Abstract
An immortalized interleukin-3 (IL-3)-dependent progenitor cell line, BAF-3, undergoes programmed cell death (apoptosis) when deprived of IL-3. This program is characterized by an early degradation of DNA into oligonucleosome-length fragments that precedes by several hours the loss of cell viability. In the absence of IL-3, DNA fragmentation and cell death can be prevented by the calcium ionophores A23187 (1 microM) and ionomycin (0.5 microM). This addition of calcium ionophore maintains cell viability while reversibly arresting the cell cycle. Apoptosis by growth factor deprivation is also a mechanism of cell elimination in bone marrow cells removed from the stromal micro-environment, as DNA fragmentation and cell death was shown to take place in primary cultures of IL-3-responsive bone marrow cells after IL-3 removal.
Full text
PDF
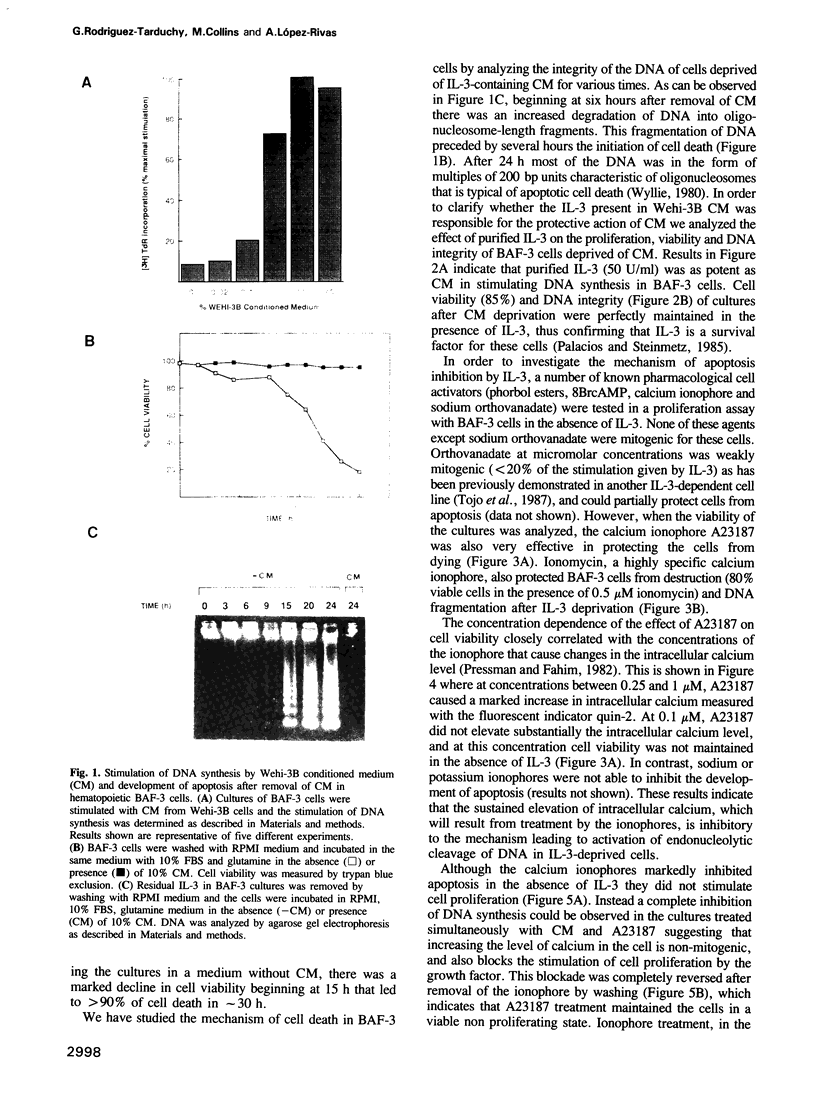


Images in this article
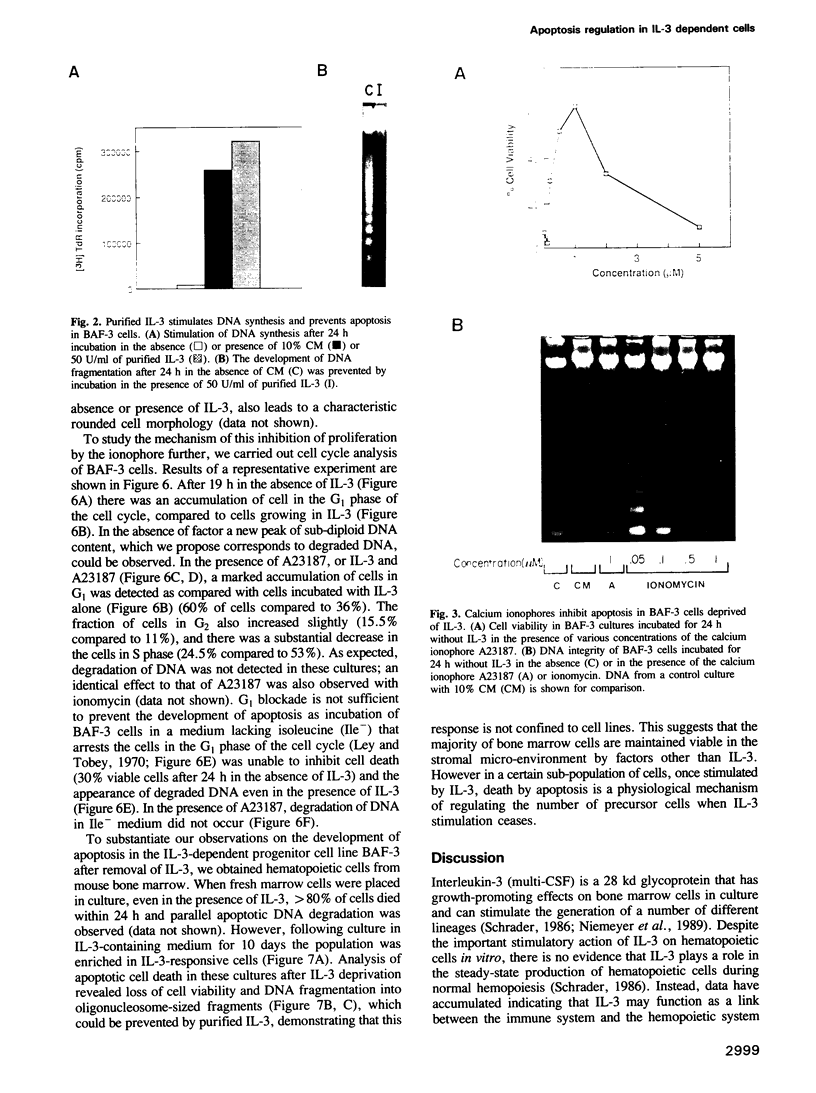
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Cook N., Dexter T. M., Lord B. I., Cragoe E. J., Jr, Whetton A. D. Identification of a common signal associated with cellular proliferation stimulated by four haemopoietic growth factors in a highly enriched population of granulocyte/macrophage colony-forming cells. EMBO J. 1989 Oct;8(10):2967–2974. doi: 10.1002/j.1460-2075.1989.tb08446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean M., Cleveland J. L., Rapp U. R., Ihle J. N. Role of myc in the abrogation of IL3 dependence of myeloid FDC-P1 cells. Oncogene Res. 1987 Aug;1(3):279–296. [PubMed] [Google Scholar]
- Duke R. C., Cohen J. J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986 Fall;5(4):289–299. [PubMed] [Google Scholar]
- Guba S. C., Stella G., Turka L. A., June C. H., Thompson C. B., Emerson S. G. Regulation of interleukin 3 gene induction in normal human T cells. J Clin Invest. 1989 Dec;84(6):1701–1706. doi: 10.1172/JCI114352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinchliffe J. R., Thorogood P. V. Genetic inhibition of mesenchymal cell death and the development of form and skeletal pattern in the limbs of talpid3 (ta3) mutant chick embryos. J Embryol Exp Morphol. 1974 Jun;31(3):747–760. [PubMed] [Google Scholar]
- Hodgson G. S., Bradley T. R. Properties of haematopoietic stem cells surviving 5-fluorouracil treatment: evidence for a pre-CFU-S cell? Nature. 1979 Oct 4;281(5730):381–382. doi: 10.1038/281381a0. [DOI] [PubMed] [Google Scholar]
- Kerr J. F., Harmon B., Searle J. An electron-microscope study of cell deletion in the anuran tadpole tail during spontaneous metamorphosis with special reference to apoptosis of striated muscle fibers. J Cell Sci. 1974 May;14(3):571–585. doi: 10.1242/jcs.14.3.571. [DOI] [PubMed] [Google Scholar]
- Koyasu S., Tojo A., Miyajima A., Akiyama T., Kasuga M., Urabe A., Schreurs J., Arai K., Takaku F., Yahara I. Interleukin 3-specific tyrosine phosphorylation of a membrane glycoprotein of Mr 150,000 in multi-factor-dependent myeloid cell lines. EMBO J. 1987 Dec 20;6(13):3979–3984. doi: 10.1002/j.1460-2075.1987.tb02740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Duddy S. K., Håkansson H., Orrenius S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Science. 1988 Oct 14;242(4876):256–259. doi: 10.1126/science.3262923. [DOI] [PubMed] [Google Scholar]
- Niemeyer C. M., Sieff C. A., Mathey-Prevot B., Wimperis J. Z., Bierer B. E., Clark S. C., Nathan D. G. Expression of human interleukin-3 (multi-CSF) is restricted to human lymphocytes and T-cell tumor lines. Blood. 1989 Mar;73(4):945–951. [PubMed] [Google Scholar]
- Palacios R., Karasuyama H., Rolink A. Ly1+ PRO-B lymphocyte clones. Phenotype, growth requirements and differentiation in vitro and in vivo. EMBO J. 1987 Dec 1;6(12):3687–3693. doi: 10.1002/j.1460-2075.1987.tb02702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Pressman B. C., Fahim M. Pharmacology and toxicology of the monovalent carboxylic ionophores. Annu Rev Pharmacol Toxicol. 1982;22:465–490. doi: 10.1146/annurev.pa.22.040182.002341. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Tarduchy G., López-Rivas A. Phorbol esters inhibit apoptosis in IL-2-dependent T lymphocytes. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1069–1075. doi: 10.1016/0006-291x(89)91778-6. [DOI] [PubMed] [Google Scholar]
- Schrader J. W., Clark-Lewis I., Crapper R. M., Wong G. W. P-cell stimulating factor: characterization, action on multiple lineages of bone-marrow-derived cells and role in oncogenesis. Immunol Rev. 1983;76:79–104. doi: 10.1111/j.1600-065x.1983.tb01098.x. [DOI] [PubMed] [Google Scholar]
- Schrader J. W. The panspecific hemopoietin of activated T lymphocytes (interleukin-3). Annu Rev Immunol. 1986;4:205–230. doi: 10.1146/annurev.iy.04.040186.001225. [DOI] [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987 Nov 15;139(10):3199–3206. [PubMed] [Google Scholar]
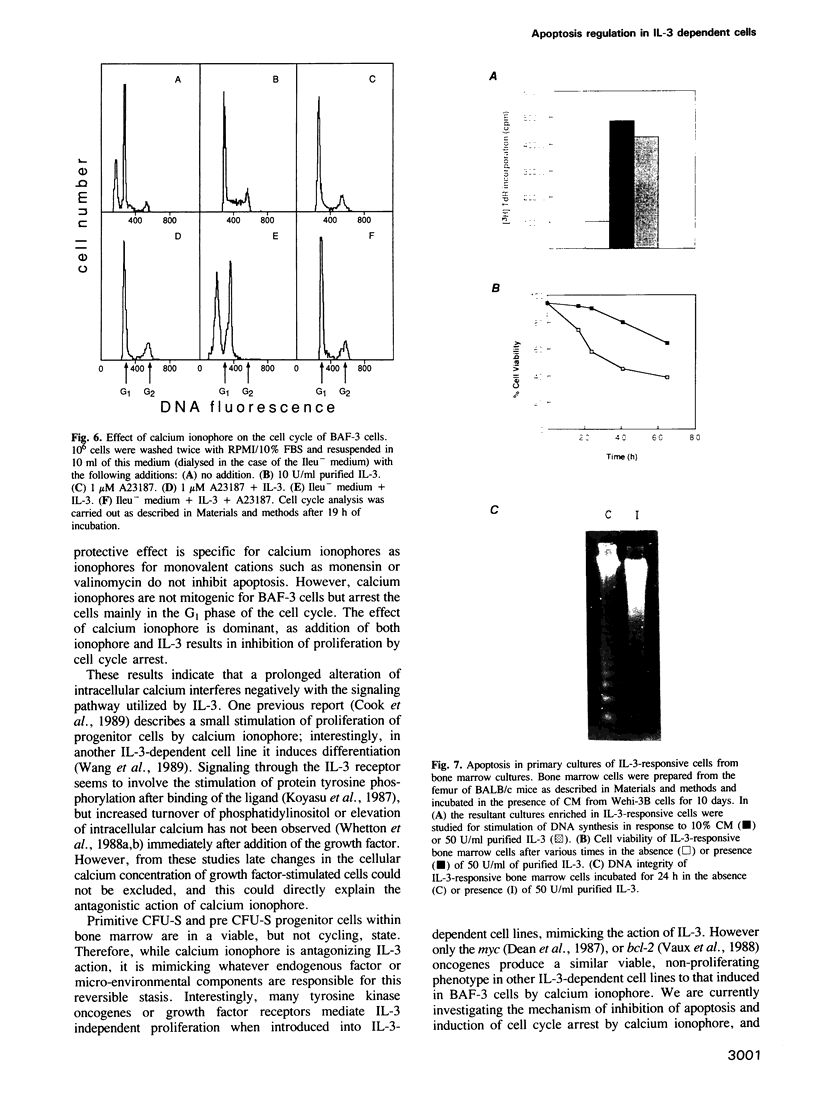
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Tojo A., Kasuga M., Urabe A., Takaku F. Vanadate can replace interleukin 3 for transient growth of factor-dependent cells. Exp Cell Res. 1987 Jul;171(1):16–23. doi: 10.1016/0014-4827(87)90247-3. [DOI] [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Van Zant G. Studies of hematopoietic stem cells spared by 5-fluorouracil. J Exp Med. 1984 Mar 1;159(3):679–690. doi: 10.1084/jem.159.3.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Wang H. M., Collins M., Arai K., Miyajima A. EGF induces differentiation of an IL-3-dependent cell line expressing the EGF receptor. EMBO J. 1989 Dec 1;8(12):3677–3684. doi: 10.1002/j.1460-2075.1989.tb08542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Bazill G. W., Dexter T. M. Haemopoietic cell growth factor mediates cell survival via its action on glucose transport. EMBO J. 1984 Feb;3(2):409–413. doi: 10.1002/j.1460-2075.1984.tb01821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Dexter T. M. Effect of haematopoietic cell growth factor on intracellular ATP levels. Nature. 1983 Jun 16;303(5918):629–631. doi: 10.1038/303629a0. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Monk P. N., Consalvey S. D., Huang S. J., Dexter T. M., Downes C. P. Interleukin 3 stimulates proliferation via protein kinase C activation without increasing inositol lipid turnover. Proc Natl Acad Sci U S A. 1988 May;85(10):3284–3288. doi: 10.1073/pnas.85.10.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetton A. D., Vallance S. J., Monk P. N., Cragoe E. J., Dexter T. M., Heyworth C. M. Interleukin-3-stimulated haemopoietic stem cell proliferation. Evidence for activation of protein kinase C and Na+/H+ exchange without inositol lipid hydrolysis. Biochem J. 1988 Dec 1;256(2):585–592. doi: 10.1042/bj2560585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T., Smith C. A., Spooncer E., Dexter T. M., Taylor D. R. Haemopoietic colony stimulating factors promote cell survival by suppressing apoptosis. Nature. 1990 Jan 4;343(6253):76–79. doi: 10.1038/343076a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980 Apr 10;284(5756):555–556. doi: 10.1038/284555a0. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]