Abstract
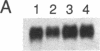
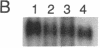
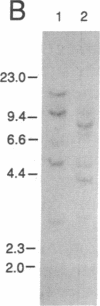
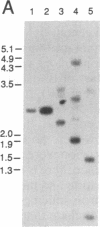
cDNA clones encoding proteins of approximately 18 kDa in which 83% of the amino acids are conserved relative to the published sequences of mammalian cyclophilin/rotamase (CyP) have been isolated from tomato, maize, and Brassica napus. In correspondence with the mammalian genes, but in contrast with the Neurospora gene and one yeast CyP gene, the plant CyP genes encode only mature proteins lacking transit peptides. RNA blot analyses demonstrate that CyP genes are expressed in all plant organs tested. Southern blots of genomic DNA indicate that there are small families (two to eight members) of CyP-related genes in maize and B. napus. A vector was constructed for expression of the tomato cDNA in E. coli. SDS/polyacrylamide gels show that extracts of appropriately induced cells harboring this vector contain nearly 40% of the protein as a single approximately 18-kDa band. While the majority of this protein is sequestered in insoluble inclusion bodies, the soluble extracts have higher levels of peptidyl-prolyl cis-trans isomerase (rotamase) activity than extracts of wild-type cells. This additional activity is sensitive to inhibition by the cyclic undecapeptide cyclosporin A.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsma D. J., Sylvester D. A Chinese hamster ovary cyclophilin cDNA sequence. Nucleic Acids Res. 1990 Jan 11;18(1):200–200. doi: 10.1093/nar/18.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächinger H. P. The influence of peptidyl-prolyl cis-trans isomerase on the in vitro folding of type III collagen. J Biol Chem. 1987 Dec 15;262(35):17144–17148. [PubMed] [Google Scholar]
- Crouse G. F., Frischauf A., Lehrach H. An integrated and simplified approach to cloning into plasmids and single-stranded phages. Methods Enzymol. 1983;101:78–89. doi: 10.1016/0076-6879(83)01006-x. [DOI] [PubMed] [Google Scholar]
- Dalgarno D. C., Harding M. W., Lazarides A., Handschumacher R. E., Armitage I. M. 1H NMR studies on bovine cyclophilin: preliminary structural characterization of this specific cyclosporin A binding protein. Biochemistry. 1986 Nov 4;25(22):6778–6784. doi: 10.1021/bi00370a008. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Dietmeier K., Tropschug M. Nucleotide sequence of a full-length cDNA coding for cyclophilin (peptidyl-prolyl cis-trans isomerase) of Saccharomyces cerevisiae. Nucleic Acids Res. 1990 Jan 25;18(2):373–373. doi: 10.1093/nar/18.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fischer G., Bang H., Berger E., Schellenberger A. Conformational specificity of chymotrypsin toward proline-containing substrates. Biochim Biophys Acta. 1984 Nov 23;791(1):87–97. doi: 10.1016/0167-4838(84)90285-1. [DOI] [PubMed] [Google Scholar]
- Fischer G., Bang H. The refolding of urea-denatured ribonuclease A is catalyzed by peptidyl-prolyl cis-trans isomerase. Biochim Biophys Acta. 1985 Mar 22;828(1):39–42. doi: 10.1016/0167-4838(85)90006-8. [DOI] [PubMed] [Google Scholar]
- Fischer G., Wittmann-Liebold B., Lang K., Kiefhaber T., Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989 Feb 2;337(6206):476–478. doi: 10.1038/337476a0. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Gasser C. S., Budelier K. A., Smith A. G., Shah D. M., Fraley R. T. Isolation of Tissue-Specific cDNAs from Tomato Pistils. Plant Cell. 1989 Jan;1(1):15–24. doi: 10.1105/tpc.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haendler B., Hofer-Warbinek R., Hofer E. Complementary DNA for human T-cell cyclophilin. EMBO J. 1987 Apr;6(4):947–950. doi: 10.1002/j.1460-2075.1987.tb04843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
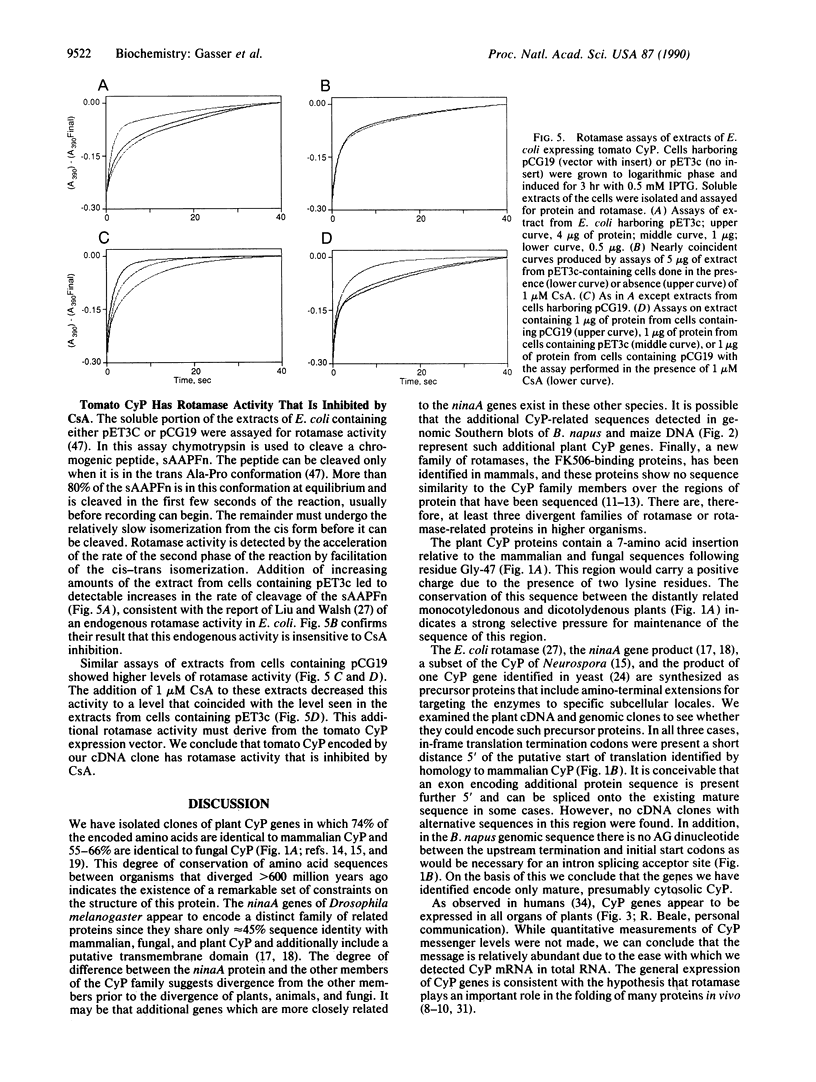
- Haendler B., Keller R., Hiestand P. C., Kocher H. P., Wegmann G., Movva N. R. Yeast cyclophilin: isolation and characterization of the protein, cDNA and gene. Gene. 1989 Nov 15;83(1):39–46. doi: 10.1016/0378-1119(89)90401-0. [DOI] [PubMed] [Google Scholar]
- Hait W. N., Harding M. W., Handschumacher R. E. Calmodulin, cyclophilin, and cyclosporin A. Science. 1986 Aug 29;233(4767):987–989. doi: 10.1126/science.3016900. [DOI] [PubMed] [Google Scholar]
- Handschumacher R. E., Harding M. W., Rice J., Drugge R. J., Speicher D. W. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984 Nov 2;226(4674):544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Galat A., Uehling D. E., Schreiber S. L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989 Oct 26;341(6244):758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Harding M. W., Handschumacher R. E. Cyclophilin, a primary molecular target for cyclosporine. Structural and functional implications. Transplantation. 1988 Aug;46(2 Suppl):29S–35S. doi: 10.1097/00007890-198808001-00006. [DOI] [PubMed] [Google Scholar]
- Harrison R. K., Stein R. L. Mechanistic studies of peptidyl prolyl cis-trans isomerase: evidence for catalysis by distortion. Biochemistry. 1990 Feb 20;29(7):1684–1689. doi: 10.1021/bi00459a003. [DOI] [PubMed] [Google Scholar]
- Harrison R. K., Stein R. L. Substrate specificities of the peptidyl prolyl cis-trans isomerase activities of cyclophilin and FK-506 binding protein: evidence for the existence of a family of distinct enzymes. Biochemistry. 1990 Apr 24;29(16):3813–3816. doi: 10.1021/bi00468a001. [DOI] [PubMed] [Google Scholar]
- Hasel K. W., Sutcliffe J. G. Nucleotide sequence of a cDNA coding for mouse cyclophilin. Nucleic Acids Res. 1990 Jul 11;18(13):4019–4019. doi: 10.1093/nar/18.13.4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heald S. L., Harding M. W., Handschumacher R. E., Armitage I. M. 1H NMR studies on bovine cyclophilin: preliminary structural characterization of its complex with cyclosporin A. Biochemistry. 1990 May 8;29(18):4466–4478. doi: 10.1021/bi00470a029. [DOI] [PubMed] [Google Scholar]
- Jenkins M. K., Schwartz R. H., Pardoll D. M. Effects of cyclosporine A on T cell development and clonal deletion. Science. 1988 Sep 23;241(4873):1655–1658. doi: 10.1126/science.241.4873.1655. [DOI] [PubMed] [Google Scholar]
- Kawamukai M., Matsuda H., Fujii W., Utsumi R., Komano T. Nucleotide sequences of fic and fic-1 genes involved in cell filamentation induced by cyclic AMP in Escherichia coli. J Bacteriol. 1989 Aug;171(8):4525–4529. doi: 10.1128/jb.171.8.4525-4529.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klee H. J., Muskopf Y. M., Gasser C. S. Cloning of an Arabidopsis thaliana gene encoding 5-enolpyruvylshikimate-3-phosphate synthase: sequence analysis and manipulation to obtain glyphosate-tolerant plants. Mol Gen Genet. 1987 Dec;210(3):437–442. doi: 10.1007/BF00327194. [DOI] [PubMed] [Google Scholar]
- Koletsky A. J., Harding M. W., Handschumacher R. E. Cyclophilin: distribution and variant properties in normal and neoplastic tissues. J Immunol. 1986 Aug 1;137(3):1054–1059. [PubMed] [Google Scholar]
- Koser P. L., Sylvester D., Livi G. P., Bergsma D. J. A second cyclophilin-related gene in Saccharomyces cerevisiae. Nucleic Acids Res. 1990 Mar 25;18(6):1643–1643. doi: 10.1093/nar/18.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lang K., Schmid F. X., Fischer G. Catalysis of protein folding by prolyl isomerase. Nature. 1987 Sep 17;329(6136):268–270. doi: 10.1038/329268a0. [DOI] [PubMed] [Google Scholar]
- Lang K., Schmid F. X. Protein-disulphide isomerase and prolyl isomerase act differently and independently as catalysts of protein folding. Nature. 1988 Feb 4;331(6155):453–455. doi: 10.1038/331453a0. [DOI] [PubMed] [Google Scholar]
- Lightowlers M. W., Haralambous A., Rickard M. D. Amino acid sequence homology between cyclophilin and a cDNA-cloned antigen of Echinococcus granulosus. Mol Biochem Parasitol. 1989 Oct;36(3):287–289. doi: 10.1016/0166-6851(89)90177-1. [DOI] [PubMed] [Google Scholar]
- Liu J., Albers M. W., Chen C. M., Schreiber S. L., Walsh C. T. Cloning, expression, and purification of human cyclophilin in Escherichia coli and assessment of the catalytic role of cysteines by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2304–2308. doi: 10.1073/pnas.87.6.2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Walsh C. T. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4028–4032. doi: 10.1073/pnas.87.11.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesniaux V. F., Schreier M. H., Wenger R. M., Hiestand P. C., Harding M. W., Van Regenmortel M. H. Cyclophilin binds to the region of cyclosporine involved in its immunosuppressive activity. Eur J Immunol. 1987 Sep;17(9):1359–1365. doi: 10.1002/eji.1830170921. [DOI] [PubMed] [Google Scholar]
- Rosenberg A. H., Lade B. N., Chui D. S., Lin S. W., Dunn J. J., Studier F. W. Vectors for selective expression of cloned DNAs by T7 RNA polymerase. Gene. 1987;56(1):125–135. doi: 10.1016/0378-1119(87)90165-x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneuwly S., Shortridge R. D., Larrivee D. C., Ono T., Ozaki M., Pak W. L. Drosophila ninaA gene encodes an eye-specific cyclophilin (cyclosporine A binding protein). Proc Natl Acad Sci U S A. 1989 Jul;86(14):5390–5394. doi: 10.1073/pnas.86.14.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh B. H., Stamnes M. A., Seavello S., Harris G. L., Zuker C. S. The ninaA gene required for visual transduction in Drosophila encodes a homologue of cyclosporin A-binding protein. Nature. 1989 Mar 2;338(6210):67–70. doi: 10.1038/338067a0. [DOI] [PubMed] [Google Scholar]
- Siekierka J. J., Hung S. H., Poe M., Lin C. S., Sigal N. H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989 Oct 26;341(6244):755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989 Feb 2;337(6206):473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Barthelmess I. B., Neupert W. Sensitivity to cyclosporin A is mediated by cyclophilin in Neurospora crassa and Saccharomyces cerevisiae. Nature. 1989 Dec 21;342(6252):953–955. doi: 10.1038/342953a0. [DOI] [PubMed] [Google Scholar]
- Tropschug M., Nicholson D. W., Hartl F. U., Köhler H., Pfanner N., Wachter E., Neupert W. Cyclosporin A-binding protein (cyclophilin) of Neurospora crassa. One gene codes for both the cytosolic and mitochondrial forms. J Biol Chem. 1988 Oct 5;263(28):14433–14440. [PubMed] [Google Scholar]
- Tropschug M. Nucleotide sequence of the gene coding for cyclophilin/peptidyl-prolyl cis-trans isomerase of Neurospora crassa. Nucleic Acids Res. 1990 Jan 11;18(1):190–190. doi: 10.1093/nar/18.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]