Abstract
Substance P (SP) is involved in the proliferation of cholangiocytes in bile duct ligated (BDL) mice and human cholangiocarcinoma growth by interacting with the neurokinin-1 receptor (NK-1R). To identify whether SP regulates liver fibrosis during cholestasis, wild type (WT) or NK-1R knockout (NK-1R−/−) mice that received BDL or sham surgery and Mdr2−/− mice treated with either an NK-1R antagonist (L-733,060) or saline were used. Additionally, WT mice were treated with SP or saline intraperitoneally. In vivo, there was increased expression of TAC1 (coding SP) and NK-1R in both BDL and Mdr2−/− mice compared to WT mice. The expression of TAC1 and NK-1R was significantly higher in liver samples from PSC patients compared to healthy controls. Knockout of NK-1R decreased BDL-induced liver fibrosis and treatment with L-733,060 resulted in decreased liver fibrosis in Mdr2−/− mice, which was shown by decreased Sirius red staining, fibrosis gene and protein expression and reduced transforming growth factor-β1 levels in serum and cholangiocytes supernatants. Furthermore, we observed that reduced liver fibrosis in NK-1R−/− mice with BDL surgery or Mdr2−/− mice treated with L-733,060 was associated with enhanced cellular senescence of hepatic stellate cells (HSCs) and decreased senescence of cholangiocytes. In vitro, L-733,060 inhibited SP-induced expression of fibrotic genes in HSCs and cholangiocytes. Treatment with L-733,060 partially reversed SP-induced decrease of senescence genes expression in cultured HSCs and SP-induced increase of senescence-related genes expression in cultured cholangiocytes. Collectively, our results demonstrated the regulatory effects of the SP/NK-1R axis on liver fibrosis through changes in cellular senescence during cholestatic liver injury.
Keywords: biliary damage, cholestasis, sensory innervation, substance P
Introduction
The liver is innervated by sympathetic, parasympathetic and peptidergic nerves, which contain afferent as well as efferent fibers (1). A number of neuropeptides have been identified in the liver (1). For example, neuronal fibers positive for neuropeptide Y, substance P (SP) and calcitonin gene-related peptide are abundant in the tunica media of vessels in portal tracts as well as in the intralobular connective tissue (2). SP is an 11-amino acid neuropeptide that belongs to the tachykinin family which also includes peptides such as neurokinin A and B (3). SP displays widespread distribution in both the central and peripheral nervous systems and has been characterized as a pro-inflammatory peptide that induces neurogenic inflammation. SP is also synthesized and secreted by peripheral organs such as liver and lung (3). Upon binding to its high affinity receptor, neurokinin-1 receptor (NK-1R), SP modulates a series of physiological and pathophysiological functions including inflammation, proliferation, cell excitability and anti-apoptosis (4). It has been shown that SP plasma levels are elevated in patients with non-alcoholic cirrhosis and that SP may play a role in the pathogenesis of spider angiomas (5). Inhibition of SP secretion and NK-1R signaling decreases cholangiocarcinoma growth (6). Knockout of NK-1R reduces cholangiocyte hyperplasia and Col1α1 (collagen, type I, alpha 1), α-smooth muscle actin (α-SMA) expression in bile duct ligated (BDL) mice (7). There are in vivo studies showing that NK-1R antagonists not only suppress the secretion of pro-inflammatory cytokines in the liver but also protect liver cells from apoptosis (8). However, no studies exist regarding the role of the SP/NK-1R axis in the development of liver fibrosis in BDL and multidrug resistance protein 2 (Mdr2−/−) mice, widely used as models of primary sclerosing cholangitis (PSC) (9).
Liver fibrosis is characterized by the deposition of extracellular matrix (ECM) proteins including collagen fibers that cause liver injury, inflammation and ultimately tissue scarring. Activation of hepatic stellate cells (HSCs) in response to liver injury is recognized as a central event in the development of liver fibrosis. The activation of HSCs is characterized by a switch from a quiescent vitamin A-rich phenotype to a myofibroblastic fibrogenic phenotype, proliferating and producing a network of ECM (10). Studies have shown that senescent HSCs accumulate in the liver following damage induced by carbon tetrachloride (CCl4) and limit the extent of fibrosis through expression of anti-fibrotic proteins, suggesting that senescent HSCs may be associated with the regression of fibrosis during tissue repair (10). After becoming senescent, activated HSCs stop proliferating, down-regulate production of ECM, and increase the levels of ECM-degrading enzymes to limit liver fibrosis (10–12). Cholangiocyte senescence is a hallmark in the progression of liver fibrosis and has been found in mouse models of PSC as well as the livers of the patients with PSC and primary biliary cirrhosis (PBC) (13, 14). In these pathologies, cholangiocytes express a number of profibrogenic and chemotactic proteins that attract both inflammatory cells and HSCs, modulating the inflammatory microenvironment (15). On the basis of these findings, we hypothesize that the SP/NK-1R axis regulates liver fibrosis through differential changes in the senescence of cholangiocytes and HSCs during BDL and Mdr2−/−-induced cholestasis.
Materials and Methods
Materials
Reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) unless otherwise indicated. For other reagents and antibodies used in the experiments, please see more-detailed information in Supplementary Materials and Methods section.
Animal Models
All animal experiments were performed in accordance with protocols approved by the Baylor Scott & White Healthcare IACUC Committee. Male C57BL/6 wild-type (WT) mice were purchased from Charles River (Wilmington, MA). NK-1R−/− mice were generated by targeted disruption of the NK-1R gene in embryonic stem cells in the Mary Lyon Centre (Oxfordshire, UK). The animals were generated at the Baylor Scott & White animal facility from heterozygous breeding pairs; animal genotype was verified by PCR using the specific primers provided by the Mary Lyon Centre. Animals were maintained in a temperature-controlled environment (20–22°C) with 12:12-hr light/dark cycles and were fed standard mouse chow with free access to drinking water ad libitum. Male WT and NK-1R−/− mice (approximately 25–30 g, 12 weeks of age,) of the N5 generation underwent BDL(7) or sham operation for 1 week. In other experiments, WT mice were treated with saline or SP (2.1 μg/hr) (16) for 2 weeks by IP implanted minipumps. In selected experiments we used male Mdr2−/− mice (17) (12 weeks of age) or their WT counterparts (FVB/NJ) (25–30 g, purchased from Jackson Laboratories, Sacramento, CA) that were treated with saline or L-733,060 (20 mg/kg BW by IP implanted mini-pumps) (8) for 2 weeks before evaluating liver fibrosis and senescence. We collected serum, total liver samples, cholangiocytes and HSCs from the selected groups of animals.
Isolated mouse cholangiocytes and HSCs, intrahepatic murine cholangiocyte and human hepatic stellate cell lines
Mouse cholangiocytes were obtained by immunoaffinity separation as described (7, 18). Mouse HSCs were isolated by Histodenz density gradient centrifugation (19) or laser capture microdissection (LCM) using an antibody against desmin (20). Following immunofluorescent staining, desmin-positive cells were dissected from the slides by a LCM system Leica LMD7000 (Buffalo Grove, IL) and collected into a PCR tube. Then, the RNA from LCM-isolated HSCs was extracted with the Arcturus PicoPure RNA isolation kit (Thermo Fisher Scientific CO, Mountain View, CA). Protein (10 μg) from whole cell lysate from mouse HSCs (isolated by Histodenz density gradient centrifugation) was used for immunoblots; HSC purity was evaluated by immunofluorescence for α-SMA (21) (Supplementary Figure 2B). In vitro, studies were performed in murine immortalized biliary cell lines (IMCLs) and human hepatic stellate cell lines (HHSteCs, Sciencell, Carlsbad, CA).
Normal and PSC male human samples
The use of human tissue was approved by the Texas A&M HSC College of Medicine Institutional Review Board. Human samples (see Supplementary Table 1 for patients’ information) were obtained from Dr. Pietro Invernizzi (Humanitas Clinical and Research Center, Rozzano, Milan, Italy) under a protocol by the Ethics Committee of the Humanitas Research Hospital; the protocol was reviewed by the Veterans’ Administration IRB and International Research Committee. The RNeasy FFPE kit was used to extract total RNA from paraffin-embedded liver sections (4–5 μm) from patients (n=3) with advanced stage (3–4) of PSC. PSC stage was defined in accordance with international guidelines (22). The control healthy liver RNA (n=3) were purchased from Biochain Inc. (Newark, CA).
Evaluation of SP serum levels and hepatic expression of TAC1 and NK-1R in cholestatic animal models and human PSC samples
SP serum levels from the selected samples (n=6) were measured by EIA kits (see above). The hepatic mRNA expression of TAC1 and NK-1R in the selected groups of mice (n=3) as well as control (n=3) and human PSC samples (n=3) was evaluated by real-time PCR. Data are expressed as relative mRNA levels ± SEM of the selected gene to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) ratio.
Assessment of serum chemistry, intrahepatic bile ductal mass (IBDM) and liver fibrosis
The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and total bilirubin were measured by a Dimension RxL Max Integrated Chemistry system (Dade Behring Inc., Deerfield IL), Chemistry Department, Baylor Scott & White Health. IBDM in liver sections (4–5 μm thick, 10 different fields analyzed from 3 different samples from 4 different animals) was measured as the area occupied by CK-19 positive-bile ducts/total area × 100. Sections were examined by the Olympus Image Pro-Analyzer software (Olympus, Tokyo, Japan). The immunoreactivity for CK-19 was also assayed by immunofluorescence in frozen liver sections (6–8 μm thick) from WT, NK-1R−/−, BDL WT and BDL NK-1R−/− mice. Fibrosis in liver sections (4–5 μm thick) was assessed by Sirius red staining (10 different fields were analyzed from 3 different samples from 3 different animals). The immunoreactivity for α-SMA was evaluated by: (i) immunofluorescence in frozen liver sections (6–8 μm thick) and (ii) immunochemistry in paraffin-embedded liver sections (4–5 μm thick); images were obtained by using Leica AF 6000 Modular Systems and Leica scanner (Buffalo Grove, IL). We evaluated the expression of α-SMA, Fn-1, and Col1α1 in total liver by real-time PCR and/or immunoblots (20), and measured TGF-β1 levels in serum and cholangiocyte supernatant by ELISA and TGF-β1gene expression in cholangiocytes and HSCs from the selected groups of mice.
Measurement of cellular senescence
Senescence was measured in frozen liver sections (10 μm thick) by staining for SA-β-galactosidase using a commercially available kit (MilliporeSigma, Billerica, MA). All the experiments were performed in 3 different liver samples from 3 different animals. Senescence was also evaluated by immunofluorescence for CCL2 in frozen liver sections (6–8 μm thick). CCL2, one of the key inflammatory chemokines that regulate migration and infiltration of monocytes/macrophages, was selected as senescence marker because it is induced by and promotes cellular senescence during liver injury (23). Following staining, images were obtained by using Leica AF 6000 Modular Systems. We also evaluated the expression of senescence markers: (i) p16 and CCL2 in total liver samples; and (ii) p16 and p21 in isolated cholangiocytes and HSCs by real-time PCR and immunoblots (18). To provide further evidence for the presence of senescence-associated secretory phenotypes (SASP) in cholangiocytes and HSCs, we measured the expression of IL-6, MMPs by real-time PCR in these two cell types, and IL-6 levels in cholangiocyte supernatant by ELISA. We isolated HSCs by LCM from human liver sections from late stage PSC samples (n = 3) and healthy controls (n = 3) and measured the mRNA levels of NK-1R and PAI-1 (another senescent marker).
Effect of SP and L-733,060 on the expression of fibrosis and senescence genes in IMCLs and HHSteCs
We evaluated the expression of NK-1R in HHSteCs by immunofluorescence. IMCLs and HHSteCs were treated with 0.2% BSA (basal) or SP (10−9 M) (7) for 24 hr in the absence/presence of L-733,060 (25 μM) (8) before evaluating the expression of fibrosis and senescent markers by real-time PCR. To determine the cellular relationship between SP and NK-1R, IMCLs were treated with BSA or L-733,060 (25 μM) or transfected with NK-1R siRNA or control siRNA at 37°C for 24 hr before evaluating SP levels in the supernatant of these cell samples by ELISA kits. Additionally, HHSteCs were treated with cholangiocyte supernatant from WT, NK-1R−/−, BDL WT and BDL NK-1R−/− mice before evaluating: (i) the expression of fibrosis and senescence genes; and (ii) the expression of TGF-β1 and Smad2 (a downstream signaling pathway specific to TGF-β1 signaling during the progression of liver fibrosis) (20) by real-time PCR.
Statistical analysis
All data are expressed as the mean ± SEM. Differences between groups were analyzed by Student’s unpaired t-test when two groups were analyzed or ANOVA when more than two groups were analyzed. A value of p<0.05 was considered significant.
Results
Enhanced hepatic mRNA expression of TAC1, NK-1R and increased SP serum levels in mice with cholestatic liver injuries and PSC patients
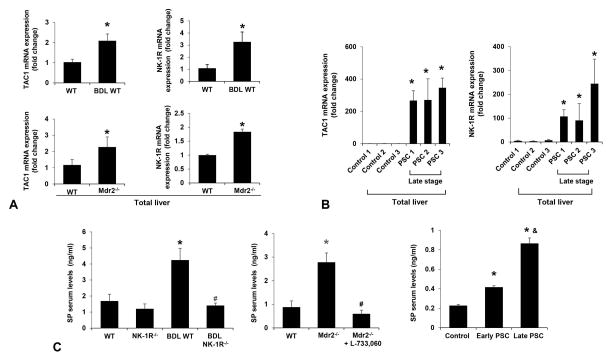
There was increased mRNA expression of TAC1 and NK-1R in total liver from BDL WT and Mdr2−/− mice compared to control mice (Figure 1A). The hepatic mRNA expression of TAC1 and NK-1R was higher in late stage PSC patients compared to healthy controls (Figure 1B). SP serum levels were higher in BDL WT and Mdr2−/− mice compared to control mice, but these levels decreased in BDL NK-1R−/− mice compared to BDL WT mice as well as in Mdr2−/− mice treated with L-733,060 compared to Mdr2−/− mice (Figure 1C). There was not much difference in SP levels in normal NK-1R−/− and Mdr2−/− mice treated with L-733,060 compared to the corresponding control mice (Figure 1C). Enhanced SP serum levels were observed in PSC patients compared to healthy controls (Figure 1C). SP serum levels were higher in late stage PSC patients than those in early stage PSC patients (Figure 1C).
Figure 1.
[A] There was increased TAC1 and NK-1R mRNA expression in total liver from BDL WT and Mdr2−/− mice compared to respective control mice (n=3). [B] There was enhanced mRNA expression of TAC1 and NK-1R in total liver samples from late stage PSC patients compared to controls (n=3). [C] SP serum levels were higher in BDL WT and Mdr2−/− mice compared to the control mice, but decreased in BDL NK-1R−/− mice and Mdr2−/− mice treated with L-733,060 compared to BDL WT and Mdr2−/− mice, respectively (n=6). There were increased SP serum levels in PSC patients compared to healthy controls. SP serum levels were higher in late stage PSC patients than those in early stage PSC patients (n=3). *P<0.05 vs. normal WT or health control samples. #P<0.05 vs. BDL WT or Mdr2−/− mice; &P<0.05 vs. early stage PSC samples.
Evaluation of serum chemistry, IBDM and liver fibrosis in liver sections and total liver samples
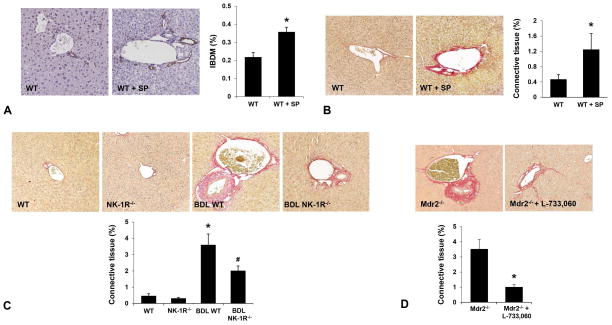
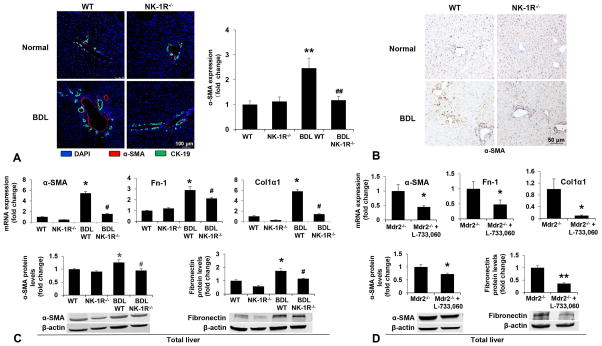
The serum levels of ALT, AST, ALP and total bilirubin increased in Mdr2−/− mice compared to WT mice and significantly decreased in Mdr2−/− mice treated with L-733,060 compared to Mdr2−/− mice (Supplementary Table 2). The administration of SP to WT mice significantly increased IBDM and collagen liver deposition (Figure 2A–B). We have previously demonstrated reduced IBDM in BDL NK-1R−/− mice compared to BDL WT mice (7). As expected, there was enhanced liver fibrosis in BDL WT compared to WT mice. Meanwhile, we observed reduced fibrosis in BDL NK-1R−/− mice compared to BDL WT mice (Figure 2C). In Mdr2−/− mice treated with L-733,060, there was decreased liver fibrosis compared to Mdr2−/− mice (Figure 2D). The immunoreactivity of α-SMA was lower in liver sections from BDL NK-1R−/− compared to BDL WT mice (Figure 3A–B). Following BDL, there was increased mRNA expression of α-SMA, Col1α1 and Fn-1 as well as increased α-SMA and Fibronectin protein levels in total liver samples compared to WT mice (Figure 3C). The gene and protein expression of these fibrosis markers was reduced in: (i) BDL NK-1R−/− mice compared to BDL WT mice (Figure 3C); and (ii) Mdr2−/− mice treated with L-733,060 compared to Mdr2−/− mice (Figure 3D). With regard to the general health of the animals receiving L-733,060, we did not observe significant changes in body weight or increased mortality in Mdr2−/− mice treated with the NK-1R antagonist (data not shown).
Figure 2.
[A–B] Administration of SP to normal WT mice increased IBDM and collagen deposition compared to control WT mice (n=4, Orig., magn, 20×). [C] There was enhanced liver fibrosis in BDL WT mice compared to normal WT mice, but reduced fibrosis in BDL NK-1R−/− mice compared to BDL WT mice (n=4). [D] In Mdr2−/− mice treated with L-733,060, there was decreased liver fibrosis compared to Mdr2−/− mice (n=3, Orig., magn., 20×). *P<0.05 vs. normal WT mice or Mdr2−/− mice; #P<0.05 vs. BDL WT mice.
Figure 3.
[A–B] There was decreased immunoreactivity for α-SMA in liver sections from BDL NK-1R−/− mice compared to BDL WT mice reflected by immunofluorescence for α-SMA and immunochemistry staining for α-SMA in liver sections (n=3, Orig. magn. 20×). [C] There was increased mRNA expression of α-SMA, Col1α1 and Fn-1 as well as α-SMA and Fibronectin protein expression in total liver from BDL WT mice compared to WT mice, that was reduced in BDL NK-1R−/− mice compared to BDL WT mice. [D] The mRNA expression of these fibrosis markers was also decreased in Mdr2−/− mice treated with L-733,060 compared to Mdr2−/− mice (n=3). *P<0.05; **P<0.01 vs. normal WT mice or Mdr2−/− mice. #P<0.05, ##P<0.01 vs. BDL WT mice.
Measurement of cellular senescence in liver sections and total liver samples
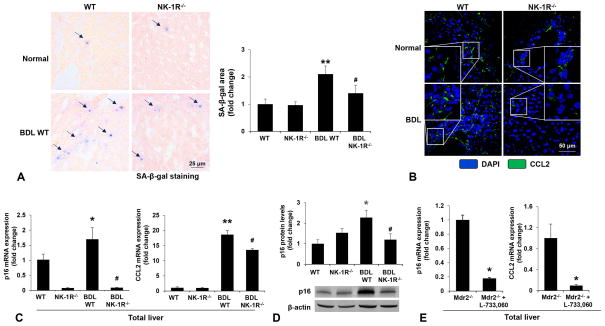
There was enhanced cellular senescence evidenced by SA-β-gal staining in liver sections from BDL WT mice compared to WT mice (Figure 4A). On the contrary, cellular senescence was reduced in BDL NK-1R−/− mice compared to BDL WT mice (Figure 4A). By immunofluorescence, we observed increased expression of CCL2 in BDL WT mice compared to WT mice, but decreased CCL2 expression in BDL NK-1R−/− mice compared to BDL WT mice (Figure 4B). Through real-time PCR, we observed: (i) increased expression of p16 and CCL2 in total liver from BDL WT compared to WT mice; and (ii) decreased expression of p16 and CCL2 in total liver from BDL NK-1R−/− mice compared to BDL WT mice (Figure 4C) as well as in Mdr2−/− mice treated with L-733,060 compared to Mdr2−/− mice (Figure 4E). The p16 protein levels were decreased in BDL NK-1R−/− mice compared to BDL WT mice (Figure 4D)
Figure 4.
[A] There was enhanced cellular senescence evidenced by SA-β-gal staining in liver sections from BDL WT mice compared to WT mice. Cellular senescence was reduced in BDL NK-1R−/− mice compared to BDL WT mice (n=4, Orig., magn., 40×). [B] There was increased expression of CCL2 in the livers of BDL WT mice (evidenced by immunofluorescence staining) compared to normal WT mice but decreased CCL2 expression in BDL NK-1R−/− mice compared to BDL WT mice. (n=3, Orig. magn., 40×). [C–D] There was increased mRNA expression of p16 and CCL2 as well as p16 protein levels in total liver from BDL WT mice compared to normal WT mice, but decreased expression of p16 and CCL2 in total liver from BDL NK-1R−/− mice compared to BDL WT mice (n=4). [E] The expression of p16 and CCL2 was also decreased in Mdr2−/− mice treated with L-733,060 compared to Mdr2−/− mice (n=3). *P<0.05; **P<0.01 vs. normal WT or Mdr2−/− mice. #P<0.05 vs. BDL WT mice.
Evaluation of senescence genes expression in cholangiocytes and HSCs
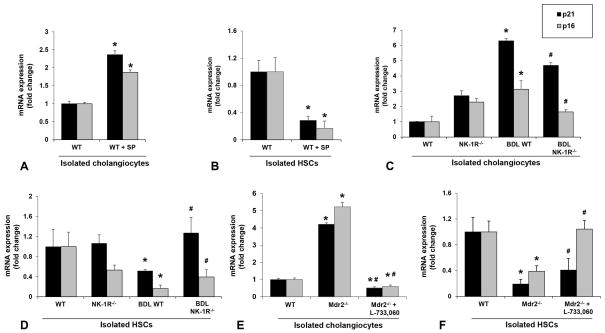
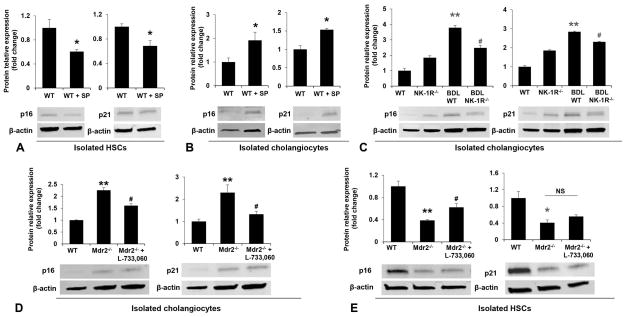
To evaluate the mechanisms by which SP contributes to liver fibrosis during cholestatic liver injury, we measured the expression of cellular senescence markers in isolated cholangiocytes and HSCs. We observed that the profibrogenic effects of SP were associated with enhanced senescence of cholangiocytes, but decreased senescence of HSCs (Figure 5A–B; Figure 6A–B). Similarly, the expression of p21 and p16 was increased in cholangiocytes but reduced in HSCs from BDL WT mice and Mdr2−/− mice compared to control mice; these changes were partially reversed in BDL NK-1R−/− mice and Mdr2−/− mice treated with L-733,060 as evidenced by decreased cellular senescence in cholangiocytes and increased senescence in HSCs (Figure 5C–F; Figure 6C–E). There was also reduced expression of IL-6, MMP2, MMP3 and MMP9 in cholangiocytes but increased expression of IL-6 and MMP2 in HSCs from BDL NK-1R−/− mice compared to BDL WT mice (Supplementary Figure 1A–C). There was enhanced expression of NK-1R but decreased expression of PAI-1 (another senescence marker) in LCM-isolated HSCs from late stage PSC patients compared to control samples, further supporting the concept that HSCs become more activated and less senescent in PSC (Supplementary Figure 1D).
Figure 5.
[A–B] The mRNA expression of p21 and p16 was increased in cholangiocytes but reduced in HSCs isolated from WT mice treated with SP compared to normal WT mice (n=3). [C–F] The mRNA expression of p21 and p16 was increased in cholangiocytes but reduced in HSCs isolated from BDL WT mice and Mdr2−/− mice compared to respective control mice, changes that were partly reversed in BDL NK-1R−/− mice and Mdr2−/− mice treated with L-733,060. *P<0.05 vs. normal WT mice. #P<0.05 vs. BDL WT or Mdr2−/− mice.
Figure 6.
[A–B] There was decreased protein expression of p16 and p21 in HSCs and increased expression of these two proteins in cholangiocytes from WT mice treated with SP compared to normal WT mice (n=3). [C–D] The p16 and p21 protein levels were increased in cholangiocytes from BDL WT and Mdr2−/− mice compared to control mice, changes that were partly reversed in BDL NK-1R−/− mice and Mdr2−/− mice treated with L-733,060. [E] The protein expression of p16 and p21 was decreased in HSCs from Mdr2−/− mice compared to WT mice, and the p16 protein levels were partly reversed in Mdr2−/− mice treated with L-733,060. *P<0.05, **P<0.01 vs. normal WT mice. #P<0.05 vs. BDL WT or Mdr2−/− mice.
Effect of SP and L-733,060 on the expression of fibrosis and senescence genes in IMCLs and HHSteCs
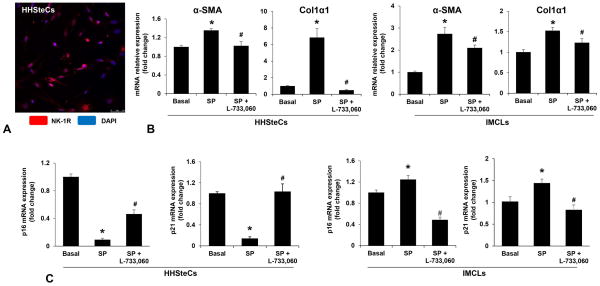
We have previously demonstrated that IMCLs express NK-1R (7); in the current study we showed by immunofluorescence that HHSteCs express NK-1R (Figure 7A). We also demonstrated that SP (10−9 M for 24 hr) increased the expression of selected fibrosis genes in both IMCLs and HHSteCs, which was prevented by L-733,060 (Figure 7B). The profibrogenic effects of SP on IMCLs and HHSteCs were associated with increased senescence of IMCLs but reduced senescence of HHSteCs; the effects were prevented by incubation with L-733,060 (Figure 7C). No significant changes in SP levels were observed in the supernatants from IMCLs treated with L-733,060 or NK-1R siRNA compared to basal or IMCLs treated with control siRNA (Supplementary Figure 2A).
Figure 7.
[A] Representative immunofluorescence picture of NK-1R in HHSteCs (n=4, Orig., Magn. 40×). [B] SP increased the expression of selected fibrosis genes in both IMCLs and HHSteCs, which was prevented by L-733,060 (n=4). [C] The expression of p16 and p21 was decreased in HHSteCs while increased in ICMLs simulated by SP; these effects were prevented by incubation with L-733,060 (n=4). *P<0.05 vs. basal, #P<0.05 vs. SP-treated group.
Measurement of TGF-β1 levels in serum and cholangiocyte supernatant and evaluation of the effect of cholangiocyte supernatant on fibrosis and senescence gens expression in HHSteCs
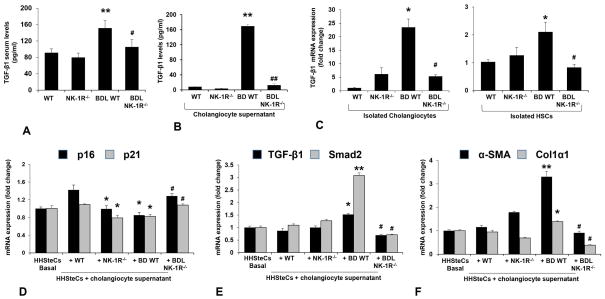
In addition, we explored if TGF-β1 is involved in the effects of SP/NK-1R axis on liver fibrosis. We observed increased TGF-β1 levels in serum and cholangiocyte supernatant as well as increased mRNA expression of TGF-β1 in HSCs and cholangiocytes from BDL WT mice compared to WT mice, but decreased in BDL NK-1R−/− mice compared to BDL WT mice (Figure 8A–C). When HHSteCs were treated with cholangiocyte supernatant from selected mice, we observed there was reduced expression of p16 and p21 (Figure 8D), CCL2 (Supplementary Figure 2C) but increased expression of fibrosis genes (Figure 8F), TGF-β1 and Smad2 (Figure 8E) and Ki67 (Supplemental Figure 2C) in HHSteCs treated with cholangiocyte supernatant from BDL WT mice compared to HHSteCs treated with cholangiocyte supernatant from normal mice. However, there were contrary changes of above genes expression in HHSteCs treated with cholangiocyte supernatant from BDL NK-1R−/− mice compared to HHSteCs treated with cholangiocyte supernatant from BDL WT mice. These findings suggest that SP/NK-1R modulation of liver fibrosis may be partly due to enhanced TGF-β1 expression.
Figure 8.
[A–C] The TGF-β1 levels increased in serum and cholangiocyte supernatant as well as the mRNA expression of TGF-β1 increased in cholangiocytes and HSCs from BDL WT mice compared to normal WT mice, but decreased in BDL NK-1R−/− mice compared to BDL WT mice (n=7). *P<0.05, **P<0.01 vs. normal WT mice, #P<0.05, **P<0.01 vs. BDL WT mice. [D–F] There was reduced expression of p16 and p21 but increased expression of fibrosis genes (α-SMA and Col1α1), TGF-β1 and Smad2 in HHSteCs treated with cholangiocyte supernatant from BDL WT mice compared to HHSteCs treated with cholangiocyte supernatant from WT mice. There was increased expression of p16 and p21 but reduced expression of fibrosis genes, TGF-β1 and Smad2 in HHSteCs treated with cholangiocyte supernatant from BDL NK-1R−/− mice compared to HHSteCs treated with cholangiocyte supernatant from BDL mice. *P<0.05, **P<0.01 vs. HHSteCs treated with cholangiocyte supernatant from normal WT mice. #P<0.05 vs. HHSteCs treated with cholangiocyte supernatant from BDL WT mice.
Discussion
Liver fibrosis occurs in many chronic liver diseases including hepatitis B and C, alcoholic liver diseases and cholestasis (24). Progressive liver fibrosis may result in cirrhosis, hepatic failure and ultimately death. Although the pathogenesis of liver fibrosis is not completely elucidated, the activation of HSCs is recognized as a key step in its progress. In this study, we examined the mechanisms by which the SP/NK-1R axis contributes to the development of liver fibrosis during cholestatic liver injury. We have provided the first piece of evidence that HSCs express NK-1R and that the expression of TAC1 and NK-1R is increased in the livers of BDL and Mdr2−/− mice compared to control mice. The expression of TAC1 and NK-1R is also higher in late stage human PSC liver samples compared to healthy controls. We also demonstrated that: (i) administration of SP to WT mice increased liver fibrosis; (ii) there was decreased liver fibrosis and enhanced cellular senescence of HSCs after knockout of NK-1R in BDL mice; and (iii) administration of L-733,060 reduced liver fibrosis and increased cellular senescence of HSCs in Mdr2−/− mice. In vitro, L-733,060 decreased SP-induced fibrogenic gene expression and differentially regulated senescence in IMCLs and HHSteCs. We propose that the SP/NK-1R axis contributes to liver fibrosis differentially regulating cellular senescence of cholangiocytes and HSCs during cholestatic liver injury.
SP, a neuropeptide released from central and peripheral nerves, potentially has fibroblast and keratinocyte proliferating activity (25) in addition to the well-recognized histamine releasing activity. Other studies have also shown that SP augments fibrogenic cytokine-induced fibroblast proliferation and modulates colitis-associated intestinal fibrosis (26). Therefore, considering that our results are consistent with these previous studies, it is likely that SP plays an important role in many kinds of tissue fibrosis. Other studies have shown that SP, via NK-1R, contributes to progression of myelofibrosis (27). In addition, NK-1R antagonists have been shown to protect mice from cytokine-mediated liver injury and reduce hepatocyte apoptosis (8, 28). In support of these previous findings, our results introduce the novel concept that NK-1R antagonists decrease liver fibrosis in Mdr2−/− mice reducing biliary damage and increasing the senescence of HSCs.
With regard to the mechanisms underlying the effects of the SP/NK-1R axis on liver fibrosis, we observed increased senescence of cholangiocytes and decreased senescence of HSCs, which could explain how the activation of the SP/NK-1R axis contributes to the development of liver fibrosis during cholestasis. Cellular senescence is a programmed reaction that occurs in response to cellular stresses such as telomere erosion, chromatin disruption, DNA damage, oxidative stress and oncogene activation (29, 30). The hallmarks of senescent cells include an essentially irreversible growth arrest, expression of p16 and beta-galactosidase; the formation of SASP, which means robust secretion of growth factors, cytokines, proteases and other proteins leading cells to a proinflammatory state, nuclear foci containing damaged DNA, and senescence associated heterochromatin foci. A previous study has shown that senescence of activated HSCs can limit fibrosis during CCl4-induced liver injury, which supports our findings (10). As well, the antiproliferative and apoptotic agent, dioscin, has been shown to inhibit liver fibrosis through increasing senescence of activated HSCs evidenced by an increased number of SA-β-galactosidase HSCs as well as the enhanced expression of the senescence markers, p16, p21 and p53 (12). In this study, we provided evidence suggesting that decreased senescence of cholangiocytes and enhanced senescence of HSCs due to NK-1R knockout during BDL-induced cholestasis or administration of L-733,060 to Mdr2−/− mice may explain why blocking of NK-1R decreases liver fibrosis. We found a concomitant increase in IBDM (index of biliary proliferation) and liver fibrosis as well as cellular senescence of cholangiocytes in WT mice treated with SP. In addition to an SP-dependent mechanism, this discrepancy may also be explained in terms of the heterogeneity of the biliary epithelium (31, 32), as the SP/NK1R axis may target different subsets of cholangiocytes that may respond differentially to SP with simultaneous increases in biliary proliferation and cellular senescence in specific subsets of different-sized cholangiocytes. However, other studies are required in order to further confirm this speculation. Similarly, the increase in biliary senescence, observed in the BDL mouse model that is characterized by the proliferation of large (but not small) cholangiocytes (31, 32) is likely related to the heterogeneous proliferative, apoptotic and senescent responses of the biliary epithelium to liver injury (31–33). This point is also supported by our recent study showing that alpha-naphthylisothiocyanate feeding induces simultaneous proliferation and apoptosis of both small and large cholangiocytes (33). Due to the fact that the proliferative and senescent responses of cholangiocytes are dynamic processes with smaller cholangiocytes differentiating into large cholangiocytes during the damage of the latter cells, we speculate that there is a subset of large cholangiocytes (within the large cholangiocyte compartment) that do not proliferate and undergo cellular senescence. Our speculation related to the differential proliferative and senescent capacities of large cholangiocytes is supported by our recent findings (Glaser and Alpini, unpublished observations, 2016) showing that in Mdr2−/− mice there is both enhanced proliferation and senescence in specific subsets of large cholangiocytes. This concept is also supported by previous studies in PSC patients showing concomitant biliary proliferation (although lowered) as well as cellular senescence (13). However, this is conjectural and requires additional studies to better isolate and characterize these different subsets of large cholangiocytes.
In the in vitro experiments, L-733,060 prevented SP-induced activation of HHSteCs and increased cellular senescence of HHSteCs. Thus, by triggering cellular senescence in HHSteCs, inhibition of SP/NK-1R signal blocks the proliferation of these ECM-producing cells and converts them into ECM-degrading cells, thereby contributing to fibrosis regression. Interestingly, our findings demonstrate that cellular senescence in total liver is decreased due to knockout of NK-1R during BDL or inhibition of NK-1R by L-733,060 in Mdr2−/− mice, while there is increased cellular senescence simultaneously in HSCs. We also speculate that inhibition of the SP/NK-1R axis decreases the damage/senescence of other liver cells such as hepatocytes that may contribute to decreased activation of HSCs. However, this speculation needs to be confirmed by further studies.
Increased biliary proliferation has been suggested to trigger liver fibrosis in various chronic liver diseases (20, 34). Cholangiocytes can also express a number of profibrogenic and chemotactic proteins attracting both inflammatory cells and HSCs (35). A recent study has demonstrated cholangiocyte senescence and SASP as an important pathogenic mechanism in PSC (36). On the basis of these previous studies, we propose that decreased cholangiocyte senescence and enhanced HSCs senescence after blocking or inhibition of NK-1R, both contribute to the reduction of liver fibrosis during cholestasis. In our study, we also observed that cholangiocyte supernatant from BDL NK-1R−/− mice decreased the proliferation and increased the senescence of HSCs compared to HSCs treated with cholangiocyte supernatant from BDL WT mice, which suggests that decreased chemotactic factors secreted by cholangiocytes, due to knockout of NK-1R, reduced the activation and enhanced the senescence of HSCs during cholestasis.
In conclusion, SP may be an important regulator of liver fibrosis and cellular senescence during the progression of cholestatic disorders. Cholestatic liver damage increases the expression of SP and activates the SP/NK-1R axis, leading to activation of HSCs and liver fibrosis. Knockout or blocking of NK-1R, reverses liver fibrosis following cholestatic liver injury through reduced senescence of cholangiocytes and increased senescence of HSCs. Further studies are needed to pinpoint the molecular mechanisms by which the SP/NK-1R axis regulates the cross-talk between cholangiocytes and HSCs leading to differential changes in biliary and HSCs senescence and ultimately to liver fibrosis. Since commercially available NK-1R antagonists (e.g., fosaprepitant) have been used to treat nausea and vomiting in humans (37), it is important to develop clinical studies to determine whether NK-1R antagonists prevent the progression of liver fibrosis in human cholestatic liver diseases.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Baylor Scott & White, a VA Research Career Scientist Award and a VA Merit award to Dr. Alpini (5I01BX000574), a VA Merit Award (5I01BX002192) to Dr. Glaser, a VA Merit Award (1I01BX001724) to Dr. Meng, a VA Merit Award (1I01BX003031) to Dr. Francis, and the NIH grants DK58411, DK07698, DK095291 and DK062975 to Drs. Alpini, Meng and Glaser and the NIH grant DK108959 to Dr. Francis. We acknowledge Adam C. Stephens in the Publications Office, Baylor Scott & White Health for editing assistance. This material is the result of work supported by resources at the Central Texas Veterans Health Care System. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Abbreviations
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- AST
aspartate aminotrasfease
- α-SMA
α-smooth muscle actin
- BDL
bile duct ligation
- CCL2
monocyte chemoattractant protein-1
- CCl4
carbon tetrachloride
- CK-19
cytokeratin 19
- Col1α1
collagen, type I, alpha 1
- ECM
extracellular matrix
- Fn-1
fibronectin-1
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HSCs
hepatic stellate cells
- HHSteCs
human hepatic stellate cells
- IBDM
intrahepatic bile ductal mass
- IL-6
interleukin-6
- IMCLs
murine immortalized biliary cell lines
- LCM
laser capture microdissection
- Mdr2
multidrug resistance protein 2
- MMPs
matrix metalloproteinases
- NK-1R
neurokinin-1 receptor
- PBC
primary biliary cholangitis
- PSC
primary sclerosing cholangitis
- SASP
senescence-associated secretory phenotypes
- Smad2
small mothers of decapentaplegic 2
- SP
substance P
- TGF-β1
transforming growth factor-β1
- WT
wild type
References
- 1.Jensen KJ, Alpini G, Glaser S. Hepatic nervous system and neurobiology of the liver. Compr Physiol. 2013;3:655–665. doi: 10.1002/cphy.c120018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoyanova II, Gulubova MV. Immunocytochemical study on the liver innervation in patients with cirrhosis. Acta Histochem. 2000;102:391–402. doi: 10.1078/0065-1281-00568. [DOI] [PubMed] [Google Scholar]
- 3.Munoz M, Covenas R. Involvement of substance P and the NK-1 receptor in human pathology. Amino Acids. 2014;46:1727–1750. doi: 10.1007/s00726-014-1736-9. [DOI] [PubMed] [Google Scholar]
- 4.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94:265–301. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li CP, Lee FY, Hwang SJ, Chang FY, Lin HC, Lu RH, Hou MC, et al. Role of substance P in the pathogenesis of spider angiomas in patients with nonalcoholic liver cirrhosis. Am J Gastroenterol. 1999;94:502–507. doi: 10.1111/j.1572-0241.1999.883_l.x. [DOI] [PubMed] [Google Scholar]
- 6.Meng F, DeMorrow S, Venter J, Frampton G, Han Y, Francis H, Standeford H, et al. Overexpression of membrane metalloendopeptidase inhibits substance P stimulation of cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2014;306:G759–768. doi: 10.1152/ajpgi.00018.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaser S, Gaudio E, Renzi A, Mancinelli R, Ueno Y, Venter J, White M, et al. Knockout of the neurokinin-1 receptor reduces cholangiocyte proliferation in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2011;301:G297–305. doi: 10.1152/ajpgi.00418.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang R, Sass G, Kiemer AK, Vollmar AM, Neuhuber WL, Tiegs G. Neurokinin-1 receptor antagonists CP-96,345 and L-733,060 protect mice from cytokine-mediated liver injury. J Pharmacol Exp Ther. 2003;305:31–39. doi: 10.1124/jpet.102.043539. [DOI] [PubMed] [Google Scholar]
- 9.Baghdasaryan A, Claudel T, Kosters A, Gumhold J, Silbert D, Thuringer A, Leski K, et al. Curcumin improves sclerosing cholangitis in Mdr2−/− mice by inhibition of cholangiocyte inflammatory response and portal myofibroblast proliferation. Gut. 2010;59:521–530. doi: 10.1136/gut.2009.186528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kong X, Feng D, Wang H, Hong F, Bertola A, Wang FS, Gao B. Interleukin-22 induces hepatic stellate cell senescence and restricts liver fibrosis in mice. Hepatology. 2012;56:1150–1159. doi: 10.1002/hep.25744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Han X, Yin L, Xu L, Qi Y, Xu Y, Sun H, et al. Potent effects of dioscin against liver fibrosis. Sci Rep. 2015;5:9713. doi: 10.1038/srep09713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tabibian JH, Trussoni CE, O’Hara SP, Splinter PL, Heimbach JK, LaRusso NF. Characterization of cultured cholangiocytes isolated from livers of patients with primary sclerosing cholangitis. Lab Invest. 2014;94:1126–1133. doi: 10.1038/labinvest.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng L, Quezada M, Levine P, Han Y, McDaniel K, Zhou T, Lin E, et al. Functional role of cellular senescence in biliary injury. Am J Pathol. 2015;185:602–609. doi: 10.1016/j.ajpath.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakanuma Y, Sasaki M, Harada K. Autophagy and senescence in fibrosing cholangiopathies. J Hepatol. 2015;62:934–945. doi: 10.1016/j.jhep.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 16.Everard MJ, Macaulay VM, Millar JL, Smith IE. [D-Arg1, D-Phe5, D-Trp7,9, Leu11] substance P inhibits the growth of human small cell lung cancer xenografts in vivo. Eur J Cancer. 1993;29A:1450–1453. doi: 10.1016/0959-8049(93)90019-c. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida S, Ikenaga N, Liu SB, Peng ZW, Chung J, Sverdlov DY, Miyamoto M, et al. Extrahepatic platelet-derived growth factor-beta, delivered by platelets, promotes activation of hepatic stellate cells and biliary fibrosis in mice. Gastroenterology. 2014;147:1378–1392. doi: 10.1053/j.gastro.2014.08.038. [DOI] [PubMed] [Google Scholar]
- 18.Glaser S, Meng F, Han Y, Onori P, Chow BK, Francis H, Venter J, et al. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–1808. doi: 10.1053/j.gastro.2014.02.030. e1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dangi A, Sumpter TL, Kimura S, Stolz DB, Murase N, Raimondi G, Vodovotz Y, et al. Selective expansion of allogeneic regulatory T cells by hepatic stellate cells: role of endotoxin and implications for allograft tolerance. J Immunol. 2012;188:3667–3677. doi: 10.4049/jimmunol.1102460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu N, Meng F, Invernizzi P, Bernuzzi F, Venter J, Standeford H, Onori P, et al. The secretin/secretin receptor axis modulates liver fibrosis through changes in TGF-beta1 biliary secretion. Hepatology. 2016 doi: 10.1002/hep.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumpter TL, Dangi A, Matta BM, Huang C, Stolz DB, Vodovotz Y, Thomson AW, et al. Hepatic stellate cells undermine the allostimulatory function of liver myeloid dendritic cells via STAT3-dependent induction of IDO. J Immunol. 2012;189:3848–3858. doi: 10.4049/jimmunol.1200819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liver EAftSot. EASL Clinical Practice Guidelines: Management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Sasaki M, Miyakoshi M, Sato Y, Nakanuma Y. Chemokine-chemokine receptor CCL2-CCR2 and CX3CL1-CX3CR1 axis may play a role in the aggravated inflammation in primary biliary cirrhosis. Dig Dis Sci. 2014;59:358–364. doi: 10.1007/s10620-013-2920-6. [DOI] [PubMed] [Google Scholar]
- 24.Borkham-Kamphorst E, Schaffrath C, Van de Leur E, Haas U, Tihaa L, Meurer SK, Nevzorova YA, et al. The anti-fibrotic effects of CCN1/CYR61 in primary portal myofibroblasts are mediated through induction of reactive oxygen species resulting in cellular senescence, apoptosis and attenuated TGF-beta signaling. Biochim Biophys Acta. 2014;1843:902–914. doi: 10.1016/j.bbamcr.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 25.Nilsson J, von Euler AM, Dalsgaard CJ. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985;315:61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- 26.Koon HW, Shih D, Karagiannides I, Zhao D, Fazelbhoy Z, Hing T, Xu H, et al. Substance P modulates colitis-associated fibrosis. Am J Pathol. 2010;177:2300–2309. doi: 10.2353/ajpath.2010.100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang VT, Yook C, Rameshwar P. Synergism between fibronectin and transforming growth factor-beta1 in the production of substance P in monocytes of patients with myelofibrosis. Leuk Lymphoma. 2013;54:631–638. doi: 10.3109/10428194.2012.722218. [DOI] [PubMed] [Google Scholar]
- 28.Bang R, Biburger M, Neuhuber WL, Tiegs G. Neurokinin-1 receptor antagonists protect mice from CD95- and tumor necrosis factor-alpha-mediated apoptotic liver damage. J Pharmacol Exp Ther. 2004;308:1174–1180. doi: 10.1124/jpet.103.059329. [DOI] [PubMed] [Google Scholar]
- 29.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 30.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–233. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Alpini G, Glaser S, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 32.Han Y, Glaser S, Meng F, Francis H, Marzioni M, McDaniel K, Alvaro D, et al. Recent advances in the morphological and functional heterogeneity of the biliary epithelium. Exp Biol Med (Maywood) 2013;238:549–565. doi: 10.1177/1535370213489926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeSage G, Glaser S, Ueno Y, Alvaro D, Baiocchi L, Kanno N, Phinizy JL, et al. Regression of cholangiocyte proliferation after cessation of ANIT feeding is coupled with increased apoptosis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G182–190. doi: 10.1152/ajpgi.2001.281.1.G182. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki M, Ikeda H, Yamaguchi J, Miyakoshi M, Sato Y, Nakanuma Y. Bile ductular cells undergoing cellular senescence increase in chronic liver diseases along with fibrous progression. Am J Clin Pathol. 2010;133:212–223. doi: 10.1309/AJCPWMX47TREYWZG. [DOI] [PubMed] [Google Scholar]
- 35.Beuers U. Crosstalk of liver, bile ducts and the gut. Clin Rev Allergy Immunol. 2009;36:1–3. doi: 10.1007/s12016-008-8084-z. [DOI] [PubMed] [Google Scholar]
- 36.Tabibian JH, O’Hara SP, Splinter PL, Trussoni CE, LaRusso NF. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–2275. doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herrstedt J, Roila F, Group EGW. Chemotherapy-induced nausea and vomiting: ESMO clinical recommendations for prophylaxis. Ann Oncol. 2008;19(Suppl 2):ii110–112. doi: 10.1093/annonc/mdn105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.