Abstract
Purpose
Ocular and systemic measurement and imaging of the macular carotenoids lutein and zeaxanthin have been employed extensively as potential biomarkers of AMD risk. In this study, we systematically compare dual wavelength retinal autofluorescence imaging (AFI) of macular pigment with skin resonance Raman spectroscopy (RRS) and serum carotenoid levels in a clinic-based population.
Methods
Eighty-eight patients were recruited from retina and general ophthalmology practices from a tertiary referral center and excluded only if they did not have all three modalities tested, had a diagnosis of macular telangiectasia (MacTel) or Stargardt disease, or had poor AFI image quality. Skin, macular, and serum carotenoid levels were measured by RRS, AFI, and HPLC, respectively.
Results
Skin RRS measurements and serum zeaxanthin concentrations correlated most strongly with AFI macular pigment volume under the curve (MPVUC) measurements up to 9° eccentricity relative to MPVUC or rotationally averaged macular pigment optical density (MPOD) measurements at smaller eccentricities. These measurements were reproducible and not significantly affected by cataracts. We also found that these techniques could readily identify subjects taking oral carotenoid-containing supplements.
Conclusions
Larger macular pigment volume AFI and skin RRS measurements are noninvasive, objective, and reliable methods to assess ocular and systemic carotenoid levels. They are an attractive alternative to psychophysical and optical methods that measure MPOD at a limited number of eccentricities. Consequently, skin RRS and MPVUC at 9° are both reasonable biomarkers of macular carotenoid status that could be readily adapted to research and clinical settings.
Keywords: macular pigment, carotenoids, macula
Three carotenoids, lutein, zeaxanthin, and their metabolite, meso-zeaxanthin, are concentrated within the retina to form the yellow spot centered at the fovea known as the macula lutea. These diet-derived xanthophylls are thought to filter the more deleterious blue wavelengths of light and scavenge free radicals to reduce reactive oxygen species damage to host retinal tissue.1–3 Epidemiologic data have suggested that individuals with lower concentrations of serum carotenoids and lower macular pigment optical density (MPOD) are at an increased risk of developing AMD.1,4,5 The Age-Related Eye Disease Study 2 (AREDS2) trial reported that nutritional supplementation with lutein and zeaxanthin can reduce risk of progression to advanced AMD, and more recent prospective data have further supported this notion by showing that people with diets high in these specific carotenoids have a reduced risk of developing advanced AMD compared with age-matched controls.6–8 Thus, there has been considerable interest in developing rapid and reliable noninvasive methods to quantify and/or image ocular and systemic carotenoid status as potential biomarkers for assessing presymptomatic risk of developing AMD and for monitoring the effect of dietary and supplement interventions in the clinic and in clinical trials.9
If one wishes to measure carotenoid levels and distributions in the human macula, multiple methods are available.1,9,10 High-performance liquid chromatography analysis of macular carotenoids is quantitative and chemically specific, but it is time-consuming to perform, has low spatial resolution, and is tissue destructive. Heterochromatic flicker photometry (HFP) and other related psychophysical methods have been commonly used to measure MPOD noninvasively at one or a few foveal eccentricities, but HFP requires substantial subject training and time to yield reproducible results and provides very limited spatial information on macular pigment distributions. Imaging-based methods have been introduced as an alternative to HFP because they can provide high-resolution quantitative spatial distributions of the macular carotenoids using reflectometry, autofluorescence attenuation, or resonance Raman spectroscopy (RRS). These methods can be rapid and enlist minimal patient cooperation beyond fixating on a target, but they have typically required nonstandardized, custom-built laboratory equipment. Recently, two ophthalmic instrument manufacturers have added macular pigment measurement to their commercial imaging platforms. A dual wavelength autofluorescence technique based on the Heidelberg Spectralis correlated well with HFP, while a reflectometry technique based on the Zeiss VisuCam exhibited significant discordance with HFP.11
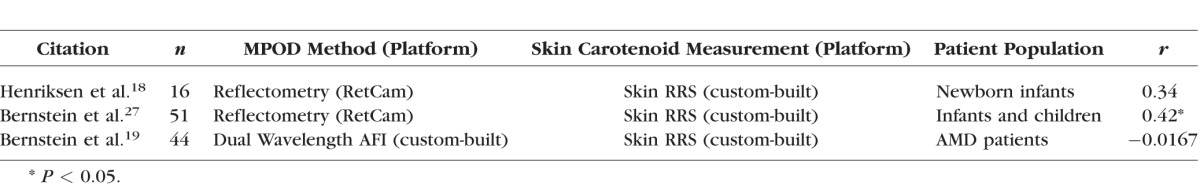
As an alternative to ocular assessment, some researchers have looked at systemic measures of carotenoid status, including HPLC analysis of serum samples, dietary surveys, and noninvasive measurement of skin carotenoids by resonance Raman spectroscopy. Serum HPLC analysis is chemically specific, but it requires a blood draw and time-consuming extractions and analyses. Dietary assessments are tedious, have limited databases, and are subject to recall errors and biases.12 Skin Raman requires specialized equipment and cannot readily distinguish between the various carotenoids, but it is rapid, painless, and correlates well with HPLC total serum and biopsied tissue concentrations, and moderately well with dietary surveys.13,14 Unfortunately, serum and skin carotenoid measurements have not correlated particularly well with assessments of ocular carotenoid status (Tables 1, 2),15–28 and dietary correlations have fared even worse. In the published literature, although these correlations with macular pigment may be statistically significant, the r values are typically very low. None of these correlation studies used the newer imaging methods of macular pigment measurement, however. Because the Spectralis autofluorescence attenuation method is highly reproducible and provides a wealth of quantitative and spatial data,29 we hypothesized that macular and systemic biomarkers of carotenoid status may be more strongly correlated than previously appreciated, and we conducted a prospective, clinic-based study to test this hypothesis.
Table 1.
Comparisons of Macular Pigment and Serum Carotenoid Concentrations Published Since 2001
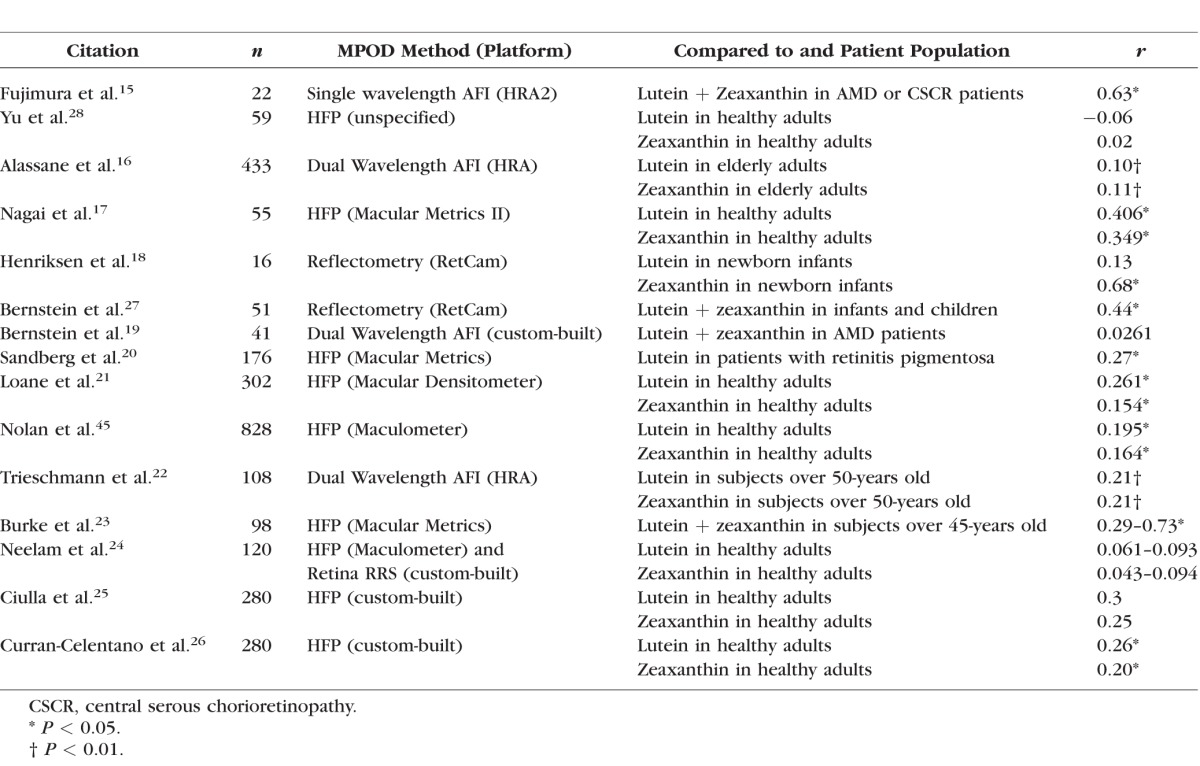
Table 2.
Comparisons of Macular Pigment and Skin Carotenoid Measurements Published Since 2001
Methods
Study Subjects
Subjects were recruited from retinal and general ophthalmology practices of a tertiary referral center under institutional review board approval and underwent evaluation of skin, macular, and serum carotenoid levels after informed consent had been obtained. This study complied with the Declaration of Helsinki and all relevant Health Insurance and Portability Act of 1996 regulations throughout its duration. Patients were excluded if they did not have all three modalities tested (serum carotenoids by HPLC; YMC, Inc., Allentown, PA, USA; skin resonance Raman spectroscopy [RRS]; and Spectralis autofluorescence imaging [AFI; Heidelberg Engineering GmbH, Heidelberg, Germany]), if they had a clinical diagnosis known to be associated with abnormal macular pigment distributions or levels such as macular telangiectasia type II (MacTel) or albinism,30,31 or if they had ocular conditions associated with severe disturbances of macular autofluorescence, which would make AFI measurement of macular pigment unreliable (i.e., Stargardt disease or bilateral central geographic atrophy due to AMD). At the time of recruitment, patients were asked to answer a short survey assessing smoking status, supplement use, and family history of macular degeneration, but no dietary surveys were performed.
Macular Pigment Imaging
Macular pigment imaging by dual wavelength AFI was performed on a Heidelberg MultiColor Spectralis as previously described.11 After pupil dilation, the subject's macula was raster scanned over 30° centered on the fovea by alternating blue and green laser light (485.6 and 516.7 nm, respectively) for approximately 30 seconds while AFIs of RPE lipofuscin for each excitation wavelength were collected and averaged. Autofluorescence detection was restricted to wavelengths above 530 nm with the help of a barrier filter. Specialized software then performed digital subtraction of the green excitation AFI from the blue excitation AFI using a correction factor to account for the fact that the blue excitation is not quite at the peak of macular pigment absorption (460 nm) and that there is still a substantial amount of macular pigment absorbance with the green excitation. The instrument's effective extinction coefficients, Kmp(Λ), are 0.789 for 485.6 nm and 0.205 for 516.7 nm, and the correction factor is: 1/[Kmp(485.6) − Kmp(516.7)] = 1.71, based on the image processing method described by Delori and colleagues32 using the macular pigment extinction coefficients calculated by Stockman and Sharp.33 In order to compensate for background signal, an offset parameter (“OFF”) is subtracted. This value is recorded by the system internally during the acquisition of the blue/green AFI with the lasers turned off.
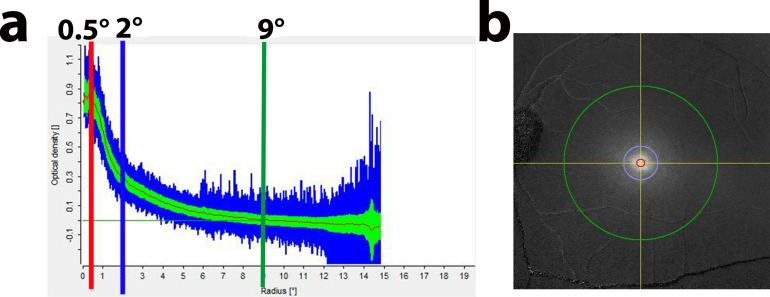
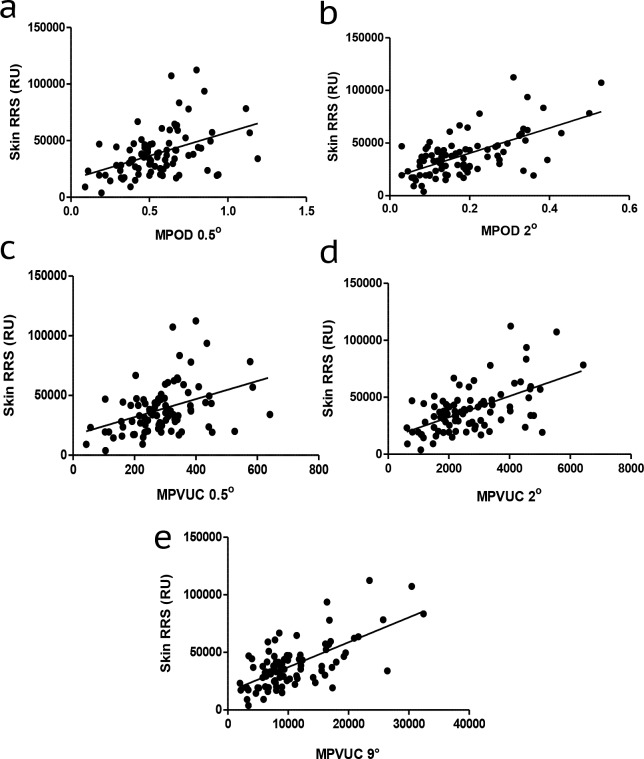
A subtracted macular pigment autofluorescence attenuation image is produced showing a white region centered on the fovea corresponding to the macular carotenoid pigment (Fig. 1). The instrument calculates the average MPOD, SD, and range of MPOD levels along a series of concentric one-pixel width circles. The results are then plotted on a graph from 0° to 15° with a red curve corresponding to the average MPOD at each eccentricity, a green region corresponding to the SD of the average MPOD, and a blue region corresponding to the high and low range of MPOD. The user must choose a reference eccentricity where the MPOD is defined as zero. We chose 9.0° because the vast majority of subjects had near baseline measurements at this distance from the fovea, and readings beyond this eccentricity would likely be affected by retinal vasculature or the optic nerve, typically manifested as an increase of SD and range at eccentricities beyond 9°. For the instrument's automated results table, the user not only selects the zero point radius (green vertical line; 233 pixels at 9°; “plateau” column on the report) but can also choose two other analysis eccentricities. We routinely used 0.5° (red vertical line; 12 pixel radii at 0.47°) and 2° (blue vertical line; 51 pixel radii at 1.99°). The most important parameters from the report that we used for our analyses were the “average OD on radius,” which we report as “MPOD X°” (macular pigment optical density at X°) corresponding to the 360° averaged MPOD at that particular radius/eccentricity and “OD sum of volumes,” which we report as “macular pigment volume under the curve at X°” (MPVUCX°), which is the integral of the total MPOD within X° of the fovea and should correspond to the total macular pigment within that particular region always using 9° as the reference eccentricity.
Figure 1.
Macular pigment tracing from a healthy subject. (a) Macular pigment tracing at 0.5° (red), 2° (blue), and 9° (green) demarcated by the solid lines as indicated. (b) Macular pigment image showing the fovea and the degrees (0.5°, red; 2°, blue; 9°, green) from the center of the macula lutea.
Skin Carotenoid Measurements
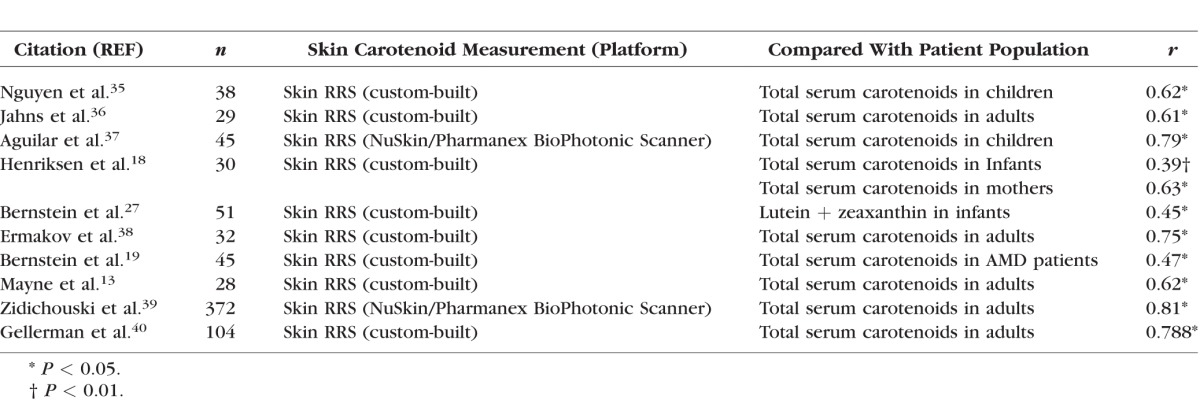
Total skin carotenoid levels were measured by RRS using a laboratory-grade instrument with extended sensitivity and range to facilitate quantitative measurements of individuals with very high or very low skin carotenoid levels.34 Resonance Raman spectroscopy has been used extensively in several nutritional epidemiology studies and is considered an excellent noninvasive biomarker of fruit and vegetable intake and has been validated by skin biopsy studies to correspond well with skin carotenoid content measured by HPLC13 and serum carotenoid levels (Table 3).13,18,19,27,35–40 After daily calibration, a 488-nm blue laser light illuminates a small patch of the subject's palm for approximately 30 seconds. Back-scattered light is collected, and a holographic notch filter rejects Rayleigh-scattered light. The remaining fluorescence- and Raman-shifted light is then analyzed using a Peltier-cooled spectrograph. The peak height/intensity of the characteristic C=C vibration of carotenoids at approximately 1525 cm−1 (reported as Raman units [RU]) is directly proportional to the tissue's carotenoid content and can be converted to microgram carotenoid levels per gram of tissue, as previously described.41 Each subject had three independent skin RRS measurements, which were then averaged.
Table 3.
Comparisons of Skin and Serum Carotenoid Concentrations Published Since 2001
Serum Carotenoid Levels
Serum carotenoid concentrations were quantified by HPLC on a C30 column as previously described.42 Because this was a clinic-based study, subjects were not fasting, but due to carotenoids' long serum and tissue half-lives, variation in serum levels throughout the day is considered minimal.
Statistical Analyses
Both eyes were measured, and data were averaged whenever possible unless the fellow eye could not be imaged properly (i.e., only one eye dilated, macular hole, macular scar). The data were then analyzed by linear regression using GraphPad Prism (La Jolla, CA, USA) with a P less than 0.05 considered statistically significant.
Results
Study Population and Method Validation
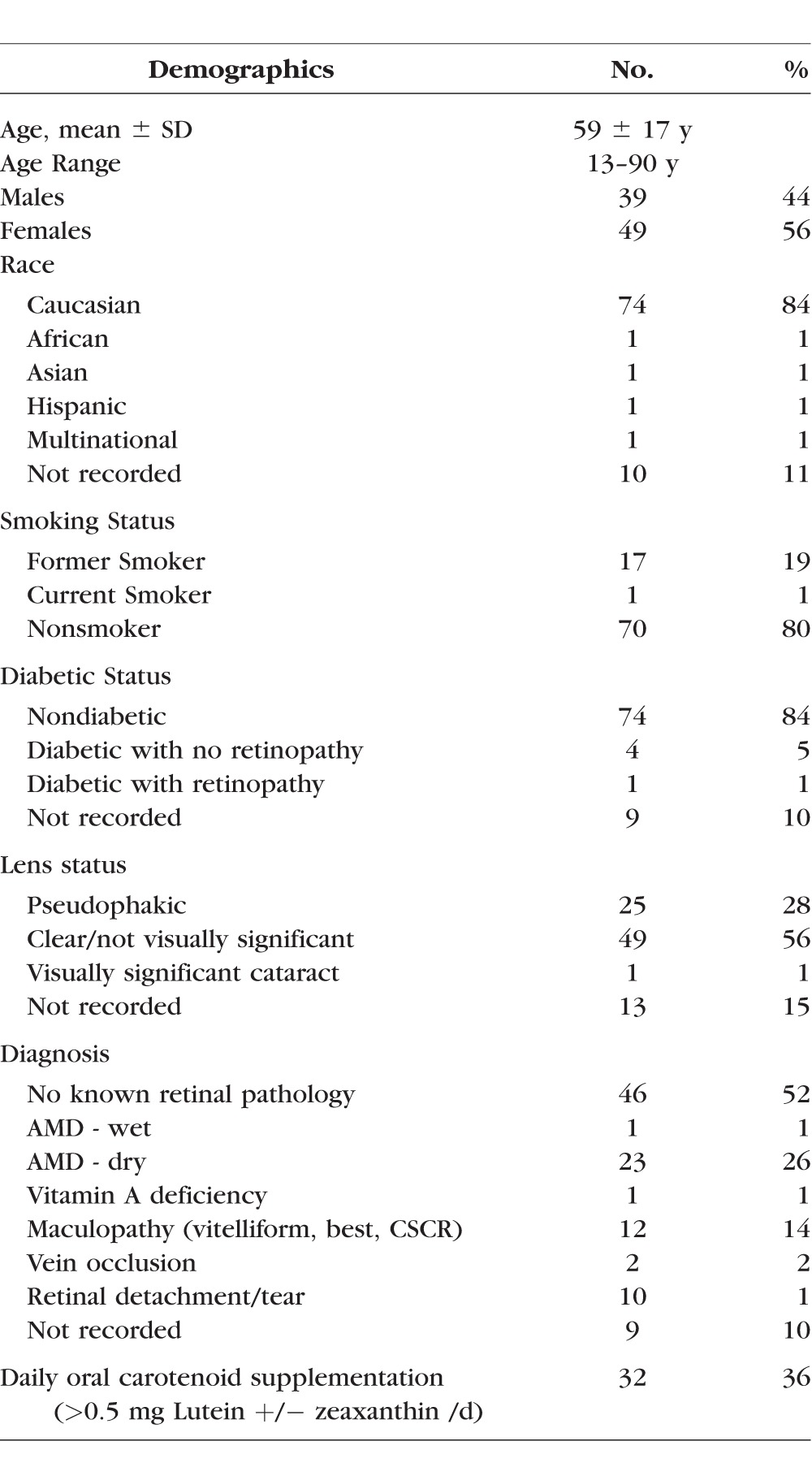
From retinal and general ophthalmology practices at the Moran Eye Center, 88 subjects were recruited for the study between June 2015 and April 2016 (Table 4). We tried to be as inclusive as possible and specifically excluded only those subjects with conditions known to be associated with anomalous macular pigment levels or distributions such as MacTel, macular holes, or albinism, or with bilateral conditions likely to produce unanalyzable AFI images, such as foveal geographic atrophy or Stargardt disease. Normal exams were had by 52%, and 48% had ongoing retinal disease. Regularly consumed oral supplements containing greater than 0.5 mg/day of lutein and/or zeaxanthin was reported by 36%.
Table 4.
Demographics of Study Subjects
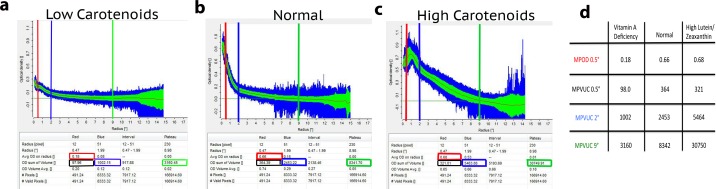
We immediately noted a wide range of macular pigment profiles for our subjects. Figure 2 shows two extreme patients relative to a healthy unsupplemented individual. The subject on the left had self-induced vitamin A deficiency (serum retinol concentration of 0.09 mg/L; normal 0.30–1.20 mg/L), and had some of the lowest macular pigment readings that we have ever recorded along with very low skin RRS levels (3788 RU) and nearly undetectable total serum carotenoids (30.67 ng/mL), yet he still had a detectable but low central peak of macular pigment (MPVUC 9° of 3413). The subject on the right had exceedingly high levels of daily carotenoid intake (20 mg of lutein supplement per day and a spinach, avocado, broccoli, and kale smoothie for breakfast each day), and was recently reported as the first case of crystalline maculopathy associated with high-dose lutein consumption.43 Her macular pigment, skin, and serum total carotenoid levels were among the highest we have ever recorded (MPVUC 9° of 30,482, 107,339 RU, and 5029 ng/mL, respectively). Of note, her macular pigment profile exhibits a “central dip,”44,45 a feature seen in 19 members of our study population. Although her MPOD 0.5° is nearly identical to the unsupplemented normal, her MPVUC 9° is 3.7 times higher than his and exhibits a much broader distribution that appears to extend past the 9° zero eccentricity.
Figure 2.
Extremes of macular pigment. A vitamin A–deficient subject (a) was compared with a healthy subject not taking any carotenoid supplements (b) and a patient consuming an excessive amount of carotenoids from her diet and supplements (c) to emphasize differences in MPOD and MPVUC measurements at various eccentricities as indicated in (d).
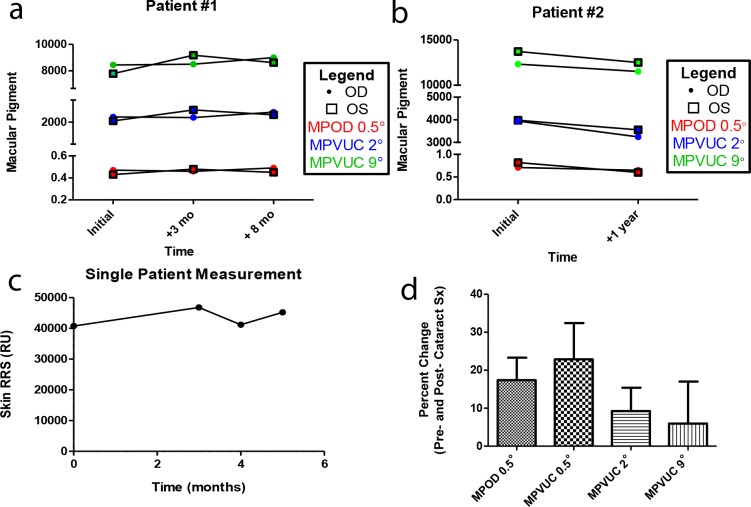
Two subjects underwent multiple macular pigment scans over a year showing excellent bilateral stability and reproducibility of values (Figs. 3a, 3b). Resonance Raman spectroscopy skin measurements in a third subject were followed over 6 months and remained within 15% of the original reading (Fig. 3c). Due to concern that optical opacities within the visual axis may reduce accuracy and attenuate signals of macular pigment AFI,46–48 four patients underwent evaluation of macular pigment pre- and postcataract surgery. Visually significant cataracts did attenuate MPOD and MPVUC at 0.5° by approximately 20%, while MPVUC at 2° and 9° were attenuated by 8% and 5%, respectively (Fig. 3d).
Figure 3.
Reproducibility of RRS and macular pigment and the effect of cataracts. (a, b) MPOD at 0.5° (red) and MPVUC at 2° (blue) and 9° (green) were followed over a year period in two individuals with independent recordings for each eye plotted. The readings showed very consistent results over time and between eyes. (c) Another patient had repeat measurements of skin carotenoids over a 6-month time-period and were relatively consistent as well. (d) Four patients underwent macular pigment evaluation pre- and postcataract surgery. The percent change between these two measurements is shown with MPOD at 0.5° and MPVUC at 0.5°, 2°, and 9°. Red points, MPOD at 0.5°; Blue points, MPVUC at 2°; Green points, MPVUC at 9°.
Correlations of Macular, Skin, and Serum Carotenoids
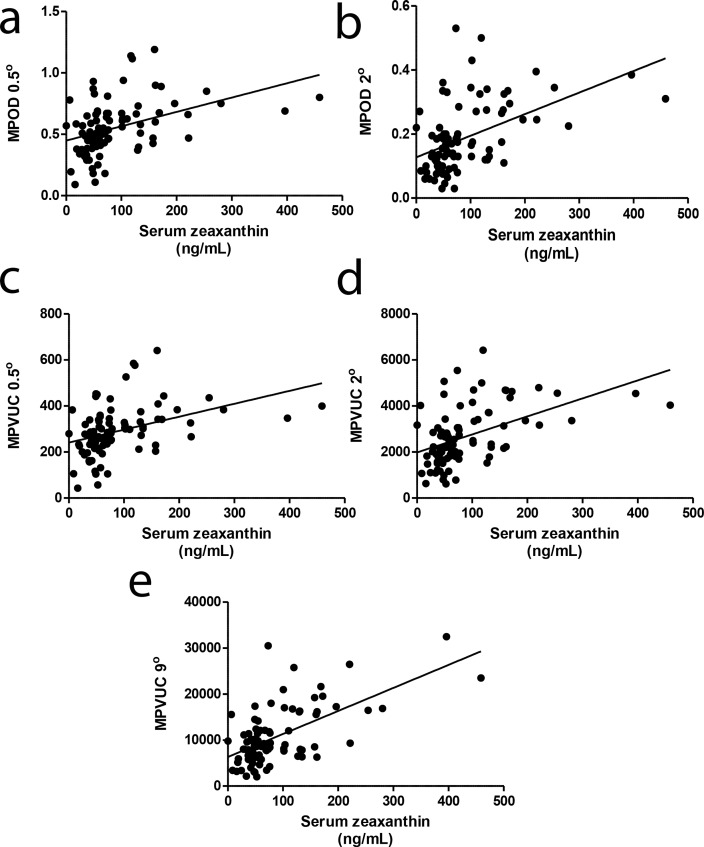
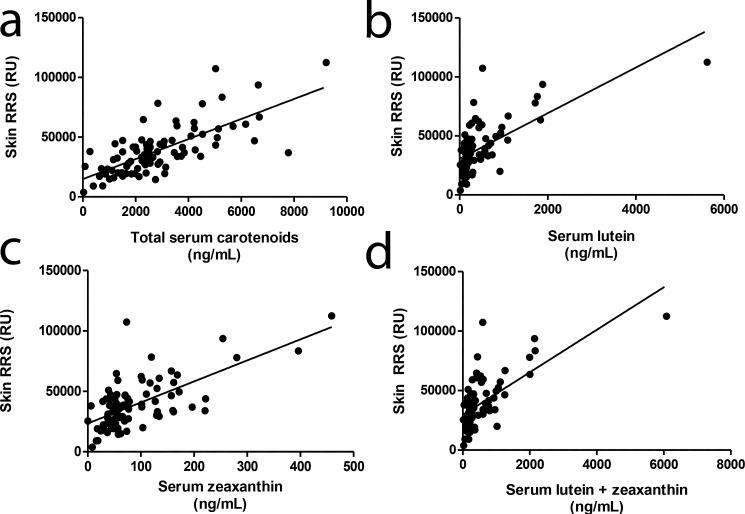
We hypothesized that volume-integrated measurements would more closely correlate with serum and skin measurements of carotenoid status than commonly used MPOD measurements at single eccentricities due to volume integration's estimation of total carotenoid content of the macula rather than MPOD measurements that may be taken on a steep slope of the macular pigment profile. We first compared correlation coefficients for our study population's MPOD at 0.5° and 2° and MPVUC at 0.5°, 2°, and 9° with serum lutein, zeaxanthin, lutein + zeaxanthin, and total carotenoids (Table 5). We found the strongest correlations with MPVUC at 9.0° with serum zeaxanthin and weaker correlations with MPOD and MPVUC at 0.5° and 2° with any of the serum carotenoid measurements. Regression plots of the various macular pigment measures versus serum zeaxanthin are shown in Figure 4. Next, we compared skin RRS with serum lutein, zeaxanthin, lutein + zeaxanthin, and total carotenoids (Table 5, Fig. 5), and not surprisingly, we found the best correlations with total serum carotenoids because skin RRS is driven by the diverse array of carotenoids found in the skin, not just lutein and zeaxanthin. Finally, we compared skin RRS with the various measures of macular pigment, and we found the rank order of correlations to be MPVUC 9° > MPOD 2° > MPVUC 2° > MPOD 0.5° > MPVUC 0.5° (Table 5, Fig. 6).
Table 5.
R Values of Macular, Skin, and Serum Carotenoid Comparisons by Linear Regression*
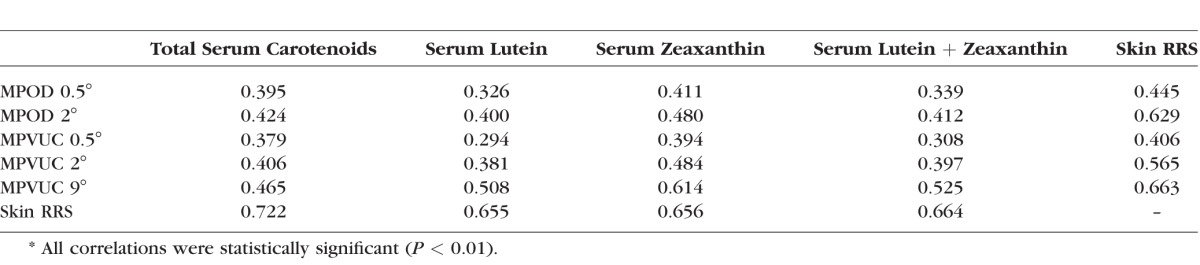
Figure 4.
Comparison of macular pigment and serum zeaxanthin. Linear regression analyses of serum zeaxanthin with (a) MPOD at 0.5° (r = 0.411, P < 0.0001), or (b) MPOD at 2° (r = 0.480, P < 0.0001). (c) Macular pigment volume under the curve at 0.5° (r = 0.394, P = 0.0001), (d) MPVUC at 2° (r = 0.484, P < 0.0001), and (e) MPVUC at 9° (r = 0.614, P < 0.0001). All serum concentrations of carotenoids are in nanograms per milliliter in (a–e).
Figure 5.
Comparison of skin RRS and serum carotenoid concentrations. Resonance Raman spectroscopy was compared with (a) total serum carotenoids (r = 0.722, P < 0.0001) and (b) lutein (r = 0.655, P < 0.0001), (c) zeaxanthin (r = 0.656, P < 0.0001), and (d) lutein + zeaxanthin (r = 0.664, P < 0.0001). All serum concentrations of carotenoids are in nanograms per milliliter in (a–d).
Figure 6.
Comparison of skin RRS and macular pigment. Resonance Raman spectroscopy was compared with (a) MPOD at 0.5° (r = 0.445, P < 0.0001) and (b) 2° (r = 0.629, P < 0.0001) and (c) MPVUC at 0.5° (r = 0.406, P < 0.0001), and (d) 2° (r = 0.565, P < 0.0001), and (e) 9° (r = 0.663, P < 0.0001).
Effect of Oral Carotenoid Supplementation on Macula, Skin, and Serum Carotenoid Levels
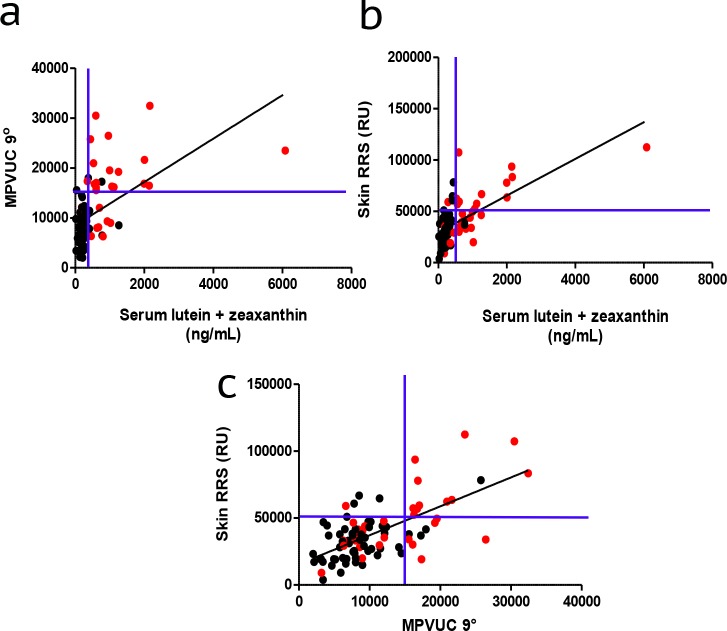
Prior reports have shown that carotenoid concentrations in serum, skin, and the macula can be readily impacted by sustained periods of oral supplements with lutein and/or zeaxanthin.49,50 Based on examination of our previously presented data (Figs. 2–6), we defined supranormal carotenoid levels as serum lutein + zeaxanthin above 500 ng/mL, skin RRS readings above 50,000 RU, and MPVUC 9° greater than 15,000. Patients who self-reported long-term use of supplements containing lutein and/or zeaxanthin represented 16 of 17 patients with serum concentrations of lutein + zeaxanthin above 500 ng/mL and MPVUC 9° greater than 15,000. Furthermore, 12 of 12 patients with serum concentrations of lutein + zeaxanthin above 500 ng/mL and RRS values above 50,000 were on oral supplementation (Figs. 7a, 7b). Additionally, 11 of 12 subjects with the highest skin RRS and MPVUC 9° were on oral supplementation (Fig. 7c). Unusually high serum lutein + zeaxanthin concentrations were more likely to be found in subjects who reported oral supplementation (24 subjects) compared with supranormal macular pigment (21 subjects) or skin RRS (17 subjects; Figs. 7a, 7b).
Figure 7.
Effect of oral supplementation on skin, serum, and macular carotenoids. (a, b) Linear regression plots of MPVUC 9° and skin RRS were plotted against serum lutein + zeaxanthin concentrations. (c) Skin RRS and MPVUC 9° were then compared. Red data points represent those patients reporting daily consumption of supplements containing >0.5 mg of lutein and/or zeaxanthin. Blue lines delineate the empirically defined borders between normal and supranormal measurements. Serum carotenoid concentrations are in nanograms per milliliters.
Discussion
Serum levels of carotenoids are commonly used biomarkers for ocular carotenoid status that have been employed in many epidemiologic and cross-sectional studies of eye disease, such as the Eye Disease Case-Control Study Group and AREDS2,19,51 but blood draws are invasive and HPLC analysis is tedious and expensive, and until now, the correlations of blood carotenoid concentrations and macular pigment levels have been generally unimpressive (Table 1). In this study, correlations of macular pigment levels measured by the Heidelberg Spectralis dual wavelength autofluorescence attenuation technique with serum carotenoid concentrations were in the 0.3 to 0.6 range and were always statistically significant. Whether for lutein, zeaxanthin, lutein + zeaxanthin, or total carotenoids, MPVUC 9° always gave the strongest correlations, demonstrating the potential importance of capturing all of the carotenoid content of the macula rather than focusing on the MPOD at just one or a few eccentricities, as has been commonly done in the past. The discrepancy of these two measurements approaches (MPOD versus MPVUC) is epitomized by the carotenoid-supplemented patient in Figure 2 with extraordinarily high MPVUC levels at 9°, but with a nearly identical MPOD at 0.5° relative to an unsupplemented control. It was also interesting that serum zeaxanthin consistently had the strongest correlations with MPOD and MPVUC at all eccentricities. We have seen this before in newborn infants when we measured central MPOD with reflectometry, and this is consistent with the foveal predominance of zeaxanthin relative to the broader distribution of lutein in the retina.18
Skin RRS measurement is a convenient and validated method to measure systemic carotenoid status (Table 3), but correlations with ocular carotenoid status performed by others and us have been disappointingly nonsignificant in the past in adults (Table 2). In this study, skin once again correlated strongly with serum carotenoids, especially when total serum carotenoids were considered (r = 0.722). This makes sense because skin RRS is driven by the ensemble of serum carotenoids that are nonspecifically deposited in the skin. Possibly as a result of the superior reproducibility of Spectralis AFI imaging of macular pigment relative to other macular pigment methods, our current study found excellent correlations with skin RRS, with the strongest correlation once again with MPVUC 9° (r = 0.663).
Self-reported supplementation with lutein and/or zeaxanthin is very common in this Utah clinic-based population whether or not they actually had significant AMD. Our results show that higher levels of carotenoids in their serum, skin, and macula can readily identify these individuals. These results are certainly consistent with the hypothesis that supplementation with lutein and/or zeaxanthin can positively influence serum and tissue levels of carotenoids, but it should be noted that many subjects who claimed to be on supplements had serum and tissue levels comparable to unsupplemented subjects and that a few unsupplemented subjects had serum and/or tissue levels in the supranormal range. This implies that high levels of serum and tissue carotenoids can be achieved through diet alone and that some individuals may have genetic factors that either inhibit or enhance carotenoid uptake into the serum, skin, and macula. This has been supported by prior trials in which patients did not respond to lutein supplementation alone, but required all three macular carotenoids.22,52,53
Macular pigment and skin RRS readings could be useful methods to help detect nutritional deficiencies and excesses. The lone vitamin A–deficient subject had some of the lowest macular pigment and skin carotenoid readings of any patient within the study. Unfortunately, vitamin serology is not widely accessible worldwide, and most deficiency identifications are based on clinical diagnosis alone. With vitamin A deficiency the leading cause of preventable blindness in children worldwide and subclinical rates higher than 30% in Southeast Asia,54 it would be of interest to use noninvasive skin and macular pigment levels with portable devices to help identify subclinical cases of malnutrition in the developing world to reduce the rates of this debilitating disease process and to monitor response to treatment. Conversely, more carotenoid supplements are not always better, as evidenced by recent reports from our group of crystalline maculopathies associated with high-dose lutein and zeaxanthin supplementation,42,43 and noninvasive skin and ocular carotenoid assessments can readily identify individuals with exceedingly high levels far above the population mean levels who may want to be less aggressive with their carotenoid supplementation.
Ongoing research continues to define the clinical role of oral carotenoid supplementation for prevention of ocular disease and improvement of visual performance. The AREDS2 trial showed that supplementation with 10 mg/day of lutein and 2 mg/day of zeaxanthin reduced the risk of AMD progression and that they are a recommended alternative to the 15 mg/day of β-carotene in the original AREDS formulation, especially in current and former smokers.8 Likewise, consumption of foods high in these carotenoids lowers the risk of developing early AMD and progressing to advanced AMD.55,56 Additionally, oral supplementation readily impacts carotenoid concentrations in the eye and the body, and human eyes with the highest quartile of macular pigment have an 82% lower risk of having AMD compared with those in the lowest quartile.57,58 A recent randomized trial noted an improvement in visual acuity in those given 10 mg of lutein compared with controls, with improvements most evident in those patients with lower baseline serum concentrations of the carotenoid.59 This has been corroborated in a larger randomized, controlled clinical trial evaluating the effect of oral supplementation in subjects with low macular pigment concentrations and no retinal disease.53 Furthermore, it is clear that not all oral supplementation is benign or even equal, as evidenced by the increased risk of lung cancer in smokers with β-carotene and the wide variations in carotenoid concentrations in various over-the-counter supplements.7,60 Consequently, accurate, noninvasive in vivo techniques such as RRS and/or MPVUC could be used to identify and stratify those patients requiring additional supplementation to potentially reduce the number of new patients with AMD, decrease progression of those with the disease, and avoid putting patients at undue risk. Moreover, these biomarkers could be used to monitor response to oral supplementation and identify target carotenoid concentrations for patients once a normalized database could be created.
The strengths of our study include the fact that we recruited from a diverse clinic-based population with minimal exclusion criteria. This allowed to us find robust, reproducible correlations between serum, skin, and ocular biomarkers of carotenoid status that generally exceeded previously reported correlations, and we are confident in our conclusion that supplementation with lutein and zeaxanthin positively influences these values in a wide variety of patients. Our experience with the Heidelberg Spectralis as a macular pigment measurement device was quite positive overall. Image acquisition and processing was rapid and reproducible, and we could easily identify and exclude subjects with poor quality scans manifest by unusually high macular pigment SDs and ranges at eccentricities close to the fovea. Unlike some previous reports that used earlier generation equipment from Heidelberg with less sophisticated image acquisition and detectors, we found that visually significant cataracts did not suppress macular pigment measurements excessively, especially when we used MPVUC 9°. Although AFI of macular pigment can be done in a single-wavelength mode, especially in healthy subjects,61,62 we noted that that dual-wavelength measurements allowed for reproducible macular pigment images in the face of significant ocular pathology, such as diabetic macular edema and exudative AMD. Wide-scale adoption of this technique will be limited, however, by the equipment's high cost, the need for pupil dilation, its bright light levels, and current lack of Food and Drug Administration approval of its analytical software. Thus, we were encouraged when we found that skin RRS had surprisingly strong correlations with serum and macular carotenoid measurements. Although this technique looks at total skin carotenoids and not just lutein and zeaxanthin, skin carotenoid measurement devices can be made in a portable and inexpensive manner suitable for widespread clinical and research use.63
This study was unable to assess the ability of skin RRS and/or MPVUC to detect and/or predict retinal pathology, such as AMD due to sample size, and it was not designed to assess the influence of diet and discrete supplements on various biomarkers of carotenoid status because dietary surveys and comprehensive supplement histories were beyond the scope of this clinic-based study and this technology cannot distinguish between individual carotenoids. In the present study, we have included a diverse population with multiple pathologies present, but future studies will likely require a normative database to be created much like those for macular optical coherence tomography and retinal nerve fiber layer thickness. Future, larger-scale prospective studies could then be specifically designed to answer whether skin RRS and/or MPVUC can be used to identify patients at risk of developing AMD and whether clinical intervention studies designed to alter the course of the disease can use these methods to monitor compliance and to assess response to carotenoid-based interventions.
Methods for noninvasive assessment of carotenoid status have evolved considerably over the past few decades, and our results show that the current generation of autofluorescence-based ocular imaging systems and resonance Raman skin devices are well-suited for research studies and even routine use in busy clinical practice settings. Skin and ocular measurements could be completed within a matter of minutes after their ophthalmologists had finished their dilated eye examinations, and we found that these measurements were reproducible and only minimally influenced by visually significant cataracts, especially when using macular pigment volume measurement as opposed to MPOD. Using these techniques, we could readily identify subjects at the extremes of carotenoid intake ranging from self-induced vitamin A deficiency from near-complete avoidance of all fruit and vegetable consumption to extraordinarily high daily intake of lutein from diet and supplements that resulted in crystalline deposits in the fovea. We look forward to more widespread use of macular pigment imaging and skin carotenoid assessments in future clinical trials, and in clinical management of AMD and other ocular diseases.
Acknowledgments
Presented at the annual meeting of the Association for Research in Vision and Ophthalmology, Seattle, Washington, United States, May 2016.
P.S. Bernstein, W. Gellermann, and the University of Utah hold patents for resonance Raman spectroscopic measurement of carotenoids in retina, skin, and other tissues.
Supported by National Institutes of Health (EY11600 and EY14800; Bethesda. MD, USA), and an unrestricted grant from Research to Prevent Blindness, Inc. (New York, NY, USA) to the Department of Ophthalmology & Visual Sciences, University of Utah (Salt Lake City, UT, USA).
Disclosure: C.D. Conrady, None; J.P. Bell, None; B.M. Besch, None; A. Gorusupudi, None; K. Farnsworth, None; I. Ermakov, None; M. Sharifzadeh, None; M. Ermakova, None; W. Gellermann, P; P.S. Bernstein, P
References
- 1. Bernstein PS,, Li B,, Vachali PP,, et al. Lutein, zeaxanthin, and meso-zeaxanthin: the basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016; 50: 34–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Junghans A,, Sies H,, Stahl W. Macular pigments lutein and zeaxanthin as blue light filters studied in liposomes. Arch Biochem Biophys. 2001; 391: 160–164. [DOI] [PubMed] [Google Scholar]
- 3. Nilsson SE,, Sundelin SP,, Wihlmark U,, Brunk UT. Aging of cultured retinal pigment epithelial cells: oxidative reactions, lipofuscin formation and blue light damage. Doc Ophthalmol. 2003; 106: 13–16. [DOI] [PubMed] [Google Scholar]
- 4. Raman R,, Biswas S,, Vaitheeswaran K,, Sharma T. Macular pigment optical density in wet age-related macular degeneration among Indians. Eye (Lond). 2012; 26: 1052–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sabour-Pickett S,, Nolan JM,, Loughman J,, Beatty S. A review of the evidence germane to the putative protective role of the macular carotenoids for age-related macular degeneration. Mol Nutr Food Res. 2012; 56: 270–286. [DOI] [PubMed] [Google Scholar]
- 6. Wu J,, Cho E,, Willett WC,, et al. Intakes of lutein, zeaxanthin, and other carotenoids and age-related macular degeneration during 2 decades of prospective follow-up. JAMA Ophthalmol. 2015; 133: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Age-Related Eye Disease Study 2 Research Group Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013; 309: 2005–2015. [DOI] [PubMed] [Google Scholar]
- 8. Chew EY, Clemons TE, Sangiovanni JP, et al. ; for the Age-Related Eye Disease Study 2 Research Group. . Secondary analyses of the effects of lutein/zeaxanthin on age-related macular degeneration progression: AREDS2 report No. 3. JAMA Ophthalmol. 2014; 132: 142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernstein PS,, Delori FC,, Richer S,, et al. The value of measurement of macular carotenoid pigment optical densities and distributions in age-related macular degeneration and other retinal disorders. Vision Res. 2010; 50: 716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Creuzot-Garcher C,, Koehrer P,, Picot C,, et al. Comparison of two methods to measure macular pigment optical density in healthy subjects. Invest Ophthalmol Vis Sci. 2014; 55: 2941–2946. [DOI] [PubMed] [Google Scholar]
- 11. Dennison JL,, Stack J,, Beatty S,, Nolan JM. Concordance of macular pigment measurements obtained using customized heterochromatic flicker photometry, dual-wavelength autofluorescence, and single-wavelength reflectance. Exp Eye Res. 2013; 116: 190–198. [DOI] [PubMed] [Google Scholar]
- 12. Natarajan L,, Flatt SW,, Sun X,, et al. Validity and systematic error in measuring carotenoid consumption with dietary self-report instruments. Am J Epidemiol. 2006; 163: 770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayne ST,, Cartmel B,, Scarmo S,, et al. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr. 2010; 92: 794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ermakov IV,, Ermakova MR,, McClane RW,, Gellermann W. Resonance Raman detection of carotenoid antioxidants in living human tissues. Opt Lett. 2001; 26: 1179–1181. [DOI] [PubMed] [Google Scholar]
- 15. Fujimura S,, Ueda K,, Nomura Y,, Yanagi Y. Preliminary analysis of the relationship between serum lutein and zeaxanthin levels and macular pigment optical density. Clin Ophthalmol. 2016; 10: 2149–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alassane S,, Binquet C,, Cottet V,, et al. Relationships of macular pigment optical density with plasma lutein, zeaxanthin, and diet in an elderly population: the Montrachet Study. Invest Ophthalmol Vis Sci. 2016; 57: 1160–1167. [DOI] [PubMed] [Google Scholar]
- 17. Nagai N,, Izumi-Nagai K,, Suzuki M,, et al. Association of macular pigment optical density with serum concentration of oxidized low-density lipoprotein in healthy adults. Retina. 2015; 35: 820–826. [DOI] [PubMed] [Google Scholar]
- 18. Henriksen BS,, Chan G,, Hoffman RO,, et al. Interrelationships between maternal carotenoid status and newborn infant macular pigment optical density and carotenoid status. Invest Ophthalmol Vis Sci. 2013; 54: 5568–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bernstein PS,, Ahmed F,, Liu A,, et al. Macular pigment imaging in AREDS2 participants: an ancillary study of AREDS2 subjects enrolled at the Moran Eye Center. Invest Ophthalmol Vis Sci. 2012; 53: 6178–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sandberg MA,, Johnson EJ,, Berson EL. The relationship of macular pigment optical density to serum lutein in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2010; 51: 1086–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loane E,, Nolan JM,, Beatty S. The respective relationships between lipoprotein profile, macular pigment optical density, and serum concentrations of lutein and zeaxanthin. Invest Ophthalmol Vis Sci. 2010; 51: 5897–5905. [DOI] [PubMed] [Google Scholar]
- 22. Trieschmann M,, Beatty S,, Nolan JM,, et al. Changes in macular pigment optical density and serum concentrations of its constituent carotenoids following supplemental lutein and zeaxanthin: the LUNA study. Exp Eye Res. 2007; 84: 718–728. [DOI] [PubMed] [Google Scholar]
- 23. Burke JD,, Curran-Celentano J,, Wenzel AJ. Diet and serum carotenoid concentrations affect macular pigment optical density in adults 45 years and older. J Nutr. 2005; 135: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 24. Neelam K,, O'Gorman N,, Nolan J,, et al. Measurement of macular pigment: Raman spectroscopy versus heterochromatic flicker photometry. Invest Ophthalmol Vis Sci. 2005; 46: 1023–1032. [DOI] [PubMed] [Google Scholar]
- 25. Ciulla TA,, Curran-Celantano J,, Cooper DA,, et al. Macular pigment optical density in a midwestern sample. Ophthalmology. 2001; 108: 730–737. [DOI] [PubMed] [Google Scholar]
- 26. Curran-Celentano J,, Hammond BR, Jr,, Ciulla TA,, et al. Relation between dietary intake, serum concentrations, and retinal concentrations of lutein and zeaxanthin in adults in a Midwest population. Am J Clin Nutr. 2001; 74: 796–802. [DOI] [PubMed] [Google Scholar]
- 27. Bernstein PS,, Sharifzadeh M,, Liu A,, et al. Blue-light reflectance imaging of macular pigment in infants and children. Invest Ophthalmol Vis Sci. 2013; 54: 4034–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yu J,, Zhou H,, Zhao M,, Cui L,, Liu N. Relationship between macular pigment optical density and serum concentration of lutein and zeaxanthin in an adult population. Chinese Journal of Ocular and Fundus Disease 2016; 32. [Google Scholar]
- 29. You QS,, Bartsch DU,, Espina M,, et al. Reproducibility of macular pigment optical density measurement by two-wavelength autofluorescence in a clinical setting. Retina. 2016; 36: 1381–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Degli Esposti S,, Egan C,, Bunce C,, et al. Macular pigment parameters in patients with macular telangiectasia (MacTel) and normal subjects: implications of a novel analysis. Invest Ophthalmol Vis Sci. 2012; 53: 6568–6575. [DOI] [PubMed] [Google Scholar]
- 31. Wolfson Y,, Fletcher E,, Strauss RW,, Scholl HP. Evidence of macular pigment in the central macula in albinism. Exp Eye Res. 2016; 145: 468–471. [DOI] [PubMed] [Google Scholar]
- 32. Delori FC,, Goger DG,, Hammond BR,, et al. Macular pigment density measured by autofluorescence spectrometry: comparison with reflectometry and heterochromatic flicker photometry. J Opt Soc Am A Opt Image Sci Vis. 2001; 18: 1212–1230. [DOI] [PubMed] [Google Scholar]
- 33. Stockman A,, Sharpe LT. The spectral sensitivities of the middle- and long-wavelength-sensitive cones derived from measurements in observers of known genotype. Vision Res. 2000; 40: 1711–1737. [DOI] [PubMed] [Google Scholar]
- 34. Ermakov IV,, Sharifzadeh M,, Ermakova M,, Gellermann W. Resonance Raman detection of carotenoid antioxidants in living human tissue. J Biomed Opt. 2005; 10: 064028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nguyen LM,, Scherr RE,, Linnell JD,, et al. Evaluating the relationship between plasma and skin carotenoids and reported dietary intake in elementary school children to assess fruit and vegetable intake. Arch Biochem Biophys. 2015; 572: 73–80. [DOI] [PubMed] [Google Scholar]
- 36. Jahns L,, Johnson LK,, Mayne ST,, et al. Skin and plasma carotenoid response to a provided intervention diet high in vegetables and fruit: uptake and depletion kinetics. Am J Clin Nutr. 2014; 100: 930–937. [DOI] [PubMed] [Google Scholar]
- 37. Aguilar SS,, Wengreen HJ,, Lefevre M,, et al. Skin carotenoids: a biomarker of fruit and vegetable intake in children. J Acad Nutr Diet. 2014; 114: 1174–1180. [DOI] [PubMed] [Google Scholar]
- 38. Ermakov IV,, Ermakova MR,, Bernstein PS,, et al. Resonance Raman based skin carotenoid measurements in newborns and infants. J Biophotonics. 2013; 6: 793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zidichouski JA,, Mastaloudis A,, Poole SJ,, et al. Clinical validation of a noninvasive, Raman spectroscopic method to assess carotenoid nutritional status in humans. J Am Coll Nutr. 2009; 28: 687–693. [DOI] [PubMed] [Google Scholar]
- 40. Gellermann W,, Zidichouski JA,, Smidt CR,, Bernstein PS. Raman detection of carotenoids in human tissue. : Packer L,, Obermueller-Jevic U,, Kraemer K,, Sies H, Carotenoids and Retinoids: Molecular Aspects and Health Issues. Champaign, IL: AOCS Press, 2005: 86–114. [Google Scholar]
- 41. Ermakov IV,, Gellermann W. Validation model for Raman based skin carotenoid detection. Arch Biochem Biophys. 2010; 504: 40–49. [DOI] [PubMed] [Google Scholar]
- 42. Choi RY,, Gorusupudi A,, Wegner K,, et al. Macular pigment distribution responses to high-dose zeaxanthin supplementation in patients with macular telangiectasia type 2 [published online ahead of print January 10, 2017]. Retina. doi: http://dx.doi.org/10.1097/IAE.0000000000001450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Choi RY,, Chortkoff SC,, Gorusupudi A,, Bernstein PS. Crystalline maculopathy associated with high-dose lutein supplementation. JAMA Ophthalmol. 2016; 134: 1445–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kirby ML,, Beatty S,, Loane E,, et al. A central dip in the macular pigment spatial profile is associated with age and smoking. Invest Ophthalmol Vis Sci. 2010; 51: 6722–6728. [DOI] [PubMed] [Google Scholar]
- 45. Nolan JM,, Akkali MC,, Loughman J,, et al. Macular carotenoid supplementation in subjects with atypical spatial profiles of macular pigment. Exp Eye Res. 2012; 101: 9–15. [DOI] [PubMed] [Google Scholar]
- 46. Komar B,, Rauscher FG,, Wiedemann R,, Dawczynski J. Macular pigment optical density measurements by one-wavelength reflection photometry–influence of cataract surgery on the measurement results. Graefes Arch Clin Exp Ophthalmol. 2014; 252: 1717–1727. [DOI] [PubMed] [Google Scholar]
- 47. Sasamoto Y,, Gomi F,, Sawa M,, et al. Effect of cataract in evaluation of macular pigment optical density by autofluorescence spectrometry. Invest Ophthalmol Vis Sci. 2011; 52: 927–932. [DOI] [PubMed] [Google Scholar]
- 48. Akuffo KO,, Nolan JM,, Stack J,, et al. The impact of cataract, and its surgical removal, on measures of macular pigment using the Heidelberg Spectralis HRA+OCT MultiColor Device. Invest Ophthalmol Vis Sci. 2016; 57: 2552–2563. [DOI] [PubMed] [Google Scholar]
- 49. Zeimer M,, Dietzel M,, Hense HW,, et al. Profiles of macular pigment optical density and their changes following supplemental lutein and zeaxanthin: new results from the LUNA study. Invest Ophthalmol Vis Sci. 2012; 53: 4852–4859. [DOI] [PubMed] [Google Scholar]
- 50. Akuffo KO,, Nolan JM,, Howard AN,, et al. Sustained supplementation and monitored response with differing carotenoid formulations in early age-related macular degeneration. Eye (Lond). 2015; 29: 902–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eye Disease Case-Control Study Group. Antioxidant status and neovascular age-related macular degeneration. Arch Ophthalmol. 1993; 111: 104–109. [DOI] [PubMed] [Google Scholar]
- 52. Nolan JM,, Loughman J,, Akkali MC,, et al. The impact of macular pigment augmentation on visual performance in normal subjects: COMPASS. Vision Res. 2011; 51: 459–469. [DOI] [PubMed] [Google Scholar]
- 53. Nolan JM,, Power R,, Stringham J,, et al. Enrichment of macular pigment enhances contrast sensitivity in subjects free of retinal disease: Central Retinal Enrichment Supplementation Trials - Report 1. Invest Ophthalmol Vis Sci. 2016; 57: 3429–3439. [DOI] [PubMed] [Google Scholar]
- 54. Akhtar S,, Ahmed A,, Randhawa MA,, et al. Prevalence of vitamin A deficiency in South Asia: causes, outcomes, and possible remedies. J Health Popul Nutr. 2013; 31: 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moeller SM,, Parekh N,, Tinker L,, et al. Associations between intermediate age-related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS): ancillary study of the Women's Health Initiative. Arch Ophthalmol. 2006; 124: 1151–1162. [DOI] [PubMed] [Google Scholar]
- 56. Merle BM,, Silver RE,, Rosner B,, Seddon JM. Adherence to a Mediterranean diet, genetic susceptibility, and progression to advanced macular degeneration: a prospective cohort study. Am J Clin Nutr. 2015; 102: 1196–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Obana A,, Tanito M,, Gohto Y,, et al. Changes in macular pigment optical density and serum lutein concentration in japanese subjects taking two different lutein supplements. PLoS One. 2015; 10: e0139257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bone RA,, Landrum JT,, Mayne ST,, et al. Macular pigment in donor eyes with and without AMD: a case-control study. Invest Ophthalmol Vis Sci. 2001; 42: 235–240. [PubMed] [Google Scholar]
- 59. Murray IJ,, Makridaki M,, van der Veen RL,, et al. Lutein supplementation over a one-year period in early AMD might have a mild beneficial effect on visual acuity: the CLEAR study. Invest Ophthalmol Vis Sci. 2013; 54: 1781–1788. [DOI] [PubMed] [Google Scholar]
- 60. Prado-Cabrero A,, Beatty S,, Howard A,, et al. Assessment of lutein, zeaxanthin and meso-zeaxanthin concentrations in dietary supplements by chiral high-performance liquid chromatography. Eur Food Res Technol. 2016; 242: 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schweitzer D,, Jentsch S,, Dawczynski J,, et al. Simple and objective method for routine detection of the macular pigment xanthophyll. J Biomed Opt. 2010; 15: 061714. [DOI] [PubMed] [Google Scholar]
- 62. Sharifzadeh M,, Bernstein PS,, Gellermann W. Nonmydriatic fluorescence-based quantitative imaging of human macular pigment distributions. J Opt Soc Am A Opt Image Sci Vis. 2006; 23: 2373–2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ermakov IV,, Gellermann W. Optical detection methods for carotenoids in human skin. Arch Biochem Biophys. 2015; 572: 101–111. [DOI] [PubMed] [Google Scholar]