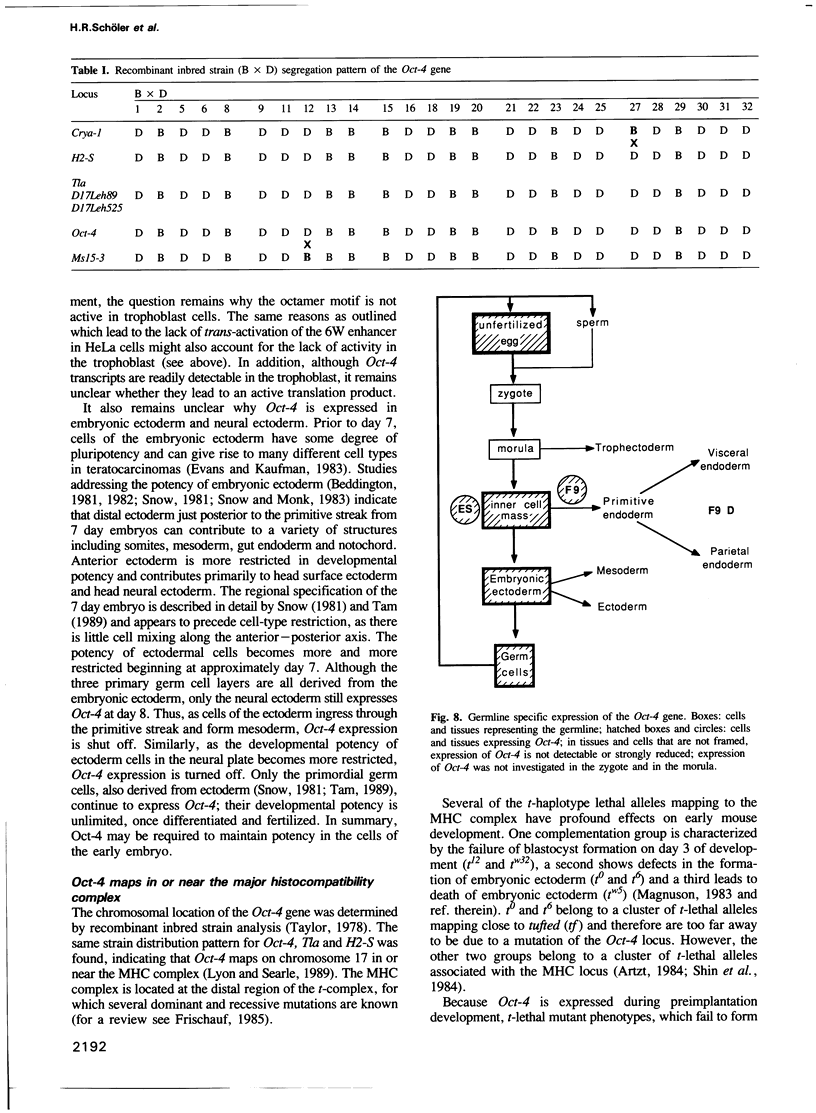
Oct-4 is a maternally expressed octamer-binding protein encoded by the murine Oct-4 gene. It is present in unfertilized oocytes, but also in the inner cell mass and in primordial germ cells. Here we show that the ectopic expression of Oct-4 in HeLa cells is sufficient for transcriptional activation from the octamer motif, indicating that Oct-4 is a transcription factor. Therefore, Oct-4 is the first transcription factor described that is specific for the early stages of mouse development. The spatial and temporal expression patterns were further determined using in situ hybridization. With this technique Oct-4 expression is detected in the oocyte, in the blastocyst and before gastrulation in the embryonic ectoderm. After day 8 Oct-4 expression decreases and is restricted to primordial germ cells from about day 8.5 onwards. Therefore Oct-4 is a transcription factor that is specifically expressed in cells participating in the generation of the germline lineage. Linkage analysis using B X D recombinant inbred mouse strains demonstrates that Oct-4 maps to chromosome 17 in or near the major histocompatibility complex. Several mouse mutants in the distal region of the mouse t-complex affecting blastocyst and embryonic ectoderm formation also map to this region.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artzt K. Gene mapping within the T/t complex of the mouse. III: t-Lethal genes are arranged in three clusters on chromosome 17. Cell. 1984 Dec;39(3 Pt 2):565–572. doi: 10.1016/0092-8674(84)90463-x. [DOI] [PubMed] [Google Scholar]
- Beddington R. S. An autoradiographic analysis of tissue potency in different regions of the embryonic ectoderm during gastrulation in the mouse. J Embryol Exp Morphol. 1982 Jun;69:265–285. [PubMed] [Google Scholar]
- Beddington S. P. An autoradiographic analysis of the potency of embryonic ectoderm in the 8th day postimplantation mouse embryo. J Embryol Exp Morphol. 1981 Aug;64:87–104. [PubMed] [Google Scholar]
- CHIQUOINE A. D. The identification, origin, and migration of the primordial germ cells in the mouse embryo. Anat Rec. 1954 Feb;118(2):135–146. doi: 10.1002/ar.1091180202. [DOI] [PubMed] [Google Scholar]
- Church G. M., Ephrussi A., Gilbert W., Tonegawa S. Cell-type-specific contacts to immunoglobulin enhancers in nuclei. 1985 Feb 28-Mar 6Nature. 313(6005):798–801. doi: 10.1038/313798a0. [DOI] [PubMed] [Google Scholar]
- Clark J. M., Eddy E. M. Fine structural observations on the origin and associations of primordial germ cells of the mouse. Dev Biol. 1975 Nov;47(1):136–155. doi: 10.1016/0012-1606(75)90269-9. [DOI] [PubMed] [Google Scholar]
- Dony C., Gruss P. Specific expression of the Hox 1.3 homeo box gene in murine embryonic structures originating from or induced by the mesoderm. EMBO J. 1987 Oct;6(10):2965–2975. doi: 10.1002/j.1460-2075.1987.tb02602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressler G. R., Gruss P. Do multigene families regulate vertebrate development? Trends Genet. 1988 Aug;4(8):214–219. doi: 10.1016/s0168-9525(88)80003-9. [DOI] [PubMed] [Google Scholar]
- Dreyfus M., Doyen N., Rougeon F. The conserved decanucleotide from the immunoglobulin heavy chain promoter induces a very high transcriptional activity in B-cells when introduced into an heterologous promoter. EMBO J. 1987 Jun;6(6):1685–1690. doi: 10.1002/j.1460-2075.1987.tb02418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A., Church G. M., Tonegawa S., Gilbert W. B lineage--specific interactions of an immunoglobulin enhancer with cellular factors in vivo. Science. 1985 Jan 11;227(4683):134–140. doi: 10.1126/science.3917574. [DOI] [PubMed] [Google Scholar]
- Fisher D. A., Hunt S. W., 3rd, Hood L. Structure of a gene encoding a murine thymus leukemia antigen, and organization of Tla genes in the BALB/c mouse. J Exp Med. 1985 Aug 1;162(2):528–545. doi: 10.1084/jem.162.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh J. Cultivation, characterization, and identification of human tumor cells with emphasis on kidney, testis, and bladder tumors. Natl Cancer Inst Monogr. 1978 Dec;(49):5–9. [PubMed] [Google Scholar]
- Gerster T., Matthias P., Thali M., Jiricny J., Schaffner W. Cell type-specificity elements of the immunoglobulin heavy chain gene enhancer. EMBO J. 1987 May;6(5):1323–1330. doi: 10.1002/j.1460-2075.1987.tb02371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham A., Papalopulu N., Krumlauf R. The murine and Drosophila homeobox gene complexes have common features of organization and expression. Cell. 1989 May 5;57(3):367–378. doi: 10.1016/0092-8674(89)90912-4. [DOI] [PubMed] [Google Scholar]
- Hillman N., Hillman R., Wileman G. Ultrastructural studies on cleavage stage t12-t12 mouse embryos. Am J Anat. 1970 Jul;128(3):311–340. doi: 10.1002/aja.1001280304. [DOI] [PubMed] [Google Scholar]
- Holland P. W., Hogan B. L. Expression of homeo box genes during mouse development: a review. Genes Dev. 1988 Jul;2(7):773–782. doi: 10.1101/gad.2.7.773. [DOI] [PubMed] [Google Scholar]
- Ingham P. W. The molecular genetics of embryonic pattern formation in Drosophila. Nature. 1988 Sep 1;335(6185):25–34. doi: 10.1038/335025a0. [DOI] [PubMed] [Google Scholar]
- Jeffreys A. J., Wilson V., Kelly R., Taylor B. A., Bulfield G. Mouse DNA 'fingerprints': analysis of chromosome localization and germ-line stability of hypervariable loci in recombinant inbred strains. Nucleic Acids Res. 1987 Apr 10;15(7):2823–2836. doi: 10.1093/nar/15.7.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., Kobayashi T., Staudt L., Baltimore D., Sharp P. A. Octamer-binding proteins from B or HeLa cells stimulate transcription of the immunoglobulin heavy-chain promoter in vitro. Genes Dev. 1988 Oct;2(10):1227–1237. doi: 10.1101/gad.2.10.1227. [DOI] [PubMed] [Google Scholar]
- Lenardo M. J., Staudt L., Robbins P., Kuang A., Mulligan R. C., Baltimore D. Repression of the IgH enhancer in teratocarcinoma cells associated with a novel octamer factor. Science. 1989 Jan 27;243(4890):544–546. doi: 10.1126/science.2536195. [DOI] [PubMed] [Google Scholar]
- Lyons K. M., Pelton R. W., Hogan B. L. Patterns of expression of murine Vgr-1 and BMP-2a RNA suggest that transforming growth factor-beta-like genes coordinately regulate aspects of embryonic development. Genes Dev. 1989 Nov;3(11):1657–1668. doi: 10.1101/gad.3.11.1657. [DOI] [PubMed] [Google Scholar]
- Müller-Immerglück M. M., Schaffner W., Matthias P. Transcription factor Oct-2A contains functionally redundant activating domains and works selectively from a promoter but not from a remote enhancer position in non-lymphoid (HeLa) cells. EMBO J. 1990 May;9(5):1625–1634. doi: 10.1002/j.1460-2075.1990.tb08282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. M., Ruppert S., Schaffner W., Matthias P. A cloned octamer transcription factor stimulates transcription from lymphoid-specific promoters in non-B cells. Nature. 1988 Dec 8;336(6199):544–551. doi: 10.1038/336544a0. [DOI] [PubMed] [Google Scholar]
- Müller U., Stephan D., Philippsen P., Steinmetz M. Orientation and molecular map position of the complement genes in the mouse MHC. EMBO J. 1987 Feb;6(2):369–373. doi: 10.1002/j.1460-2075.1987.tb04764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K., Okazawa H., Okuda A., Sakai M., Muramatsu M., Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990 Feb 9;60(3):461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- Pedersen T., Peters H. Proposal for a classification of oocytes and follicles in the mouse ovary. J Reprod Fertil. 1968 Dec;17(3):555–557. doi: 10.1530/jrf.0.0170555. [DOI] [PubMed] [Google Scholar]
- Scheidereit C., Heguy A., Roeder R. G. Identification and purification of a human lymphoid-specific octamer-binding protein (OTF-2) that activates transcription of an immunoglobulin promoter in vitro. Cell. 1987 Dec 4;51(5):783–793. doi: 10.1016/0092-8674(87)90101-2. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Identification of a novel lymphoid specific octamer binding protein (OTF-2B) by proteolytic clipping bandshift assay (PCBA). EMBO J. 1988 Dec 20;7(13):4221–4229. doi: 10.1002/j.1460-2075.1988.tb03319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Balling R., Hatzopoulos A. K., Suzuki N., Gruss P. Octamer binding proteins confer transcriptional activity in early mouse embryogenesis. EMBO J. 1989 Sep;8(9):2551–2557. doi: 10.1002/j.1460-2075.1989.tb08393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Hatzopoulos A. K., Balling R., Suzuki N., Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. EMBO J. 1989 Sep;8(9):2543–2550. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöler H. R., Ruppert S., Suzuki N., Chowdhury K., Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990 Mar 29;344(6265):435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- Severne Y., Wieland S., Schaffner W., Rusconi S. Metal binding 'finger' structures in the glucocorticoid receptor defined by site-directed mutagenesis. EMBO J. 1988 Aug;7(8):2503–2508. doi: 10.1002/j.1460-2075.1988.tb03097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. S., Bennett D., Artzt K. Gene mapping within the T/t complex of the mouse. IV: The inverted MHC is intermingled with several t-lethal genes. Cell. 1984 Dec;39(3 Pt 2):573–578. doi: 10.1016/0092-8674(84)90464-1. [DOI] [PubMed] [Google Scholar]
- Silver J. Confidence limits for estimates of gene linkage based on analysis of recombinant inbred strains. J Hered. 1985 Nov-Dec;76(6):436–440. doi: 10.1093/oxfordjournals.jhered.a110140. [DOI] [PubMed] [Google Scholar]
- Snow M. H. Autonomous development of parts isolated from primitive-streak-stage mouse embryos. Is development clonal? J Embryol Exp Morphol. 1981 Oct;65 (Suppl):269–287. [PubMed] [Google Scholar]
- Steinmetz M., Malissen M., Hood L., Orn A., Maki R. A., Dastoornikoo G. R., Stephan D., Gibb E., Romaniuk R. Tracts of high or low sequence divergence in the mouse major histocompatibility complex. EMBO J. 1984 Dec 1;3(12):2995–3003. doi: 10.1002/j.1460-2075.1984.tb02246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Stephan D., Sun H., Lindahl K. F., Meyer E., Hämmerling G., Hood L., Steinmetz M. Organization and evolution of D region class I genes in the mouse major histocompatibility complex. J Exp Med. 1986 May 1;163(5):1227–1244. doi: 10.1084/jem.163.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinnakre M. G., Evans M. J., Willison K. R., Stern P. L. Expression of Forssman antigen in the post-implantation mouse embryo. J Embryol Exp Morphol. 1981 Feb;61:117–131. [PubMed] [Google Scholar]
- Tam P. P. Regionalisation of the mouse embryonic ectoderm: allocation of prospective ectodermal tissues during gastrulation. Development. 1989 Sep;107(1):55–67. doi: 10.1242/dev.107.1.55. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Grossniklaus U., Herr W., Hernandez N. Activation of the U2 snRNA promoter by the octamer motif defines a new class of RNA polymerase II enhancer elements. Genes Dev. 1988 Dec;2(12B):1764–1778. doi: 10.1101/gad.2.12b.1764. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990 Feb 9;60(3):375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- Thali M., Müller M. M., DeLorenzi M., Matthias P., Bienz M. Drosophila homoeotic genes encode transcriptional activators similar to mammalian OTF-2. Nature. 1988 Dec 8;336(6199):598–601. doi: 10.1038/336598a0. [DOI] [PubMed] [Google Scholar]
- Vincek V., Kawaguchi H., Mizuno K., Zaleska-Rutczynska Z., Kasahara M., Forejt J., Figueroa F., Klein J. Linkage map of mouse chromosome 17: localization of 27 new DNA markers. Genomics. 1989 Nov;5(4):773–786. doi: 10.1016/0888-7543(89)90119-5. [DOI] [PubMed] [Google Scholar]
- Wirth T., Staudt L., Baltimore D. An octamer oligonucleotide upstream of a TATA motif is sufficient for lymphoid-specific promoter activity. Nature. 1987 Sep 10;329(6135):174–178. doi: 10.1038/329174a0. [DOI] [PubMed] [Google Scholar]