Abstract
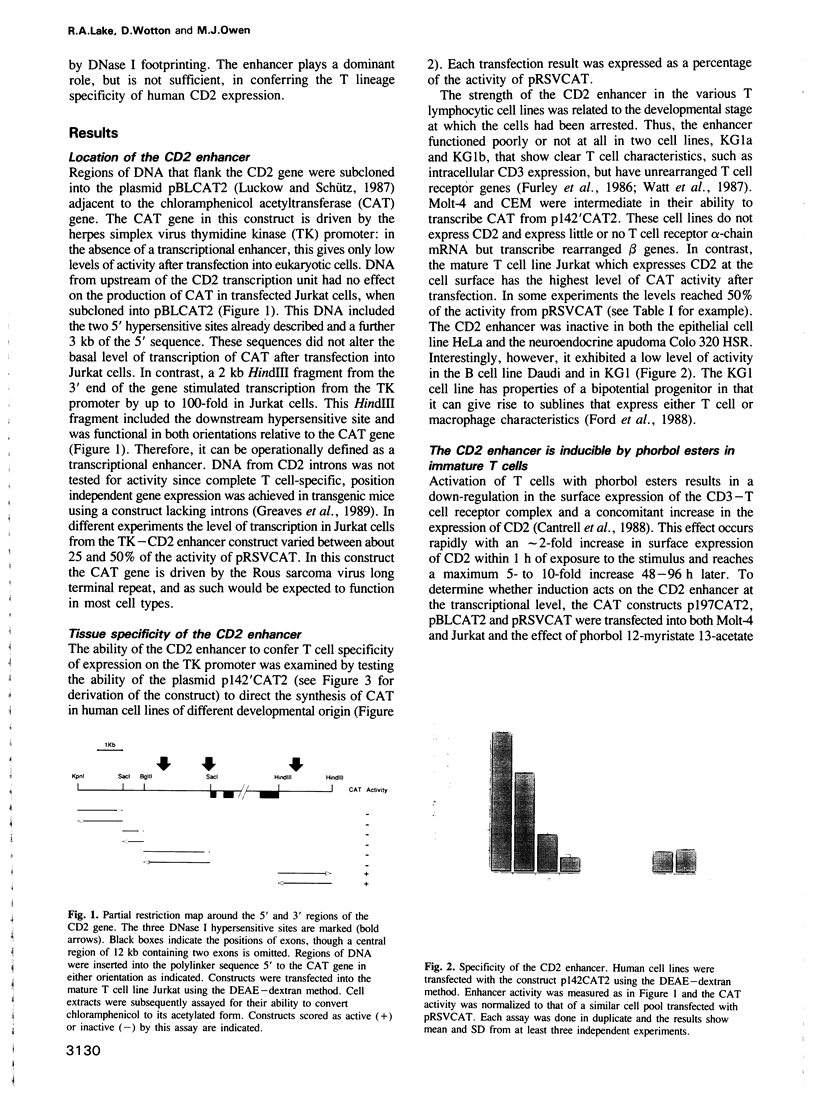
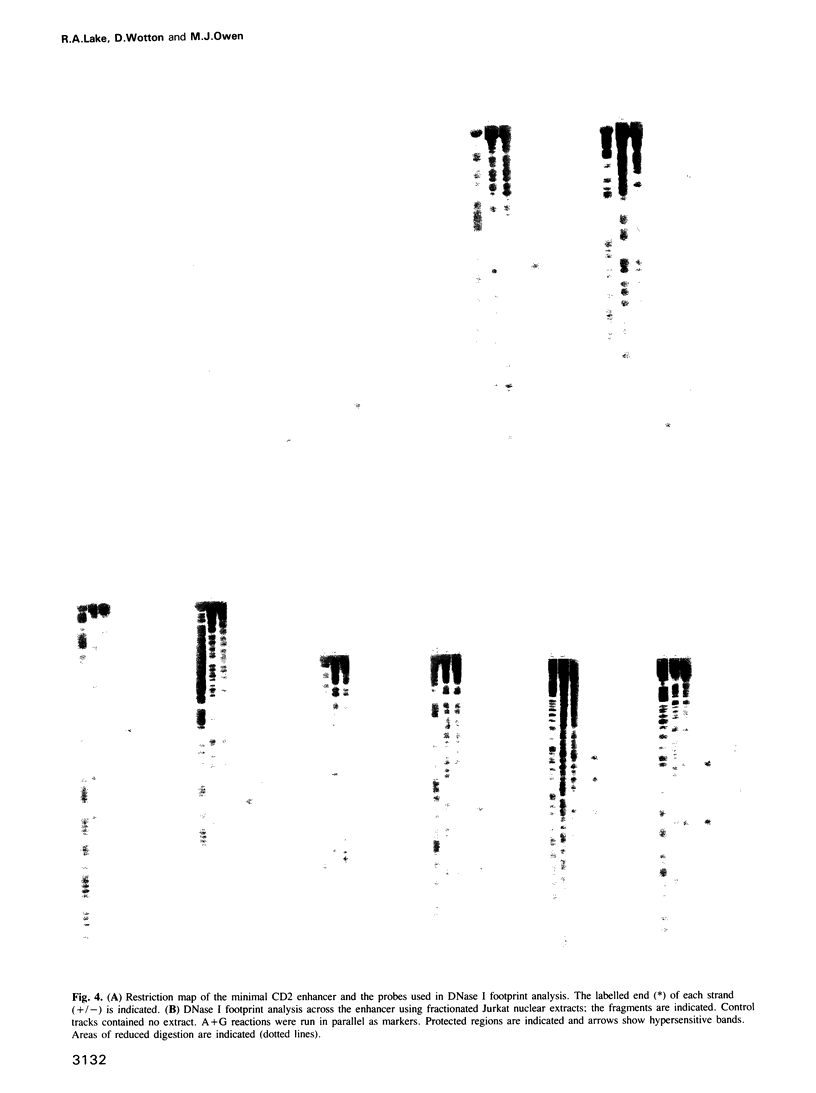
A strong lymphocyte-specific transcriptional enhancer was identified within a DNase I hypersensitive site at the 3' end of the human CD2 gene. Full activity, in a transient expression assay, was contained within a region of 550 bp (minimal enhancer). T cells which express CD2 could use the enhancer to activate transcription from the reporter gene chloramphenicol acetyltransferase in the context of a heterologous promoter. Lower levels of transcription were detected in non-CD2-expressing T cells and in B cells. In contrast, the enhancer did not function in the epithelial cell line HeLa or in Colo 320 HSR, a cell line of neuroendocrine origin. Low levels of enhancement were detectable from two core regions, which acted synergistically with other cis-acting sequences to generate the complete enhancer. DNase I footprinting studies identified six cis-acting sequences to which proteins bound. Five of these sequence motifs were novel; the sixth was a canonical cAMP response element. Topoisomerase II, and scaffold attachment region consensus sequences were also found within an A/T-rich area downstream of the minimal enhancer. Neither region was bound to the nuclear matrix. The CD2 enhancer is modular in structure, it is constructed of novel cis-acting sequences and it is a major component of the regulatory system that controls expression of the CD2 gene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi Y., Käs E., Laemmli U. K. Preferential, cooperative binding of DNA topoisomerase II to scaffold-associated regions. EMBO J. 1989 Dec 20;8(13):3997–4006. doi: 10.1002/j.1460-2075.1989.tb08582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altevogt P., Michaelis M., Kyewski B. Identical forms of the CD2 antigen expressed by mouse T and B lymphocytes. Eur J Immunol. 1989 Aug;19(8):1509–1512. doi: 10.1002/eji.1830190826. [DOI] [PubMed] [Google Scholar]
- Atchison M. L. Enhancers: mechanisms of action and cell specificity. Annu Rev Cell Biol. 1988;4:127–153. doi: 10.1146/annurev.cb.04.110188.001015. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby M., Liou H. C., Glimcher L. H. Differences in DNA sequence specificity among MHC class II X box binding proteins. J Immunol. 1989 Feb 1;142(3):1005–1014. [PubMed] [Google Scholar]
- Cantrell D. A., Verbi W., Davies A., Parker P., Crumpton M. J. Evidence that protein kinase C differentially regulates the human T lymphocyte CD2 and CD3 surface antigens. Eur J Immunol. 1988 Sep;18(9):1391–1396. doi: 10.1002/eji.1830180914. [DOI] [PubMed] [Google Scholar]
- Clevers H., Lonberg N., Dunlap S., Lacy E., Terhorst C. An enhancer located in a CpG-island 3' to the TCR/CD3-epsilon gene confers T lymphocyte-specificity to its promoter. EMBO J. 1989 Sep;8(9):2527–2535. doi: 10.1002/j.1460-2075.1989.tb08390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerill P. N., Yuen M. H., Garrard W. T. The enhancer of the immunoglobulin heavy chain locus is flanked by presumptive chromosomal loop anchorage elements. J Biol Chem. 1987 Apr 15;262(11):5394–5397. [PubMed] [Google Scholar]
- Diamond D. J., Clayton L. K., Sayre P. H., Reinherz E. L. Exon-intron organization and sequence comparison of human and murine T11 (CD2) genes. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1615–1619. doi: 10.1073/pnas.85.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Ford A. M., Watt S. M., Furley A. J., Molgaard H. V., Greaves M. F. Cell lineage specificity of chromatin configuration around the immunoglobulin heavy chain enhancer. EMBO J. 1988 Aug;7(8):2393–2399. doi: 10.1002/j.1460-2075.1988.tb03084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furley A. J., Reeves B. R., Mizutani S., Altass L. J., Watt S. M., Jacob M. C., van den Elsen P., Terhorst C., Greaves M. F. Divergent molecular phenotypes of KG1 and KG1a myeloid cell lines. Blood. 1986 Nov;68(5):1101–1107. [PubMed] [Google Scholar]
- Georgopoulos K., van den Elsen P., Bier E., Maxam A., Terhorst C. A T cell-specific enhancer is located in a DNase I-hypersensitive area at the 3' end of the CD3-delta gene. EMBO J. 1988 Aug;7(8):2401–2407. doi: 10.1002/j.1460-2075.1988.tb03085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves D. R., Wilson F. D., Lang G., Kioussis D. Human CD2 3'-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989 Mar 24;56(6):979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- Gross D. S., Garrard W. T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Ho I. C., Yang L. H., Morle G., Leiden J. M. A T-cell-specific transcriptional enhancer element 3' of C alpha in the human T-cell receptor alpha locus. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6714–6718. doi: 10.1073/pnas.86.17.6714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoun M., Martin P. J., Hansen J. A., Brown M. A., Siadak A. W., Nowinski R. C. Identification of a human T lymphocyte surface protein associated with the E-rosette receptor. J Exp Med. 1981 Jan 1;153(1):207–212. doi: 10.1084/jem.153.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper B., Jackson P. D., Felsenfeld G. Protein-binding sites within the 5' DNase I-hypersensitive region of the chicken alpha D-globin gene. Mol Cell Biol. 1987 Jun;7(6):2059–2069. doi: 10.1128/mcb.7.6.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G., Wotton D., Owen M. J., Sewell W. A., Brown M. H., Mason D. Y., Crumpton M. J., Kioussis D. The structure of the human CD2 gene and its expression in transgenic mice. EMBO J. 1988 Jun;7(6):1675–1682. doi: 10.1002/j.1460-2075.1988.tb02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loc P. V., Strätling W. H. The matrix attachment regions of the chicken lysozyme gene co-map with the boundaries of the chromatin domain. EMBO J. 1988 Mar;7(3):655–664. doi: 10.1002/j.1460-2075.1988.tb02860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckow B., Schütz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987 Jul 10;15(13):5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Mirkovitch J., Mirault M. E., Laemmli U. K. Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell. 1984 Nov;39(1):223–232. doi: 10.1016/0092-8674(84)90208-3. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M. J., Jenkinson E. J., Brown M. H., Sewell W. A., Krissansen G. W., Crumpton M. J., Owen J. J. Murine CD2 gene expression during fetal thymus ontogeny. Eur J Immunol. 1988 Jan;18(1):187–189. doi: 10.1002/eji.1830180129. [DOI] [PubMed] [Google Scholar]
- Ptashne M. Gene regulation by proteins acting nearby and at a distance. Nature. 1986 Aug 21;322(6081):697–701. doi: 10.1038/322697a0. [DOI] [PubMed] [Google Scholar]
- Ptashne M. How eukaryotic transcriptional activators work. Nature. 1988 Oct 20;335(6192):683–689. doi: 10.1038/335683a0. [DOI] [PubMed] [Google Scholar]
- Sewell W. A., Brown M. H., Dunne J., Owen M. J., Crumpton M. J. Molecular cloning of the human T-lymphocyte surface CD2 (T11) antigen. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8718–8722. doi: 10.1073/pnas.83.22.8718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson R. T. Nucleosome positioning can affect the function of a cis-acting DNA element in vivo. Nature. 1990 Jan 25;343(6256):387–389. doi: 10.1038/343387a0. [DOI] [PubMed] [Google Scholar]
- Sleigh M. J. A nonchromatographic assay for expression of the chloramphenicol acetyltransferase gene in eucaryotic cells. Anal Biochem. 1986 Jul;156(1):251–256. doi: 10.1016/0003-2697(86)90180-6. [DOI] [PubMed] [Google Scholar]
- Stief A., Winter D. M., Strätling W. H., Sippel A. E. A nuclear DNA attachment element mediates elevated and position-independent gene activity. Nature. 1989 Sep 28;341(6240):343–345. doi: 10.1038/341343a0. [DOI] [PubMed] [Google Scholar]
- To R. Q., Kmiec E. B. Assembly of transcriptionally active chromatin in vitro: a possible role for topoisomerase II. Cell Growth Differ. 1990 Jan;1(1):39–45. [PubMed] [Google Scholar]
- Watt S. M., Karhi K., Gatter K., Furley A. J., Katz F. E., Healy L. E., Altass L. J., Bradley N. J., Sutherland D. R., Levinsky R. Distribution and epitope analysis of the cell membrane glycoprotein (HPCA-1) associated with human hemopoietic progenitor cells. Leukemia. 1987 May;1(5):417–426. [PubMed] [Google Scholar]
- Wotton D., Flanagan B. F., Owen M. J. Chromatin configuration of the human CD2 gene locus during T-cell development. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4195–4199. doi: 10.1073/pnas.86.11.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagita H., Nakamura T., Karasuyama H., Okumura K. Monoclonal antibodies specific for murine CD2 reveal its presence on B as well as T cells. Proc Natl Acad Sci U S A. 1989 Jan;86(2):645–649. doi: 10.1073/pnas.86.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]






