Abstract
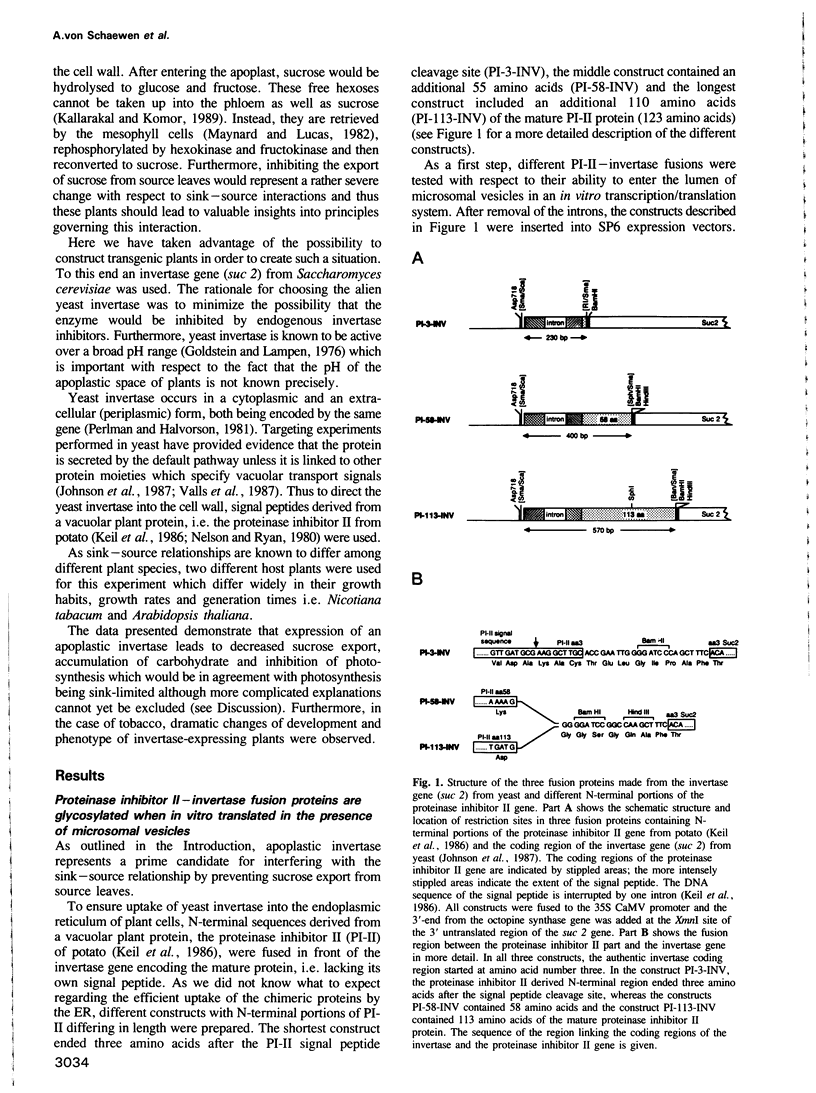
Chimeric genes consisting of the coding sequence of the yeast invertase gene suc 2 and different N-terminal portions of the potato-derived vacuolar protein proteinase inhibitor II fused to the 35S CaMV promoter and the poly-A site of the octopine synthase gene were transferred into tobacco and Arabidopsis thaliana plants using Agrobacterium based systems. Regenerated transgenic plants display a 50- to 500-fold higher invertase activity compared to non-transformed control plants. This invertase is N-glycosylated and efficiently secreted from the plant cell leading to its apoplastic location. Whereas expression of the invertase does not lead to drastic changes in transgenic Arabidopsis thaliana plants, transgenic tobacco plants show dramatic changes with respect to development and phenotype. Expression of the invertase leads to stunted growth due to reduction of internodal distances, to development of bleached and/or necrotic regions in older leaves and to suppressed root formation. In mature leaves, high levels of soluble sugars and starch accumulate. These carbohydrates do not show a diurnal turnover. The accumulation of carbohydrate is accompanied by an inhibition of photosynthesis, and in tobacco, by an increase in the rate of respiration. Measurements in bleached versus green areas of the same leaf show that the bleached section contains high levels of carbohydrates and has lower photosynthesis and higher respiration than green sections. It is concluded that expression of invertase in the cell wall interrupts export and leads to an accumulation of carbohydrates and inhibition of photosynthesis.
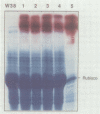
Full text
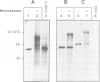
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azcón-Bieto J. Inhibition of photosynthesis by carbohydrates in wheat leaves. Plant Physiol. 1983 Nov;73(3):681–686. doi: 10.1104/pp.73.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carlson M., Osmond B. C., Botstein D. Mutants of yeast defective in sucrose utilization. Genetics. 1981 May;98(1):25–40. doi: 10.1093/genetics/98.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough J. M., Peet M. M., Kramer P. J. Effects of High Atmospheric CO(2) and Sink Size on Rates of Photosynthesis of a Soybean Cultivar. Plant Physiol. 1981 May;67(5):1007–1010. doi: 10.1104/pp.67.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm B., Schmidt R., Willmitzer L. Efficient transformation of Arabidopsis thaliana using direct gene transfer to protoplasts. Mol Gen Genet. 1989 May;217(1):6–12. doi: 10.1007/BF00330935. [DOI] [PubMed] [Google Scholar]
- Deblaere R., Bytebier B., De Greve H., Deboeck F., Schell J., Van Montagu M., Leemans J. Efficient octopine Ti plasmid-derived vectors for Agrobacterium-mediated gene transfer to plants. Nucleic Acids Res. 1985 Jul 11;13(13):4777–4788. doi: 10.1093/nar/13.13.4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorel C., Voelker T. A., Herman E. M., Chrispeels M. J. Transport of proteins to the plant vacuole is not by bulk flow through the secretory system, and requires positive sorting information. J Cell Biol. 1989 Feb;108(2):327–337. doi: 10.1083/jcb.108.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R. Effect of Rapid Changes in Sink-Source Ratio on Export and Distribution of Products of Photosynthesis in Leaves of Beta vulgaris L. and Phaseolus vulgaris L. Plant Physiol. 1980 Nov;66(5):945–949. doi: 10.1104/pp.66.5.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel O., Wang S. F. Determination of enzymatic activity in polyacrylamide gels. I. Enzymes catalyzing the conversion of nonreducing substrates to reducing products. Anal Biochem. 1969 Mar;27(3):545–554. doi: 10.1016/0003-2697(69)90068-2. [DOI] [PubMed] [Google Scholar]
- Goldstein A., Lampen J. O. Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Huber S. C. Biochemical Mechanism for Regulation of Sucrose Accumulation in Leaves during Photosynthesis. Plant Physiol. 1989 Oct;91(2):656–662. doi: 10.1104/pp.91.2.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höfgen R., Willmitzer L. Storage of competent cells for Agrobacterium transformation. Nucleic Acids Res. 1988 Oct 25;16(20):9877–9877. doi: 10.1093/nar/16.20.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. M., Bankaitis V. A., Emr S. D. Distinct sequence determinants direct intracellular sorting and modification of a yeast vacuolar protease. Cell. 1987 Mar 13;48(5):875–885. doi: 10.1016/0092-8674(87)90084-5. [DOI] [PubMed] [Google Scholar]
- Keil M., Sanchez-Serrano J., Schell J., Willmitzer L. Primary structure of a proteinase inhibitor II gene from potato (Solanum tuberosum). Nucleic Acids Res. 1986 Jul 25;14(14):5641–5650. doi: 10.1093/nar/14.14.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maynard J. W., Lucas W. J. Sucrose and Glucose Uptake into Beta vulgaris Leaf Tissues : A Case for General (Apoplastic) Retrieval Systems. Plant Physiol. 1982 Nov;70(5):1436–1443. doi: 10.1104/pp.70.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nafziger E. D., Koller H. R. Influence of Leaf Starch Concentration on CO(2) Assimilation in Soybean. Plant Physiol. 1976 Apr;57(4):560–563. doi: 10.1104/pp.57.4.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. E., Ryan C. A. In vitro synthesis of pre-proteins of vacuolar compartmented proteinase inhibitors that accumulate in leaves of wounded tomato plants. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1975–1979. doi: 10.1073/pnas.77.4.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Halvorson H. O. Distinct repressible mRNAs for cytoplasmic and secreted yeast invertase are encoded by a single gene. Cell. 1981 Aug;25(2):525–536. doi: 10.1016/0092-8674(81)90071-4. [DOI] [PubMed] [Google Scholar]
- Reiss B., Sprengel R., Schaller H. Protein fusions with the kanamycin resistance gene from transposon Tn5. EMBO J. 1984 Dec 20;3(13):3317–3322. doi: 10.1002/j.1460-2075.1984.tb02297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosahl S., Schell J., Willmitzer L. Expression of a tuber-specific storage protein in transgenic tobacco plants: demonstration of an esterase activity. EMBO J. 1987 May;6(5):1155–1159. doi: 10.1002/j.1460-2075.1987.tb02348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufty T. W., Huber S. C. Changes in Starch Formation and Activities of Sucrose Phosphate Synthase and Cytoplasmic Fructose-1,6-bisphosphatase in Response to Source-Sink Alterations. Plant Physiol. 1983 Jun;72(2):474–480. doi: 10.1104/pp.72.2.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasek T. W., Delucia E. H., Strain B. R. Reversibility of Photosynthetic Inhibition in Cotton after Long-Term Exposure to Elevated CO(2) Concentrations. Plant Physiol. 1985 Jul;78(3):619–622. doi: 10.1104/pp.78.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnewald U., von Schaewen A., Willmitzer L. Expression of mutant patatin protein in transgenic tobacco plants: role of glycans and intracellular location. Plant Cell. 1990 Apr;2(4):345–355. doi: 10.1105/tpc.2.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Bulpin P. V., ap Rees T. Pathway of starch breakdown in photosynthetic tissues of Pisum sativum. Biochim Biophys Acta. 1978 Nov 15;544(1):200–214. doi: 10.1016/0304-4165(78)90223-4. [DOI] [PubMed] [Google Scholar]
- Stitt M., Gerhardt R., Kürzel B., Heldt H. W. A role for fructose 2,6-bisphosphate in the regulation of sucrose synthesis in spinach leaves. Plant Physiol. 1983 Aug;72(4):1139–1141. doi: 10.1104/pp.72.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimble R. B., Maley F. Optimizing hydrolysis of N-linked high-mannose oligosaccharides by endo-beta-N-acetylglucosaminidase H. Anal Biochem. 1984 Sep;141(2):515–522. doi: 10.1016/0003-2697(84)90080-0. [DOI] [PubMed] [Google Scholar]
- Valls L. A., Hunter C. P., Rothman J. H., Stevens T. H. Protein sorting in yeast: the localization determinant of yeast vacuolar carboxypeptidase Y resides in the propeptide. Cell. 1987 Mar 13;48(5):887–897. doi: 10.1016/0092-8674(87)90085-7. [DOI] [PubMed] [Google Scholar]
- Van Haute E., Joos H., Maes M., Warren G., Van Montagu M., Schell J. Intergeneric transfer and exchange recombination of restriction fragments cloned in pBR322: a novel strategy for the reversed genetics of the Ti plasmids of Agrobacterium tumefaciens. EMBO J. 1983;2(3):411–417. doi: 10.1002/j.1460-2075.1983.tb01438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervliet G., Holsters M., Teuchy H., Van Montagu M., Schell J. Characterization of different plaque-forming and defective temperate phages in Agrobacterium. J Gen Virol. 1975 Jan;26(1):33–48. doi: 10.1099/0022-1317-26-1-33. [DOI] [PubMed] [Google Scholar]
- Voelker T. A., Herman E. M., Chrispeels M. J. In vitro mutated phytohemagglutinin genes expressed in tobacco seeds: role of glycans in protein targeting and stability. Plant Cell. 1989 Jan;1(1):95–104. doi: 10.1105/tpc.1.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]