Abstract
Cannabinoids, when co-administered with opioids, may enable reduced opioid doses without loss of analgesic efficacy (ie, an opioid-sparing effect). The aim of this study was to conduct a systematic review to determine the opioid-sparing potential of cannabinoids. Eligible studies included pre-clinical and clinical studies for which the outcome was either analgesia or opioid dose requirements. Clinical studies included controlled studies and case series. We searched Scopus, Cochrane Database of Systematic Reviews, Medline, and Embase. Nineteen pre-clinical and nine clinical studies met the search criteria. Seventeen of the 19 pre-clinical studies provided evidence of synergistic effects from opioid and cannabinoid co-administration. Our meta-analysis of pre-clinical studies indicated that the median effective dose (ED50) of morphine administered in combination with delta-9-tetrahydrocannabinol (delta-9-THC) is 3.6 times lower (95% confidence interval (CI) 1.95, 6.76; n=6) than the ED50 of morphine alone. In addition, the ED50 for codeine administered in combination with delta-9-THC was 9.5 times lower (95% CI 1.6, 57.5, n=2) than the ED50 of codeine alone. One case series (n=3) provided very-low-quality evidence of a reduction in opioid requirements with cannabinoid co-administration. Larger controlled clinical studies showed some clinical benefits of cannabinoids; however, opioid dose changes were rarely reported and mixed findings were observed for analgesia. In summary, pre-clinical studies provide robust evidence of the opioid-sparing effect of cannabinoids, whereas one of the nine clinical studies identified provided very-low-quality evidence of such an effect. Prospective high-quality-controlled clinical trials are required to determine the opioid-sparing effect of cannabinoids.
Introduction
Chronic pain is associated with enormous personal, social, and economic burden and is the largest contributor to years lived with disability globally (Rice et al, 2015). Despite this, existing medications provide only modest relief. Opioids in particular have considerable side effects, including constipation, impaired sleep, and respiratory depression (Chou et al, 2015). The last two decades have seen an increase in the prescription of opioids, which has been associated with an increase in opioid use disorders and opioid-related mortality (Chou et al, 2015; Volkow and McLellan, 2016; Zedler et al, 2014). This has been termed as an ‘opioid crisis’, and has caused regulators, health professionals, and the public to begin seeking means to reduce problems associated with high-dose opioid use. Consequently, there is a need for evidence-based strategies for reducing reliance on high-dose opioids without compromising pain management.
Using combinations of medications to harness complementary but distinct mechanisms of action can maximize the analgesic response, enabling the use of a lower dose of each medication and resulting in an improved side effect profile. One promising area for medication combinations is the use of opioid-sparing medications. Opioid-sparing medications, when co-administered with opioids, enable a reduced opioid dose without loss of analgesic efficacy. Cannabinoid medications are increasingly being studied for their analgesic- and opioid-sparing potential. The endocannabinoid system represents an ideal target because it is a key endogenous system in modulating pain-processing pathways (Woodhams et al, 2015).
The endocannabinoid system is composed of the cannabinoid CB1 and CB2 receptors, the endocannabinoid ligands anandamide and 2-arachidonoylglycerol, and their synthesis and degradation system (Pertwee, 2006). CB1 and CB2 receptors are differentially expressed on the central nervous system (Cencioni et al, 2010; Herkenham et al, 1991) and play important roles in pain processes. Both cannabinoid receptors and endocannabinoids are present in the primary afferent pain circuits to the brain (Manzanares et al, 1999; Woodhams et al, 2015). Cannabinoid and opioid receptors have similar signal transduction systems (Cichewicz, 2004; Howlett et al, 2002; Vigano et al, 2005) and are expressed in several brain regions involved in antinociception, including the periaqueductal gray, raphe nuclei, and central-medial thalamic nuclei (Cichewicz, 2004). In addition, mu-opioid receptors and CB1 receptors co-localize in the spinal cord at the first synaptic contact for peripheral nociceptive afferent neurons (Hohmann et al, 1999; Salio et al, 2001).
It has previously been observed that CB2 receptors indirectly stimulate opioid receptors located in primary afferent pathways (Ibrahim et al, 2005). Therefore, in addition to their direct analgesic effects, cannabinoids may work synergistically to enhance opioid analgesia. The behavioral, anatomical, and biochemical similarities between opioid and cannabinoid receptor systems and their endogenous ligands are well documented. For example, activation of either cannabinoid or opioid receptors produces comparable neurobehavioral and physiological effects, including antinociception (Manzanares et al, 1999). This is highlighted by both CB1 and CB2 agonists being able to induce antinociception by increasing opioid precursors’ gene expression or via release of endogenous opioids (Houser et al, 2000; Ibrahim et al, 2005; Valverde et al, 2001). Further, pharmacological modulation of the opioid system can modify the effects of delta-9-tetrahydrocannabinol (delta-9-THC)—a partial agonist at the CB1 and CB2 receptor—on nociception (Mason et al, 1999; Pugh et al, 1997; Smith et al, 1994) and vice versa. Finally, cannabinoid antagonists have been shown to reverse the antinociception induced by morphine (da Fonseca Pacheco et al, 2008). Collectively, this strongly supports shared mechanisms between both systems in regard to analgesia.
Animal models have identified a role for CB1 receptor activation in reducing neuropathic, visceral, and inflammatory pain (Pertwee, 2008; Walker et al, 1999). Several pre-clinical studies have demonstrated that systemic administration of cannabinoid receptor ligands produces analgesia in acute and chronic pain models (Walker and Huang, 2002). In addition, the role of CB2 receptors has been explored in pre-clinical studies, suggesting that these receptors may mediate effects in inflammatory pain states (Ibrahim et al, 2006; Quartilho et al, 2003), and reduce inflammation and neuropathic pain (Gui et al, 2015).
Further to these pre-clinical findings, clinical studies indicate that cannabinoid administration may reduce pain and improve other symptoms such as sleep disturbances associated with chronic pain (Ware et al, 2010a; Ware et al, 2010b). This effect could be mediated by delta-9-THC, which is the main psychoactive ingredient present in cannabis (Cichewicz, 2004; Jensen et al, 2015). Despite the growing body of relevant literature, to date no systematic review has focused on the opioid-sparing effects of cannabinoids. To address this gap, we conducted a systematic review of pre-clinical and clinical studies to examine the strength of existing evidence demonstrating the opioid-sparing effect of cannabinoids in the context of analgesia.
Materials and methods
Search
We conducted a systematic search of the literature in accordance with recommendations by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Moher et al, 2009). The search aimed to identify clinical and pre-clinical studies using the following electronic databases: Scopus, Cochrane Database of Systematic Reviews, Medline, and Embase. Search terms are listed and a sample search strategy is reported in Supplementary Appendix 1. No date limits were included. Searches were run on 29 October 2015. In addition, reference lists from identified studies and review articles were searched to find additional studies not identified by the main search.
Eligible studies included:
Human or animal studies.
Outcomes of either pain/analgesia or opioid requirements/opioid-sparing effects from concurrently administered opioids and cannabinoids.
Controlled clinical studies and case series.
Titles were screened by two authors (SN and PS). Where inconsistencies were identified, the authors were able to reach consensus on each occasion.
Data Extraction and Outcomes
Data extraction forms were developed and circulated to the author group before piloting and refining. All data were extracted by one of the authors (SN, PS, or JMT) and checked by a second author (SN, PS, or JMT). These same authors reviewed and resolved any inconsistencies, with input from the authorship group as required. When required data were missing, attempts were made to contact authors of published reports to collect additional information.
Outcome Measures
For pre-clinical studies, the primary outcome was the dose of opioid required to give an equivalent antinociceptive effect in the presence and absence of cannabinoids. For clinical studies, the primary outcome was evidence of the opioid-sparing effect of cannabinoids. Data were extracted on opioid dose and/or analgesic outcome where cannabinoids were co-administered. Secondary outcome measures examined included analgesia, sleep, and quality of life.
Analysis
Pre-clinical studies
Data were extracted and a narrative review was conducted. Ten studies were identified as sufficiently similar in design and outcome measures to be eligible for meta-analysis. Of these, six reported sufficient data to enable meta-analysis; that is, the dose of opioid required to produce comparable analgesia in the presence and absence of cannabinoids, the variance of the observed dose, and the sample size. Authors of the other studies were contacted in an attempt to include additional studies in the meta-analysis; however, no additional data were identified to enable the inclusion of any additional studies.
To prepare the data for the meta-analysis, the effective dose (ED50) and either confidence limits or SE were extracted from the relevant literature. ED50 is calculated on the log10 scale. Therefore, to meet the assumption of normality, the log10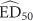 must be used in the meta-analysis. The log10 of the confidence limits must also be determined to calculate the SD of the log10
must be used in the meta-analysis. The log10 of the confidence limits must also be determined to calculate the SD of the log10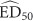 :
:
where UL is the upper confidence limit.
When only SE was reported, the confidence limits were calculated using the method of Litchfield and Wilcoxon (1949) and the above procedure was repeated to calculate the SD. This method also allowed for the inclusion of studies that did not report exact sample sizes for all treatment groups, as sample size was not required for the calculation of SD.
Data for the meta-analysis were analyzed using Review Manager 5.1 (Cochrane Collaboration, Oxford, UK). When calculating the continuous outcome of an equally effective opioid dose (eg, the log10ED50 for morphine when administered alone vs when administered with a cannabinoid), the inverse variance statistical method and random effects model were used to compensate for study heterogeneity.
No statistical difference was found in outcomes between the studies that used different species or nociceptive assays. Therefore, the mean difference of log 10ED50 of and the corresponding 95% confidence intervals (CI) were calculated. Due to the nature of log calculations, the mean difference—when back-transformed to the original units—represents the response ratio. For easier interpretation, we present the reciprocal of the response rate.
Assessment of bias in pre-clinical studies
A funnel plot was produced to examine publication bias and small study effects in the pre-clinical studies included in the meta-analysis.
Clinical studies
The nine clinical studies identified were heterogeneous in design and outcomes, and therefore not suitable for meta-analysis. Thus, a narrative synthesis was conducted instead, with all studies scored for quality using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) criteria (Guyatt et al, 2008).
Results
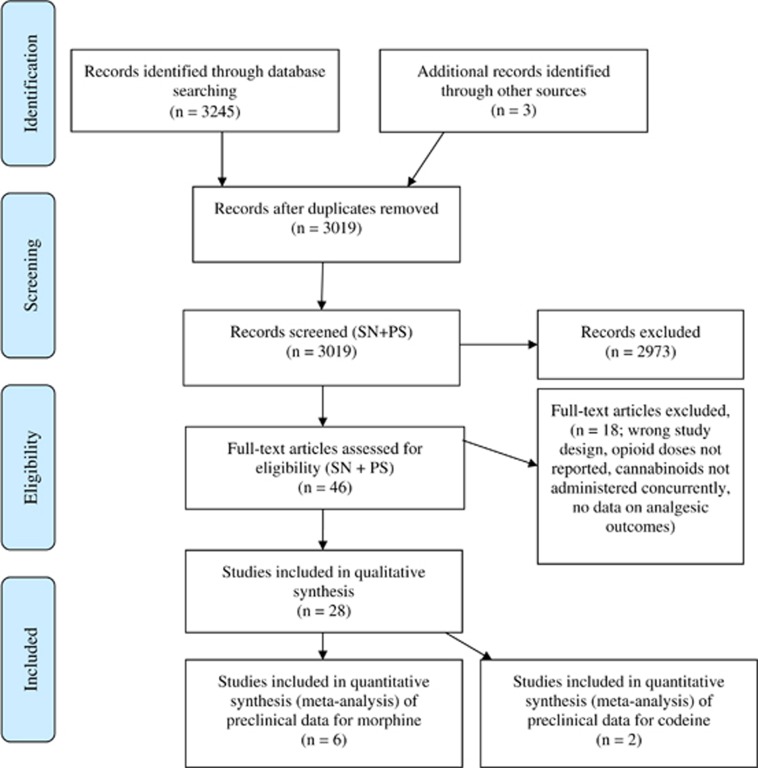
The initial searches identified 3019 records after duplicates were removed, with 19 pre-clinical and nine clinical studies identified for inclusion in the final review (see Figure 1 for the PRISMA diagram).
Figure 1.
PRISMA diagram showing study identification. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Summary of Pre-Clinical Studies
Nineteen pre-clinical studies were identified in which the analgesic effect of opioid and cannabinoid co-administration was examined (Cichewicz et al, 1999, 2005; Cichewicz and McCarthy, 2003; Cox et al, 2007; Finn et al, 2004; Katsuyama et al, 2013; Li et al, 2008; Maguire et al, 2013; Pugh Jr et al, 1996; Reche et al, 1996; Smith et al, 1998, 2007; Tham et al, 2005; Wakley and Craft, 2011; Welch and Stevens, 1992; Williams et al, 2006, 2008; Wilson et al, 2008; Yesilyurt et al, 2003) (Table 1). Fourteen of these studies examined delta-9-THC, whereas one to two studies examined each of 10 other cannabinoid agonists, including beta-caryophyllene, CP 55 940, CP 56 667, delta-8-THC, 11-hydroxy-delta-9-THC, dextronantradol, levonantradol, WIN 55, 212-2, and HU-210. Seventeen studies examined morphine, three studies examined codeine, and one to two studies examined buprenorphine, fentanyl, oxycodone, morphine, hydromorphone, methadone, LAAM, meperidine, and pentazocine. Most of the studies used rodents; however, two used rhesus monkeys and one used guinea pigs. The most common antinociceptive assays were tail-flick tests (n=10) and hot plate tests (n=5), although individual studies also used other forms of mechanical, thermal, and chemical nociception.
Table 1. Summary of Evidence of Opioid-Sparing Effects from Pre-Clinical Studies.
|
Equipotent opioid dose represented as ED50(95% CL) or ± SEM, unless measured otherwise specified | |||||||
|---|---|---|---|---|---|---|---|
| Study reference | Pain model (species) | Opioid administered | Cannabinoid administered | Cannabinoid condition | Vehicle condition | Potency ratio or evidence of synergism | Other notes |
| Cichewicz et al, 1999 | Tail-flick test (male ICR mice) | Morphine p.o. | Delta-9-THC (20 mg/kg p.o.) | 13.1 mg/kg (8.8, 19.5) | 28.8 mg/kg (20.2, 41) | Potency ratio: 2.2 | |
| Codeine p.o. | Delta-9-THC (20 mg/kg p.o.) | 5.9 mg/kg (1.4, 24.9) | 139.9 mg/kg (75.2, 260.5) | Potency ratio: 25.8 | |||
| Oxymorphone p.o. | Delta-9-THC (20 mg/kg p.o.) | 0.5 mg/kg (0.3, 0.8) | 2.6 mg/kg (1.7, 3.9) | Potency ratio: 5.0 | |||
| Hydromorphone p.o. | Delta-9-THC (20 mg/kg p.o.) | 0.4 mg/kg (0.2, 0.8) | 5.6 mg/kg (3.2, 9.7) | Potency ratio: 12.6 | |||
| Methadone p.o. | Delta-9-THC (20 mg/kg p.o.) | 2.7 mg/kg (1.4, 5.2) | 12.0 mg/kg (8.1, 17.9) | Potency ratio: 4.1 | |||
| LAAM p.o. | Delta-9-THC (20 mg/kg p.o.) | 2.6 mg/kg (1.7, 3.9) | 8.0 mg/kg (6.4, 10.1) | Potency ratio: 2.5 | |||
| Heroin p.o. | Delta-9-THC (20 mg/kg p.o.) | 5.4 mg/kg (1.7, 16.9) | 26.1 mg/kg (12.7, 53.4) | Potency ratio: 4.1 | |||
| Meperidine p.o. | Delta-9-THC (20 mg/kg p.o.) | 11.1 mg/kg (4.2, 29.4) | 86.2 mg/kg (52.8, 140.6) | Potency ratio: 8.9 | |||
| Fentanyl p.o. | Delta-9-THC (20 mg/kg p.o.) | 0.5 mg/kg (0.3, 0.8) | 6.1 mg/kg (estimated from an extrapolated curve) | Not determined (50% MPE not seen) | |||
| Pentazocine p.o. | Delta-9-THC (20 mg/kg p.o.) | 838.6 mg/kg (estimated from an extrapolated curve) | 625.9 mg/kg (estimated from an extrapolated curve) | Not determined (50% MPE not seen) | |||
| Cichewicz and Welch, 2003 | Tail-flick test (male ICR mice) | Morphine p.o. Codeine p.o. | Delta-9-THC (5–35 mg/kg and 1–27 mg/kg p.o.) Delta-9-THC (5–30 and 5–18 mg/kg p.o.) | 13.6 mg/kg±1.94 20.1 mg/kg±3.0 | 24.5 mg/kg±4.8 78.2 mg/kg ±14.4 | For each ratio tested, experimental values were less than the calculated additive values (synergism) For each ratio tested, experimental values were less than the calculated additive values (synergism) | Fixed-ratio combinations of 9-THC with either morphine or codeine were tested for antinociceptive effects. The experimentally derived ED50 for each combination was compared with the theoretical additive ED50, using an isobolographic analysis. All the fixed-ratio combinations tested produced greater antinociception (synergy) than predicted from simple additivity |
| Cichewicz et al, 2005 | Pin-prick test (IAF hairless guinea pigs) | Fentanyl s.c. | Delta-9-THC (50 mg/kg i.p.) | 6.8 μg/kg (3.3, 14.2) | 50.8 μg/kg (41.0, 63.0) | Greater than additive effect on antinociception. Potency ratio: 6.7 (1.8–17.0) | |
| Buprenorphine s.c. | Delta-9-THC (50 mg/kg i.p.) | 0.02 mg/kg (0.01, 0.05) | 2.97 mg/kg (1.84, 4.81) | Greater than additive effect on antinociception. Enhanced potency in a non-parallel fashion | Not possible to compare the change in potency produced by delta-9-THC due to the non-parallel nature of the two dose–response curves for buprenorphine | ||
| Fentanyl t.d. | Delta-9-THC (400 mg/kg t.d.) | 2 h: 254.9 μg/kg (202.90, 320.6) 4 h: 176.3 μg/kg (144.3, 215.5) | 2 h: 928.6 μg/kg (599.5, 1438.3) 4 h: 1067.0 μg/kg (840.4, 1356.1) | Potency ratio at 2 h: 3.7 Potency ratio at 4 h: 5.8 | |||
| Buprenorphine t.d. | Delta-9-THC (400 mg/kg t.d.) | 2 h: 4.3 mg/kg (2.8, 6.8) 4 h: 2.2 mg/kg (1.1, 4.6) | 2 h: 26.1 mg/kg (17.1, 39.9) 4 h: 15.6 mg/kg (10.0, 24.5) | Potency ratio at 2 h: 8.2 Potency ratio at 4 h: 7.2 | |||
| Cox et al, 2007 | Paw pressure test (Male Sprague–Dawley rats) | Morphine i.p. (normal rats) Morphine i.p. (arthritic rats) | Delta-9-THC (0.4 mg/kg±0.5 i.p.) (1 : 1 ratio THC : Morphine) Delta-9-THC (0.6 mg/kg±0.55 i.p.) (1 : 1 ratio THC : Morphine) | 0.4 mg/kg±0.5 0.6 mg/kg±0.55 | 2.4 mg/kg (2.2, 2.8) 2.2 mg/kg (1.9, 2.4). | The combination of delta-9-THC and morphine showed synergism in both non-arthritic and arthritic rats | Results from normal rats included in the meta-analysis only |
| Finn et al, 2004 | Formalin-evoked nociceptive behavior (adult male Lister-Hooded rats) | Morphine i.p. | Delta-9-THC (1 mg/kg i.p.) | Not reported | Not reported | Not clearly synergistic. Potentially additive. Morphine (2 mg/kg) + delta-9-THC (1 mg/kg) had a significant effect on nociceptive behavior (compared to morphine alone but not delta-9-THC alone). | |
| Katsuyama et al, 2013 | Capsaicin test (Male mice of ddY strain) | Morphine (1.0 mg/kg s.c. and 100 pmol i.t.) | Beta-caryophyllene (2.25 mg i.pl., CB2 receptor agonist) | ID50 1.16 mg/kg (1.03, 1.32, systemic, s.c.) and 130.1 pmol (111.9, 156.4, spinal, i.t.) | ID50 2.51 mg/kg (2.17, 2.97) (systemic, s.c.) and 193.7 pmol (165.7, 225.6, spinal, i.t.) | Morphine + beta-caryophyllene decreased licking/biting response p<0.05 compared to morphine + saline or beta-caryophyllene + jojoba wax. | Ineffective doses of beta-caryophyllene significantly enhanced morphine-induced antinociception. |
| Li et al, 2008 | Thermal antinociception (rhesus monkeys) | Morphine s.c. | Delta-9-THC (0.32 and 1.0 mg/kg s.c.) | ED80 2.42 mg/kg | ED80 6.36 mg/kg (3.81, 8.91) | Pre-treatment with delta-9-THC enhanced the antinociceptive effects of morphine. | Morphine dose dependently increased the latency for monkeys to remove their tails from 50°C and 55°C water. |
| Maguire et al, 2013 | Warm water tail withdrawal (rhesus monkeys) | Morphine s.c. | CP 55 940 (0.01 mg/kg s.c.) WIN 55 212 (0.32 mg/kg s.c.) | Mean (n=3) CP 0.23 mg/kg WIN 0.24 mg/kg | 1.26 mg/kg (mean, n=3) | Pre-treatment with CP 55 940 resulted in a mean leftward shift to of –6.73-fold. Pre-treatment with WIN 55 212 resulted in mean leftward shift of –5.5-fold. | Antinociception from the combination appeared to be achieved without an increase in abuse liability. |
| Pugh et al, 1996 | Tail-flick test (mail ICR mice) | Morphine i.t. | Delta-9-THC (6 μcg/mouse i.t., inactive analgesic dose) | 0.01 mcg/mouse | 0.318 mcg/mouse (2.825, 0.036) | Greater than additive effect observed, clear leftward shift of graph. | |
| Reche et al, 1996 | Tail-flick and hot plate test (Swiss albino mice) | Morphine i.p. | Delta-9-THC i.p. | NA Only one dose of morphine (2 mg/kg i.p.) examined. Study measured change in ED50 of delta-9-THC. | NA | Morphine pre-administration shifted the dose–response curve for delta-9-THC to the left (a 2.5-fold shift for the tail-flick test and a three-fold shift for the hot plate test). Analgesic effect blocked by SR-141 716 (cannabinoid antagonist) and naloxone. | |
| Smith et al, 1998 | Tail-flick and hot plate test (male ICR mice) | Morphine s.c. Morphine p.o. | Tail-flick: delta-9-THC (4 mg/kg s.c.) Tail-flick: delta-9-THC (20 mg/kg p.o.) | 0.29 mg/kg (95% CI 0.04, 1.94) 2.8 mg/kg (2.0, 3.9) | 2.81 mg/kg (2.24, 3.53) 31.7 mg/kg (22.4, 44.9) | Potency ratio: 8.5 Potency ratio: 7.6 | Multiple conditions tested different combinations of s.c and p.o morphine. Only s.c. + s.c. and p.o. + p.o. for the tail-flick test are reported here. A paw withdrawal test was also conducted to demonstrate that enhancement of antinociception was not limited to the tail. |
| Smith et al, 2007 | Paw withdrawal test (male Sprague–Dawley rats) | Morphine s.c. | Delta-9-THC (0.75 mg /kg i.p.) | ED80 morphine + delta-9-THC (0.75 mg/kg) | ED80 morphine alone (100 mg/kg) | Tolerance to morphine alone rapidly established; no loss of effect with low-dose combinations of morphine + delta-9-THC | A morphine pellet arm and delta-9-THC alone arm were not reported in this table due to difficulties in comparing doses between morphine formulations. |
| Tham et al, 2005 | Tail-flick and hot plate test (Swiss male mice) | Morphine s.c. | Tail-flick: CP 55 940 (0.1–3 mg/kg s.c.) Hot plate: CP 55 940 (0.1–3 mg/kg s.c.) | 3.31 mg/kg 7.54 mg/kg | 11.3 mg/kg (9.6, 13.4) 29.4 mg/kg (27.3, 31.6) | Analyses showed greater than additive results (synergism). | |
| Wakley and Craft, 2011 | Paw pressure test (male Sprague–Dawley rats) | Methadone i.p. | Delta-9-THC (0.32–3.2 mg/kg i.p.) | Not reported (dose–response curve shown) | ED50 in naive rats, 1.27 mg/kg (95% CI 0.91, 1.91), ED50 in rats trained for discrimination, 3.49 mg/kg (95% CI 2.59, 5.31) | In opioid and delta-9-THC naive rats, methadone 1.0 mg/kg significantly enhanced the antinociceptive effect of delta-9-THC, however this was not observed in rats that were previously trained for drug discrimination tasks. | The rats trained for drug discrimination tasks had received repeated administration of opioids and cannabinoids over many months and may have been tolerant to drug effects at the doses administered. |
| Welch and Stevens, 1992 | Tail-flick and hot plate test (mice) | Morphine i.t. | Delta-9-THC (3.133 mcg/mouse) | 0.15 mcg/mouse (0.11, 0.21) | 0.61 mcg/mouse (0.26, 1.44) | Yes | |
| Delta-9-THC (6.25 mcg/mouse) | 0.05 mcg/mouse (0.03, 0.08) | Yes | |||||
| Delta-8-THC (25 mcg/mouse) | 0.05 mcg/mouse (0.02, 0.10) | Yes | |||||
| Levonantradol (0.005 mcg/mouse) | 0.06 mcg/mouse (0.01, 0.24) | Yes | |||||
| CP 55 940 (0.01 mcg/mouse) | 0.3 mcg/mouse (0.0, 0.10) | Additive | |||||
| CP 56 667 (0.5 mcg/mouse) | 0.26 mcg/mouse (0.08, 0.82) | Additive | |||||
| 11-hydroxy-delta-9-THC (3 mcg/mouse) | 0.08 mcg/mouse (0.04, 0.19) | Yes | |||||
| Dextronantradol (25 mcg/mouse) | 0.51 mcg/mouse (0.36, 0.89) | No | |||||
| Williams et al, 2006 | Tail-flick test (mail ICR mice) | Study 1: low-dose codeine (30 mg/kg) and morphine (20 mg/kg) and fully efficacious ED80, codeine (100 mg/kg) and morphine (80 mg/kg). Study 2: high-dose codeine (200 mg/kg) and morphine (100 mg/kg) (all p.o.) | Delta-9-THC (20 mg/kg p.o., inactive analgesic dose) | ED80 codeine (30 mg/kg) ED80 morphine (20 mg/kg) | ED80 codeine (200 mg/kg) ED80 morphine (100 mg/kg) | A low dose of morphine (20 mg/kg) or codeine (30 mg/kg) with a single pre-treatment of an inactive dose of delta-9-THC produced the same efficacy (ED80) as the high doses of each opioid alone. For codeine, delta-9-THC pre-treatment also increased the duration of action of the ED80 dose of codeine. | Study 1: pre-treatment with delta-9-THC did not enhance the fully efficacious dose of morphine but enhanced low-dose morphine and both doses of codeine, in addition to extending the time course. Study 2: delta-9-THC restored analgesic efficacy after the time that the opioids had ceased being effective on their own (360 min post dose for morphine and 120 min post dose for codeine). |
| Williams et al, 2008 | Tail-flick test (diabetic and non-diabetic mice and rats) | Morphine s.c. Morphine s.c. | Delta-9-THC (20 mg/kg p.o.) in non-diabetic mice Delta-9-THC (20 mg/kg p.o.) in diabetic mice | 2.5 mg/kg (1.8, 3.4) 0.84 mg/kg (0.79, 0.89) | 5.6 mg/kg (4.3, 7.2) 6.1 mg/kg (5.2, 7.1) | Yes Yes | Delta-9-THC significantly enhanced morphine-induced antinociception in both diabetic and non-diabetic mice. |
| Wilson et al, 2008 | Hot plate test (male Sprague–Dawley rats) | Morphine microinjections into PAG | HU-210 (5 μg) | Not reported (dose–response curve shown) | Not reported (dose–response curve shown) | No evidence of synergism. Morphine + HU-210 showed the greatest increase in hot plate latency (39.9 s±1.1 s), but was not significantly different from morphine alone (33.1 s ±4.0 s) | HU-210 shown to prevent development of tolerance to morphine’s antinociceptive effects. HU-210 pre-treatment enhanced subsequent morphine antinociception. Co-administration of HU-210 into the PAG attenuated morphine antinociception. The authors suggested that opioids and cannabinoids may have opposing actions within the PAG. |
| Yesilyurt et al, 2003 | Tail-flick test (adult female Bulb-C mice) | Morphine topical | WIN 55, 212-2 (20 mg/ml, topical, mixed CB1-CB2 receptor agonist) | Morphine (20 mg/ml) + WIN sustained analgesic effect of 50% analgesia over 4 h | Morphine (20 mg/ml) alone produced 18% analgesic effect, peak at 20 min then reduced. | Antinociceptive effects were markedly potentiated (they peaked and were sustained at 30 min) compared to morphine response alone. | |
Abbreviations: delta-9-THC, delta-9-tetrahydrocannabinol; ED, effective dose; ICR, imprinting control region; ID, inhibitory dose; i.p., intraperitoneal; i.t., intrathecal; MPE, maximum possible effect; PAG, periaqueductal gray matter; pmol, picomol; p.o., oral administration; s.c., subcutaneous; t.d, transdermal.
Most studies (17 of the 19) demonstrated that combining a cannabinoid with an opioid resulted in a synergistic effect on analgesia compared to the analgesic effects of the individual drugs. One study examined a single dose of morphine and demonstrated that morphine could potentiate the analgesic effect of intrathecally administered delta-9-THC (Reche et al, 1996). However, this study could not demonstrate an opioid-sparing effect due to the use of a single dose of opioid. Another study found that 2 mg/kg morphine administered with 1 mg/kg delta-9-THC resulted in a significant effect on nociception compared to morphine alone (p<005), but not when compared to delta-9-THC alone (Finn et al, 2004). In another study, a greater increase in hot plate latency was found for morphine combined with HU-210 (38.9 s±1.1 s) compared with HU-210 alone (33.1 s±4.0 s) (Wilson et al, 2008); however, this difference did not reach significance.
One study testing multiple opioid agonists identified clear synergistic effects for delta-9-THC for most opioid drugs, with the exception of fentanyl and pentazocine (Cichewicz et al, 1999). The potency ratio when administered alone for those opioids found to have a synergistic effect, compared to when those same opioids were co-administered with delta-9-THC varied between 2.2 and 25.8. Another study tested multiple cannabinoid agonists when co-administered with morphine and demonstrated a synergistic effect with delta-9-THC, delta-8-THC, levonantradol, and 11-hydroxy-delta-9-THC; additive effects with CP 55 940 and CP 56 667; and no observable potentiation of morphine effects with dextronantradol, which is an isomer of levonantradol (Welch and Stevens, 1992). In contrast to the finding of an additive effect for CP 55 940, two other studies of CP 55 940 in combinations with morphine demonstrated a synergistic analgesic effect (Maguire et al, 2013; Tham et al, 2005). In addition to changes in the magnitude of the analgesic effect, two studies showed that the duration of the analgesic effect can be extended by administrating a low-dose opioid and cannabinoid in combination, compared with administrating an opioid alone (Williams et al, 2006; Yesilyurt et al, 2003).
Meta-Analysis of Pre-Clinical Studies
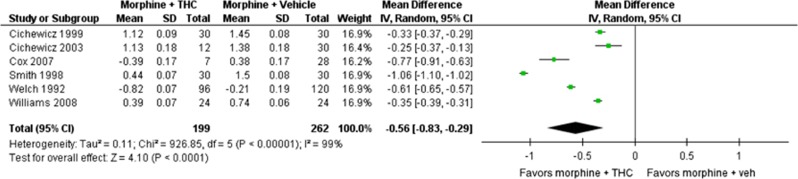
Six studies used sufficiently similar approaches to enable a meta-analysis (Cichewicz et al, 1999; Cichewicz and Welch, 2003; Cox et al, 2007; Smith et al, 1998; Welch and Stevens, 1992; Williams et al, 2008) (Figure 2). A further four studies were comparable in study design, but did not contain the required data (ED50 or variance on estimates) to enable meta-analysis (Finn et al, 2004; Pugh Jr et al, 1996; Smith et al, 2007; Williams et al, 2006). All studies included in the meta-analysis used rodents and reported comparable antinociceptive doses of morphine alone and morphine co-administered with delta-9-THC. Results from the meta-analysis are reported in terms of mean difference.
Figure 2.
Forrest plot for meta-analysis examining the opioid-sparing effect of delta-9-THC when co-administered with morphine. Note: all mean difference and SD values are of log10ED50. THC, tetrahydrocannabinol.
The meta-analysis identified a significant opioid-sparing effect with morphine and delta-9-THC co-administration, Z=5.59, p<0.001 (MD in log10ED50 –0.56 (–0.83, –0.29)). As there was significant heterogeneity in the data (I2=95%), a random effects model was used. When back-transformed to the original units, the response ratio was 3.6 (95% CI 1.95, 6.76), indicating that the median ED50 of morphine was 3.6 times lower when given in combination with delta-9-THC compared to when morphine was administered alone.
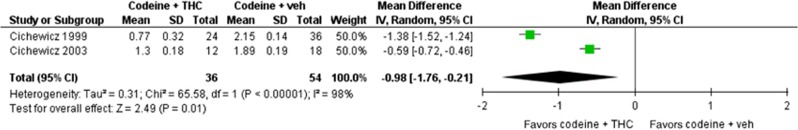
Two studies compared doses of codeine with and without delta-9-THC in rodents (Cichewicz et al, 1999; Cichewicz and Welch, 2003) (Figure 3). Both studies used male ICR mice and the tail-flick assay. Meta-analysis of these data indicated a significant opioid-sparing effect of delta-9-THC when co-administered with codeine, Z=2.49, p=0.01 (MD in the log10ED50 –0.98 (–1.76, –0.21)). Significant heterogeneity in the data (I2=98%) necessitated the use of a random effects model. When back-transformed to the original units, the response ratio was 9.5 (95% CI 1.6, 57.5), indicating that the ED50 of codeine was 9.5 times lower when given in combination with delta-9-THC compared to when codeine was administered alone.
Figure 3.
Forrest plot for meta-analysis examining the opioid-sparing effect of delta-9-THC when co-administered with codeine. Note: all mean difference and SD values are of log10ED50. THC, tetrahydrocannabinol.
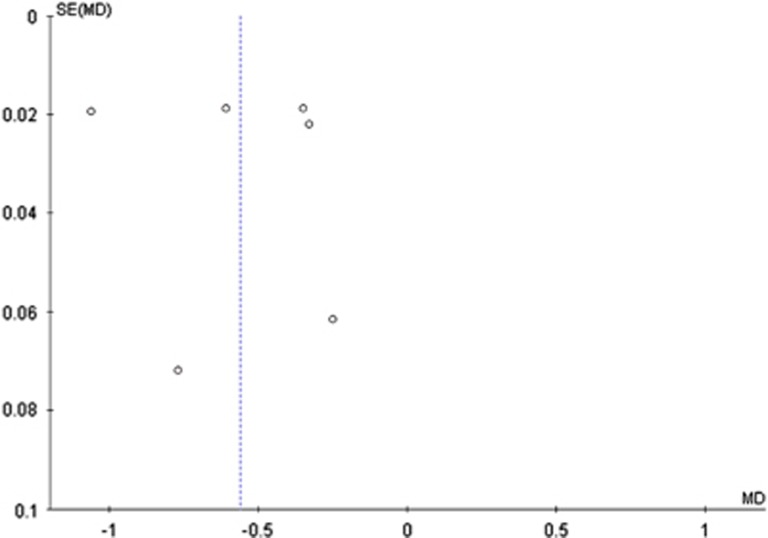
Funnel plots did not provide evidence of publication or small study bias with these pre-clinical studies (Figure 4).
Figure 4.
Funnel plot showing data from the six studies included in the meta-analysis. MD, mean difference, SE, standard error.
Results from Clinical Studies
Nine clinical studies with 750 participants provided data relevant to the research question (Table 2); however, the heterogeneous nature of the studies precluded meta-analysis. Three laboratory-based studies examined pain responses in participants concurrently being administered opioids and cannabinoids. One study recruited people with mixed chronic non-cancer pain (n=24) who were prescribed opioids (Abrams et al, 2011). A significant reduction in pain ratings was observed for the participants in this study following co-administration of cannabinoids—39.6 (95% CI 35.8, 43.3) at baseline vs 29.1 (95% CI 25.4, 32.8) following co-administration (Abrams et al, 2011). It should be noted that no placebo or control condition was used in this study for comparison (Abrams et al, 2011).
Table 2. Summary of Evidence of Opioid-sparing Effects from Clinical Studies.
| (a) Study reference | Study design | Population | Follow-up period | Opioid used | Cannabinoid used | Effect of cannabinoid on opioid dose | Outcome on analgesia observed | GRADE evidence rating and other notes |
|---|---|---|---|---|---|---|---|---|
| Laboratory studies | ||||||||
| Abrams et al, 2011 | Clinical laboratory study of self-reported pain under observed conditions (also measured pharmacokinetic effects of concurrent administration) | People (n=24) receiving chronic opioid treatment (mixed pain conditions) | 5 days | Morphine sulfate (mean daily dose 62 mg, n=13) or oxycodone hydrochloride (mean daily dose 53 mg, n=11) | Vaporized cannabis dose of 0.9 g of 3.56% delta-9-THC or as much as they could tolerate, administered three times per day. | Opioid dose held constant to examine effect of delta-9-THC on opioid pharmacokinetics (ie, no reduction from baseline opioid dose possible). | Mean pain score reduction, from 34.8 (95% CI 29.4, 40.1) at baseline to 24.1 (95% CI 18.8, 29.4) on day 5 with morphine, and from 43.8 (95% CI 38.6, 49.1) at baseline to 33.6 (95% CI 28.5, 38.6) on day 5 with oxycodone. Significant reduction overall. | Cannabis inhalation produced a subjective ‘high’. GRADE rating ‘low’ quality. Downgraded as study did not have a placebo condition, so placebo effects cannot be excluded Note: no pharmacokinetic interaction observed. |
| Naef et al, 2003 | Experimental heat, cold, pressure, single and repeated transcutaneous electrical stimulation pain, randomized, placebo-controlled, double-blinded, crossover study. | Healthy cannabis naive volunteers (n=12) | Four study sessions with at least 7 days washout between sessions | Morphine (30 mg) daily | Dronabinol (20 mg) | No significant analgesic effect of dronabinol or morphine–dronabinol combination on heat, pressure, or cold tests. Additive effect of morphine on transcutaneous electrical stimulation test. | Potentiation of analgesia not observed in this experimental pain study. | GRADE rating ‘moderate’. Placebo-controlled, blinded study. Downgraded due to indirect evidence as use of experimental pain measures. |
| Roberts et al, 2006 | Experimental thermal pain. Double-blinded, four treatment crossover design. | Healthy volunteers (n=13) with no recent opioid or cannabinoid use | Four lab sessions | Morphine (0.02 mg/kg IV, 1.4 mg dose for 70 mg adult, ie, sub-analgesic) | Dronabinol (5 mg) | NA (opioid dose held constant) | Combination of delta-9-THC and morphine did not have an effect on pain intensity. The combination resulted in lower ratings of unpleasantness of pain compared with either drug alone. | GRADE rating ‘moderate’. Placebo-controlled, blinded study. Downgraded due to indirect evidence as use of experimental pain measures. |
| Case series | ||||||||
| Lynch and Clark, 2003 | Observational case series | Mixed pain conditions (n=3) (peripheral neuropathy, multiple sclerosis, lower back pain) | 1–9-month observation period | Morphine (75–360 mg daily) | Smoked cannabis plant, unknown content | Mean baseline morphine dose of 195 mg (SD 147 mg) compared with 35 mg (SD 31 mg) after commencing smoked cannabis. Opioid dose reduction or cessation in each case. | Improved pain control described, with patients either reducing or ceasing morphine dose. | GRADE rating ‘very low’. |
| (b) Study reference | Study design | Population | Follow-up period | Opioid used | Cannabinoid used | Effect of cannabinoid on opioid dose | Outcome on analgesia observed | GRADE evidence rating and other notes |
|---|---|---|---|---|---|---|---|---|
| Controlled trials | ||||||||
| Johnson et al, 2010 | Multicenter, double-blind, randomized, placebo-controlled, parallel-group trial. | Patients with cancer pain (n=177), with inadequate analgesia despite chronic opioid dosing. | 2 weeks | Varied opioids reported as OME (IQR) 120 mg (50–213) 80 mg (30–180) 120 mg (40–240) | Patients randomized to delta-9-THC : CBD, delta-9-THC, or placebo Delta-9-THC (mean 9 sprays per day) Delta-9-THC : CBD (mean 10 sprays per day) Placebo (mean 11 sprays/day) | No change in median amount of breakthrough opioid medication in any group. | Change in pain NRS (out of 10) from baseline was statistically significantly in favor of delta-9-THC : CBD compared with the placebo –1.01 (p=0.245) –1.37 (p=0.014) –0.69 (reference group) | GRADE rating ‘high’. Placebo-controlled and randomized. |
| Lissoni et al, 2014 | Two groups (not randomized): cannabinoid tincture or melatonin | Patients with untreatable metastatic solid tumor (n=26) | Not stated | Oxycodone, median dose of 30 mg (10–60 mg), twice per day | Cannabis flos (19% delta-9-THC) was given as an infusion. 100 ml (500 mg/l water) three times per day | 5/12 patients (42%) achieved control of pain without opioid dose increase compared to the control group, where 2/14 (14%) achieved pain control | The number that achieved pain control was not significantly different between groups | GRADE rating ‘low’. Non-randomized design, no allocation concealment described. Control group received melatonin (20–100 mg). Greater disease progression documented in the cannabis group |
| Narang et al, 2008 | Phase 1: randomized, single-dose, double-blinded, placebo-controlled, crossover trial. Primary outcome measures TOTPAR score | Patients on opioids for chronic pain; BPI⩾4 (n=30). Pain diagnosis: neuropathic (n=7), nociceptive (n=7), mixed neuropathic and nociceptive (n=11), and uncategorized (n=5) | Phase 1: three 8-h lab sessions with 3 days washout | OME mean 68.1 mg (SD 57.2, range 7.5–228). Participants were taking oxycodone, morphine, methadone hydrocodone, and hydromorphone | Phase 1: single-dose placebo, dronabinol 10 and 20 mg | One subject took rescue pain medication in all conditions, one subject took rescue medication during the placebo and 10 mg dronabinol condition, and six subjects took rescue medication only with placebo. | In single-dose studies, 10 and 20 mg dronabinol significantly increased the amount of analgesic relief reported compared to placebo | GRADE rating ‘moderate’. Randomized and placebo-controlled. Downgraded as only a single dose was examined. TOTPAR 31.1 in placebo group, compared with 39.7 with dronabinol 10 mg and 41.7 with dronabinol 20 mg |
| Phase 2: open-label (no placebo) extension. Primary outcome measure change in pain intensity | Phase 2: open label for 4 weeks | Phase 2: flexible dose schedule, dronabinol 5 mg daily – 20 mg three times per day. | Opioid dose not reported | Mean baseline NRS of 6.9 compared with 5.2 after 4 weeks of dronabinol. This represents a statistically significant reduction | GRADE rating ‘low’. Open-label study. Significant improvements (p<0.05) in sleep, energy, pain relief, and social functioning. Lack of placebo control means effects may be non-specific or placebo | |||
| Portenoy et al, 2012 | Randomized, 4-arm placebo-controlled, graded-dose study | Patients with active cancer and chronic pain on a stable oral morphine regimen, plus fentanyl (n=360) | 5 weeks of medication administration | Morphine and fentanyl Median 120 mg OME Median 120 mg OME Median 180 mg OME Median 120 mg OME | Nabiximols 1–4 sprays Nabiximols 6–10 sprays Nabiximols 11–16 sprays Placebo | No change in median amount of breakthrough opioid medication in any group. Note that patients were discouraged from reducing their opioid dose, so the opioid-sparing effect could not be observed | Treatment difference (change from baseline pain score): –0.75 points (95% CI–1.28, –0.22, p=0.06 compared to placebo) –0.36 points (95% CI –.089, 0.18 points, p=0.19 compared to placebo). –0.09 points (95% CI: –0.62, 0.44 points, p=0.75 compared to placebo) Not reported (reference group) | GRADE rating ‘high’. Placebo-controlled, randomized controlled trial Opioid composite measure showed better improvements in the low-dose group. 1–4 spray group had significant improvements in analgesia. Lower tolerability of delta-9-THC : CBD in higher dose groups |
| Seeling et al, 2006 | Randomized, controlled trial (two groups) | Prostate cancer patients <70 y.o. (n=105). N=53 in intervention and 52 in control | From the day prior to surgery to two days post operation | Piritramide 1.5 mg/ml, bolus 2 mg (no continuous infusion) via patient-controlled analgesia for 48 h post operation | Dronabinol 5 mg × 8 doses over 48 h (perioperatively) | Median dose of piritramide alone was 74 mg (IQR 44–90) compared with 54 mg (IQR 46–88) when administered with dronabinol | The difference between the intervention (dronabinol) group and control group was not significant. No evidence was found of synergistic antinociceptive interaction between delta-9-THC and piritramide for acute postoperative pain | GRADE rating ‘high’. Placebo-controlled, randomized controlled trial Patients administered their own opioid doses |
Abbreviations: BPI, brief pain inventory; CBD, cannabidiol; GRADE, Grading of Recommendations Assessment, Development and Evaluation; IQR, interquartile range; NRS, numerical rating scale; OME, oral morphine equivalents; TOTPAR, total pain relief.
In another two studies, healthy volunteers (n=12 and 13, respectively) participated in crossover studies, with single doses of placebo, morphine alone, dronabinol alone, and dronabinol and morphine combined administered over four sessions (Naef et al, 2003; Roberts et al, 2006). These studies did not identify a synergistic effect on experimental pain in healthy controls, although Roberts et al (2006) found that the co-administration of dronabinol and morphine resulted in a reduced unpleasantness of pain compared to either drug alone. In a case series examining the effects of cannabinoid administration in patients with chronic non-cancer pain, three patients with mixed pain conditions (multiple sclerosis, HIV-related peripheral neuropathy, and lower back and leg pain) reported reductions in opioid requirements after initiation of smoked cannabis plant material (Lynch and Clark, 2003).
Five controlled studies were identified. One small, non-randomized study of patients with advanced cancer pain found that 5 out of 12 patients achieved pain control after receiving a cannabis infusion, compared with 2 out of 14 achieving pain control in the control group—a non-statistically different effect (Lissoni et al, 2014). Two randomized controlled trials examined delta-9-THC : Cannabidiol (THC : CBD) combination oral sprays compared to a placebo (Johnson et al, 2010; Portenoy et al, 2012) in patients with cancer pain who were taking opioids. These studies found improved analgesia with the THC : CBD combination compared to the placebo. Johnson et al (2010) found no effect of THC : CBD on breakthrough opioid dose requirements. Portenoy et al (2012) conducted a dose-ranging study, using fixed dose ranges of the THC : CBD combination. In this study, a significant analgesic effect was only found in the lowest dose group, with poorer tolerability observed for higher doses.
Two controlled studies examined the effects of dronabinol: one in patients with mixed chronic pain (Narang et al, 2008) and one in patients with prostate cancer (Seeling et al, 2006). Narang et al (2008) found significantly reduced pain intensity with the opioid–cannabinoid combination in double-blinded laboratory sessions compared to opioid alone. Additional improvements in sleep, energy, and social functioning were reported in a 4-week open-label phase of the same study (Narang et al, 2008). In the study by Seeling et al (2006), perioperative use of dronabinol compared with a placebo in patients with prostate cancer, no difference was found in self-administered opioid dose requirements between groups.
Quality Ratings of Clinical Studies
The clinical studies were rated using the GRADE criteria. One study provided very-low-quality evidence, three studies provided low-quality evidence, two studies provided moderate-quality evidence, and three randomized controlled trials provided high-quality evidence. None of the high-quality studies provided evidence of an opioid-sparing effect. The only study that provided direct evidence of an opioid-sparing effect was rated as providing very low-quality evidence (Lynch and Clark, 2003).
Discussion
Twenty-eight studies provided data relating to the potential opioid-sparing effect of cannabinoids in the context of opioid analgesia. Most of the pre-clinical studies examined reported reduced opioid requirements when co-administered with cannabinoids. Few controlled clinical studies measured opioid-sparing as an end point and findings relating to analgesia were mixed. Two controlled studies found no effect of cannabinoids on opioid dose requirements (Johnson et al, 2010; Seeling et al, 2006). One case series provided very low-quality evidence of a reduction in opioid dose requirements with cannabinoid co-administration (Lynch and Clark, 2003).
Most of the pre-clinical studies examined found synergistic effects when opioids and cannabinoids were co-administered, although two studies found that with specific opioids and cannabinoids the analgesic effect was additive rather than synergistic. Through meta-analyses, it was found that the doses of morphine and codeine required to produce the same analgesic effect were 3.6 and 9.5 times lower, respectively, when co-administered with delta-9-THC. Reductions in opioid requirements that are smaller than those seen in these pre-clinical studies may have relevance to pain treatment. Some confidence in these findings comes from the consistent observation of an opioid-sparing effect when using different nociceptive assays and in pain models of arthritis and diabetic neuropathy.
The relevance of the findings from these pre-clinical studies (with acute-dosing paradigms) to clinical chronic pain treatment must be considered. There are important limitations in translating findings from pre-clinical studies to clinical practice, particularly when evaluating doses and effect sizes. Although the outcomes of pre-clinical studies are often consistent with clinical studies, pre-clinical studies may over-represent effects. The lesser effect sizes in human studies have been attributed to the heterogeneity of clinical populations or the response being limited to sub-populations, reducing the overall effect observed (Berge, 2011). This underscores the importance of clinical studies to examine the effects found in pre-clinical work.
Controlled clinical studies demonstrated some beneficial effects of opioid and cannabinoid co-administration on outcomes of pain, sleep, and functioning in chronic pain patients (Abrams et al, 2011; Narang et al, 2008). One case series (n=3) provided very low-quality evidence of a reduction in opioid requirements with delta-9-THC administration. No randomized controlled studies were identified that provided evidence of an opioid-sparing effect of cannabinoids. Important limitations identified in these clinical studies included a lack of placebo control (Abrams et al, 2011; Lynch and Clark, 2003; Narang et al, 2008), difficulties extrapolating from experimental to clinical pain (Naef et al, 2003; Roberts et al, 2006), use of single doses (Naef et al, 2003; Roberts et al, 2006), use of small sample sizes (Lissoni et al, 2014; Lynch and Clark, 2003; Narang et al, 2008), and the mixed quality of the study design in general. In particular, Roberts et al (2006) used sub-therapeutic doses of morphine, which may have limited that study’s ability to test the effects of co-administration. Portenoy et al (2012) noted that the use of fixed dose ranges of cannabinoids may have limited that study’s ability to test the efficacy of cannabinoids for pain, as some patients may have dropped out due to tolerability. Moreover, by discouraging patients from reducing their opioid dose during the study, no opioid-sparing effect could be observed (Portenoy et al, 2012).
This review highlights some important considerations for future studies of cannabinoids. A dose-ranging study with patients with advanced cancer found that only lower doses of cannabinoids demonstrated analgesic effects (Portenoy et al, 2012). In the same study, one in four participants in the high-dose group discontinued treatment. Side effects such as nausea, drowsiness, and dizziness are more frequent with higher doses of cannabinoids (Narang et al, 2008; Portenoy et al, 2012). This suggests that dose range and tolerability are important outcomes to examine and that careful dose titration is essential. Future studies should carefully document adverse effects from concurrent opioid and cannabinoid administration to provide a better understanding of potential harms. One hypothesis to explain why patients reduce their opioid dose with cannabinoid administration is that they experience undesirable psychoactive effects from concurrent use of opioids and cannabinoids. This could be explored in future studies.
Recent observational studies provided further data on a possible opioid-sparing effect. Two studies found 44–64% reductions in self-reported opioid consumption in cohorts of patients with chronic pain who were using cannabis (Boehnke et al, 2016; Haroutounian et al, 2016). These observational studies provide further low-quality evidence supporting an opioid-sparing effect. A further observational study found that in patients with chronic pain who were prescribed opioids, greater pain relief was reported from cannabis than from their other medications (Degenhardt et al, 2015). A single case study also reported reduced requirements for breakthrough pain with oral delta-9-THC administration (Holdcroft et al, 1997). Taken together, these reports support the need for high-quality studies to directly assess the opioid-sparing effect of cannabinoids under controlled conditions.
This review identified some limitations in the literature. The pre-clinical studies examined used a range of animal populations, antinociceptive assays, opioids, and cannabinoids, and often had small numbers of animals per group. This resulted in statistical heterogeneity. Despite this, a large and significant effect was observed in the meta-analysis. No studies examined the opioid-sparing effect of cannabidiol alone, in combination with delta-9-THC outside of a 1 : 1 ratio, or with other cannabinoids. Further, the lack of high-quality studies in humans investigating the opioid-sparing effect means that the evidence for this is largely limited to pre-clinical studies. A funnel plot was produced and did not provide evidence of publication or small study bias; however, due to the small number of studies in the meta-analysis (<10) the interpretation of the funnel plot is limited.
The potential for cannabinoids to reduce opioid dose requirements and extend the duration of effective analgesia should not be understated. The rapid increase in opioid use and opioid-associated mortality is largely attributed use of opioids in chronic pain treatment (Chou et al, 2015; Zedler et al, 2014). Use of lower opioid doses has been recommended (Dowell et al, 2016); however, clinical processes to achieve this reduction are not well defined. Opioid-sparing medications may have enormous clinical relevance by enabling effective pain treatment with lower opioid doses and a potential reduction in opioid-related mortality.
In conclusion, pre-clinical studies support the opioid-sparing effect of delta-9-THC. However, the findings from clinical trials are inconsistent, with some studies found to have important limitations such as a lack of placebo control. An opioid-sparing effect of cannabinoids in chronic pain patients was observed in only one very-low-quality clinical study. These findings provide an early signal that warrants exploration. It remains to be seen if these promising pre-clinical and observational findings can be replicated in large, well-designed clinical studies.
Funding and disclosure
SN is supported by a NHMRC Research Fellowship (#1013803). The National Drug and Alcohol Research Centre at the University of New South Wales is supported by funding from the Australian Government under the Substance Misuse Prevention and Service Improvements Grant Fund. The contents of the published material are solely the responsibility of the authors and do not reflect the funding bodies. MAW has received a grant to his institution from CanniMed. BLF has received speaker fees or consulting fees from Allergan, Mettrum, CCIC, Mylan Pharmaceutical, Pfizer, Ethypharm, Richter Pharmaceuticals, and Lundbeck. He also received salary/grant support from Pfizer and Bioprojet, and in kind support from GW Pharma, Mylan Pharmaceuticals, and Brainsway. SN and NL have been investigators on untied educational grants from Reckitt-Benckiser. SN and MF have been investigators on an untied education grant from Indivior. KEK has previously received a speaker’s honorarium from Pfizer and Mundipharma, in addition to fees from an advisory board and an educational grant from Seqirus. As Director of NDARC, MF notes that the National Drug and Alcohol Research Centre has received untied educational grants from Mundipharma and Indivior. MF took part in a single research advisory board with Indivior in 2014. The remaining authors declare no conflict of interest.
Acknowledgments
SN, PS, JMT, MAW, BM, NL, KEK, MF, AS, and BLF were involved in the conceptualization of the work. SN and PS screened the abstracts. SN, PS, and JMT extracted the data and checked the extracted data. BBS transformed the data, prepared the data for meta-analysis, and provided advice on the meta-analysis. SN conducted the meta-analysis and drafted the manuscript with assistance from BM, PS, and JMT. All authors revised the manuscript and approved the final version.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abrams DI, Couey P, Shade SB, Kelly ME, Benowitz NL (2011). Cannabinoid-opioid interaction in chronic pain. Clin Pharmacol Ther 90: 844–851. [DOI] [PubMed] [Google Scholar]
- Berge O-G (2011). Predictive validity of behavioural animal models for chronic pain. Br J Pharmacol 164: 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehnke KF, Litinas E, Clauw DJ (2016). Medical cannabis use is associated with decreased opiate medication use in a retrospective cross-sectional survey of patients with chronic pain. J Pain 17: 739–744. [DOI] [PubMed] [Google Scholar]
- Cencioni MT, Chiurchiu V, Catanzaro G, Borsellino G, Bernardi G, Battistini L et al (2010). Anandamide suppresses proliferation and cytokine release from primary human T-lymphocytes mainly via CB2 receptors. PLoS ONE 5: e8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I et al (2015). The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop Effectiveness and Risks of Long-Term Opioid Therapy for Chronic Pain. Ann Intern Med 162: 276–286. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL (2004). Synergistic interactions between cannabinoid and opioid analgesics. Life Sci 74: 1317–1324. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Martin ZL, Smith FL, Welch SP (1999). Enhancement mu opioid antinociception by oral delta9-tetrahydrocannabinol: dose-response analysis and receptor identification. J Pharmacol Exp Ther 289: 859–867. [PubMed] [Google Scholar]
- Cichewicz DL, McCarthy EA (2003). Antinociceptive synergy between delta(9)-tetrahydrocannabinol and opioids after oral administration. J Pharmacol Exp Ther 304: 1010–1015. [DOI] [PubMed] [Google Scholar]
- Cichewicz DL, Welch SP (2003). Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral Delta 9-tetrahydrocannabinol. J Pharmacol Exp Ther305: 812–817.. [DOI] [PubMed]
- Cichewicz DL, Welch SP, Smith FL (2005). Enhancement of transdermal fentanyl and buprenorphine antinociception by transdermal delta9-tetrahydrocannabinol. Eur J Pharmacol 525: 74–82. [DOI] [PubMed] [Google Scholar]
- Cox ML, Haller VL, Welch SP (2007). Synergy between delta9-tetrahydrocannabinol and morphine in the arthritic rat. Eur J Pharmacol 567: 125–130. [DOI] [PubMed] [Google Scholar]
- da Fonseca Pacheco D, Klein A, de Castro Perez A, da Fonseca Pacheco CM, de Francischi JN, Duarte ID (2008). The mu-opioid receptor agonist morphine, but not agonists at delta- or kappa-opioid receptors, induces peripheral antinociception mediated by cannabinoid receptors. Br J Pharmacol 154: 1143–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Lintzeris N, Campbell G, Bruno R, Cohen M, Farrell M et al (2015). Experience of adjunctive cannabis use for chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Drug Alcohol Depend 147: 144–150. [DOI] [PubMed] [Google Scholar]
- Dowell D, Haegerich TM, Chou R (2016). CDC Guideline for Prescribing Opioids for Chronic Pain - United States, 2016. MMWR Recomm Rep 65: 1–49. [DOI] [PubMed] [Google Scholar]
- Finn DP, Beckett SR, Roe CH, Madjd A, Fone KC, Kendall DA et al (2004). Effects of coadministration of cannabinoids and morphine on nociceptive behaviour, brain monoamines and HPA axis activity in a rat model of persistent pain. Eur J Neurosci 19: 678–686. [DOI] [PubMed] [Google Scholar]
- Gui H, Tong Q, Qu W, Mao CM, Dai SM (2015). The endocannabinoid system and its therapeutic implications in rheumatoid arthritis. Int Immunopharmacol 26: 86–91. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P et al (2008). GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Br Med J 336: 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutounian S, Ratz Y, Ginosar Y, Furmanov K, Saifi F, Meidan R et al (2016). The effect of medicinal cannabis on pain and quality of life outcomes in chronic pain: a Prospective Open-label Study. Clin J Pain 32: 1036–1043. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC (1991). Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci 11: 563–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Briley EM, Herkenham M (1999). Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res 822: 17–25. [DOI] [PubMed] [Google Scholar]
- Holdcroft A, Smith M, Jacklin A, Hodgson H, Smith B, Newton M et al (1997). Pain relief with oral cannabinoids in familial Mediterranean fever. Anaesthesia 52: 483–486. [DOI] [PubMed] [Google Scholar]
- Houser SJ, Eads M, Embrey JP, Welch SP (2000). Dynorphin B and spinal analgesia: induction of antinociception by the cannabinoids CP55,940, Delta(9)-THC and anandamide. Brain Res 857: 337–342. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA et al (2002). International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 54: 161–202. [DOI] [PubMed] [Google Scholar]
- Ibrahim M, Rude M, Stagg N, Mata H, Lai J, Vanderah T et al (2006). CB2 cannabinoid receptor mediation of antinociception. Pain 122: 36–42. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A et al (2005). CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Proc Natl Acad Sci USA 102: 3093–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen B, Chen J, Furnish T, Wallace M (2015). Medical marijuana and chronic pain: a review of basic science and clinical evidence. Curr Pain Headache Rep 19: 50. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Burnell-Nugent M, Lossignol D, Ganae-Motan ED, Potts R, Fallon MT (2010). Multicenter, double-blind, randomized, placebo-controlled, parallel-group study of the efficacy, safety, and tolerability of THC:CBD extract and THC extract in patients with intractable cancer-related pain. J Pain Symptom Manage 39: 167–179. [DOI] [PubMed] [Google Scholar]
- Katsuyama S, Mizoguchi H, Kuwahata H, Komatsu T, Nagaoka K, Nakamura H et al (2013). Involvement of peripheral cannabinoid and opioid receptors in beta-caryophyllene-induced antinociception. Eur J Pain 17: 664–675. [DOI] [PubMed] [Google Scholar]
- Li JX, McMahon LR, Gerak LR, Becker GL, France CP (2008). Interactions between Delta(9)-tetrahydrocannabinol and mu opioid receptor agonists in rhesus monkeys: discrimination and antinociception. Psychopharmacology 199: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissoni P, Porro G, Messina G, Porta E, Rovelli F, Roselli MG et al (2014). Morphine, melatonin, Marijuana, Magnolia and MYRRH as the "five m" schedule in the treatment of cancer pain and the possible dose-dependency of the antitumor and analgesic effects of the pineal hormone melatonin. Anticancer Res 34: 6033–6034. [Google Scholar]
- Litchfield JA, Wilcoxon F (1949). A simplified method of evaluating dose-effect experiments. J Pharmacol Exp Ther 96: 99–113. [PubMed] [Google Scholar]
- Lynch ME, Clark AJ (2003). Cannabis reduces opioid dose in the treatment of chronic non-cancer pain. J Pain Symptom Manage 25: 496–498. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Yang W, France CP (2013). Interactions between mu-opioid receptor agonists and cannabinoid receptor agonists in rhesus monkeys: antinociception, drug discrimination, and drug self-administration.(Erratum appears in J Pharmacol Exp Ther. 2014 Mar; 348(3): 490-1 Note: Dosage error in article text). J Pharmacol Exp Ther 345: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanares J, Corchero J, Romero J, Fernandez-Ruiz JJ, Ramos JA, Fuentes JA (1999). Pharmacological and biochemical interactions between opioids and cannabinoids. Trends Pharmacol Sci 20: 287–294. [DOI] [PubMed] [Google Scholar]
- Mason DJ Jr, Lowe J, Welch SP (1999). Cannabinoid modulation of dynorphin A: correlation to cannabinoid-induced antinociception. Eur J Pharmacol 378: 237–248. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef M, Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Zbinden A, Brenneisen R (2003). The analgesic effect of oral delta-9-tetrahydrocannabinol (THC), morphine, and a THC-morphine combination in healthy subjects under experimental pain conditions. Pain 105: 79–88. [DOI] [PubMed] [Google Scholar]
- Narang S, Gibson D, Wasan AD, Ross EL, Michna E, Nedeljkovic SS et al (2008). Efficacy of dronabinol as an adjuvant treatment for chronic pain patients on opioid therapy. J Pain 9: 254–264. [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2006). The pharmacology of cannabinoid receptors and their ligands: an overview. Int J Obes 30: S13–S18. [DOI] [PubMed] [Google Scholar]
- Pertwee RG (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 153: 199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy RK, Ganae-Motan ED, Allende S, Yanagihara R, Shaiova L, Weinstein S et al (2012). Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: a randomized, placebo-controlled, graded-dose trial. J Pain 13: 438–449. [DOI] [PubMed] [Google Scholar]
- Pugh G Jr, Mason DJ Jr, Combs V, Welch SP (1997). Involvement of dynorphin B in the antinociceptive effects of the cannabinoid CP55,940 in the spinal cord. J Pharmacol Exp Ther 281: 730–737. [PubMed] [Google Scholar]
- Pugh G Jr, Smith PB, Dombrowski DS, Welch SP (1996). The role of endogenous opioids in enhancing the antinociception produced by the combination of DELTA9-tetrahydrocannabinol and morphine in the spinal cord. J Pharmacol Exp Ther 279: 608–616. [PubMed] [Google Scholar]
- Quartilho A, Mata HP, Ibrahim MM, Vanderah TW, Porreca F, Makriyannis A et al (2003). Inhibition of inflammatory hyperalgesia by activation of peripheral CB2 cannabinoid receptors. Anesthesiology 99: 955–960. [DOI] [PubMed] [Google Scholar]
- Reche I, Fuentes JA, Ruiz-Gayo M (1996). Potentiation of delta 9-tetrahydrocannabinol-induced analgesia by morphine in mice: involvement of mu- and kappa-opioid receptors. Eur J Pharmacol 318: 11–16. [DOI] [PubMed] [Google Scholar]
- Rice AS, Smith BH, Blyth FM (2015). Pain and the global burden of disease. Pain 157: 791–796. [DOI] [PubMed] [Google Scholar]
- Roberts JD, Gennings C, Shih M (2006). Synergistic affective analgesic interaction between delta-9- tetrahydrocannabinol and morphine. Eur J Pharmacol 530: 54–58. [DOI] [PubMed] [Google Scholar]
- Salio C, Fischer J, Franzoni MF, Mackie K, Kaneko T, Conrath M (2001). CB1-cannabinoid and mu-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. NeuroReport 12: 3689–3692. [DOI] [PubMed] [Google Scholar]
- Seeling W, Kneer L, Buchele B, Gschwend J, Maier L, Nett C et al (2006). DELTA9-tetrahydrocannabinol and the opioid receptor agonist piritramide do not act synergistically in postoperativepain. Anaesthesist 55: 391–400. [DOI] [PubMed] [Google Scholar]
- Smith FL, Cichewicz D, Martin ZL, Welch SP (1998). The enhancement of morphine antinociception in mice by delta9-tetrahydrocannabinol. Pharmacol Biochem Behav 60: 559–566. [DOI] [PubMed] [Google Scholar]
- Smith PA, Selley DE, Sim-Selley LJ, Welch SP (2007). Low dose combination of morphine and delta9-tetrahydrocannabinol circumvents antinociceptive tolerance and apparent desensitization of receptors. Eur J Pharmacol 571: 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PB, Welch SP, Martin BR (1994). Interactions between delta 9-tetrahydrocannabinol and kappa opioids in mice. J Pharmacol Exp Ther 268: 1381–1387. [PubMed] [Google Scholar]
- Tham SM, Angus JA, Tudor EM, Wright CE (2005). Synergistic and additive interactions of the cannabinoid agonist CP55,940 with mu opioid receptor and alpha2-adrenoceptor agonists in acute pain models in mice. Br J Pharmacol 144: 875–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde O, Noble F, Beslot F, Dauge V, Fournie-Zaluski MC, Roques BP (2001). Delta9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewardingeffect. Eur J Neurosci 13: 1816–1824. [DOI] [PubMed] [Google Scholar]
- Vigano D, Rubino T, Parolaro D (2005). Molecular and cellular basis of cannabinoid and opioid interactions. Pharmacol Biochem Behav 81: 360–368. [DOI] [PubMed] [Google Scholar]
- Volkow ND, McLellan AT (2016). Opioid abuse in chronic pain — misconceptions and mitigation strategies. N Engl J Med 374: 1253–1263. [DOI] [PubMed] [Google Scholar]
- Wakley AA, Craft RM (2011). THC-methadone and THC-naltrexone interactions on discrimination, antinociception, and locomotion in rats. Behav Pharmacol 22: 489–497. [DOI] [PubMed] [Google Scholar]
- Walker JM, Hohmann AG, Martin WJ, Strangman NM, Huang SM, Tsou K (1999). The neurobiology of cannabinoid analgesia. Life Sci 65: 665–673. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM (2002). Cannabinoid analgesia. Pharmacol Ther 95: 127–135. [DOI] [PubMed] [Google Scholar]
- Ware MA, Fitzcharles M-A, Joseph L, Shir Y (2010. a). The effects of nabilone on sleep in fibromyalgia: results of a randomized controlled trial. Anesth Analg 110: 604–610. [DOI] [PubMed] [Google Scholar]
- Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T et al (2010. b). Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 182: E694–E701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch SP, Stevens DL (1992). Antinociceptive activity of intrathecally administered cannabinoids alone, and in combination with morphine, in mice. J Pharmacol Exp Ther 262: 10–18. [PubMed] [Google Scholar]
- Williams IJ, Edwards S, Rubo A, Haller VL, Stevens DL, Welch SP (2006). Time course of the enhancement and restoration of the analgesic efficacy of codeine and morphine by δ9-tetrahydrocannabinol. Eur J Pharmacol 539: 57–63. [DOI] [PubMed] [Google Scholar]
- Williams J, Haller VL, Stevens DL, Welch SP (2008). Decreased basal endogenous opioid levels in diabetic rodents: effects on morphine and delta-9-tetrahydrocannabinoid-induced antinociception. Eur J Pharmacol 584: 78–86. [DOI] [PubMed] [Google Scholar]
- Wilson AR, Maher L, Morgan MM (2008). Repeated cannabinoid injections into the rat periaqueductal gray enhance subsequent morphine antinociception. Neuropharmacology 55: 1219–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams SG, Sagar DR, Burston JJ, Chapman V (2015). The role of the endocannabinoid system in pain. Handb Exp Pharmacol 227: 119–143. [DOI] [PubMed] [Google Scholar]
- Yesilyurt O, Dogrul A, Gul H, Seyrek M, Kusmez O, Ozkan Y et al (2003). Topical cannabinoid enhances topical morphine antinociception. Pain 105: 303–308. [DOI] [PubMed] [Google Scholar]
- Zedler B, Xie L, Wang L, Joyce A, Vick C, Kariburyo F et al (2014). Risk factors for serious prescription opioid-related toxicity or overdose among veterans health administration patients. Pain Med 15: 1911–1929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.