Abstract
Chemokines are small soluble molecules that play critical roles in wound healing, infection, and cancer progression. In particular, overexpression of the C-C motif chemokine ligand 2 (CCL2) in multiple cancer types correlates with poor patient prognosis. Animal studies have shown that CCL2 signals to macrophages and breast cancer cells to promote tumor growth, invasion, and metastasis, indicating that CCL2 is a promising therapeutic target. However, the effectiveness of human-specific neutralizing antibodies has not been fully evaluated. Furthermore, controversies remain on the use of neutralizing antibodies to target CCL2 and could be due to mode of drug delivery. Here, we investigated the effects of continuous delivery of human CCL2-neutralizing antibodies on breast cancer progression. Nude mice bearing MCF10CA1d breast tumor xenografts were implanted with osmotic pumps containing control IgG or anti-CCL2 and analyzed for CCL2 levels and tumor progression over 4 weeks. Despite inhibiting CCL2-induced migration in vitro, CCL2-neutralizing antibodies did not significantly affect tumor growth, invasion, macrophage recruitment, or tumor angiogenesis. CCL2 antibodies did not affect murine CCL2 levels but significantly increased human CCL2 levels in circulating blood and tumor interstitial fluid. CCL2-neutralizing antibodies reduced CCL2 levels in cultured cells short term at high concentrations. Enzyme-linked immunosorbent assay analysis of CCL2 in cultured fibroblasts and breast cancer cells revealed that the neutralizing antibodies sequestered CCL2 in the media. CCL2 levels were restored once the antibodies were removed. These studies reveal limitations in CCL2-neutralizing antibodies as a therapeutic agent, with important implications for translating CCL2 targeting to the clinic.
Introduction
Chemokines are a large family of small soluble proteins (8-10 kDa) that regulate homing and recruitment of immune cells through formation of molecular gradients. They play critical roles in regulating immune cell trafficking and endothelial sprouting during embryonic development, wound healing, and infection, which have been well documented [1], [2], [3]. Over 50 chemokines have been identified and are classified in multiple categories, C-C, C-X-C, C-X3-C, depending on the amino acid composition of a cysteine motif at the N terminus [1], [3]. Chemokines are highly conserved between mice and humans with up to an 80% amino acid sequence homology [4], [5]. Chemokines signal through G protein–coupled receptors, which possess a seven-transmembrane spanning domain and activate G protein–dependent and –independent pathways regulating cell migration, survival, proliferation, and gene transcription [3], [6].
Of the different classes of chemokines, C-C chemokines are known to regulate angiogenesis and recruitment of myeloid cells during acute and chronic inflammation [7], [8]. In particular, CCL2 is a critical regulator of macrophage recruitment during wound healing and infection and signals primarily through CCR2 receptors [9]. Overexpression of CCL2 has been implicated in inflammatory diseases including rheumatoid arthritis, macular degeneration, diabetes, and atherosclerosis [9], [10]. CCL2 is overexpressed in the epithelium and stroma of numerous cancer types, including gliomas, prostate cancers, ovarian cancers, and breast cancers, and expression correlates with recruitment of macrophages [10], [11], [12]. CCL2 expression correlates with tumor grade and unfavorable patient prognosis from studies of tumor biopsies and blood serum levels of cancer patients [10]. Functional studies in breast and prostate cancer animal models show that blockade of CCL2 activity through neutralizing antibodies or knockdown through small interfering RNAs inhibits tumor growth and metastasis, correlating with decreased macrophage recruitment and tumor angiogenesis [11], [12]. These studies demonstrate that CCL2 is a promising therapeutic target for many diseases.
Neutralizing antibodies and small pharmacologic agents to target cytokines in cancer have proven successful for the treatment of various cancer types, including breast and lung cancers [13]. While small pharmacologic agents are in early clinical development [14], CCL2-neutralizing antibodies have been the primary agent used to target CCL2 activity and have been extensively studied in animal models [15], [16], [17], [18]. However, recent studies have highlighted certain controversies surrounding targeting of CCL2 in cancer. Delivery of CCL2-neutralizing antibodies in animal models of breast and prostate cancer effectively inhibited tumor growth and metastasis, and decreased recruitment of macrophages [15], [16], [17], [18]. However, one recent study showed that cessation of CCL2 neutralization in a breast cancer model led to a rebound in tumor growth, associated with increased macrophage recruitment and tumor angiogenesis in the primary tumor [19]. In addition, clinical trials have reported limited to no therapeutic efficacy of the CCL2-neutralizing antibody CNTO888 either as a single agent or in combination with chemotherapy for the treatment of metastatic and nonmetastatic cancer [20], [21], [22]. Studies have suggested that a lack of efficacy was mainly due to clearance of antibody and rapid dissociation of antibody-CCL2 complex in vivo, leading to rebound of CCL2 levels during the antibody treatment [23]. The majority of studies have involved interval injections of CCL2-blocking reagents (antibody or inhibitor). Thus, this method of delivery could lead to fluctuations of inhibitor levels over time, possibly limiting therapeutic efficacy. Furthermore, preclinical studies utilized murine-specific CCL2 antibodies. To date, human-specific CCL2-neutralizing antibodies in mouse models have not been extensively tested.
This study sought to more fully characterize the effects of human-specific CCL2-neutralizing antibodies on breast cancer progression and determine whether effectiveness was related to method of delivery. Nude mice bearing MCF10CA1d breast tumor xenografts were implanted with osmotic pumps containing control IgG or anti-CCL2 and analyzed for CCL2 levels and tumor progression over 4 weeks. Despite inhibiting CCL2-induced migration in vitro, CCL2-neutralizing antibodies did not significantly affect breast tumor growth, invasion, macrophage recruitment, or tumor angiogenesis. CCL2 antibodies significantly increased human CCL2 levels in circulating blood and tumor interstitial fluid. CCL2-neutralizing antibodies reduced CCL2 levels in cultured cells short term at high concentrations. Enzyme-linked immunosorbent assay (ELISA) analysis of CCL2 in cultured fibroblasts and breast cancer cells revealed that the neutralizing antibodies sequestered CCL2 in the media and that CCL2 levels were restored once the antibodies were removed. This study demonstrates that continuous delivery of CCL2 antibodies in vivo is possible but reveals limitations to use of neutralizing antibodies as a targeting agent for CCL2, with important implications for translating targeted therapies to the clinic.
Materials and Methods
Cell Culture
The human breast cancer cell line MCF10CA1d (CA1d) [24], [25] was kindly provided by the laboratory of Dr. Fred Miller (University of Michigan). Human cancer–associated fibroblasts (hCAF-2300) were isolated from invasive ductal carcinoma tissues and characterized previously [26], [27]. Cells were cultured in Dulbecco's modified Eagle medium containing 10% fetal bovine serum (FBS), 2 mM l-glutamate, and 1% penicillin-streptomycin. Human monocyte cell line THP-1 monocytes were kindly provided by Dr. Katherine Fields (University of Kansas Medical Center) and were cultured in Roswell Park Memorial Institute medium (RPMI) containing 10% FBS and 1% penicillin-streptomycin. DNA genotyping was performed to confirm cell identity. Cells were tested for mycoplasma after thawing using a luciferase-based mycoplasma assay (Lozona, #LT07-703).
Transwell Migration Assay
Transwell migration assays were carried out in 24-well plates using Boyden chambers with 5-μm pores (VWR Inc., #10789-236). In the upper chamber, THP-1 cells were seeded at 100,000 cells per well in 100 μl of RPMI containing 0.1% bovine serum albumin (BSA). At the bottom chamber, 600 μl RPMI containing 0.1% BSA was pipetted into the bottom chamber in the presence or absence of recombinant CCL2 (10, 50, or 100 ng/ml), anti-CCL2 (0.1, 1, or 10 μg/ml), or IgG isotype control. Cells were incubated at 37°C for up to 5 hours. Phase contrast images were captured at 10× magnification of THP-1 cells migrated to the lower chamber using an EVOS FL auto imaging system, with 28 stitched fields per well. The total number of cells for each well was quantified using Image J software.
Animal Care and Orthotopic Transplantation
Athymic nu/nu female nude mice (5-6 weeks old) were obtained from Charles River (NCI #553) and maintained at the University of Kansas Medical Center animal facilities under Institutional Animal Care and Use Committee and Association for Assessment and Accreditation of Laboratory Animal Care International–approved guidelines. Breast cancer cells and cancer-associated fibroblasts were co-grafted into the mammary glands of mice as previously described [27]. Briefly, 100,000 MCF10CA1d cells and 250,000 hCAF-2300 cells were co-embedded into 50 μl of rat tail collagen I (Corning Inc., #354236) and cultured overnight at 37°C. The mice were anesthetized with 2% isoflurane. A “Y”-shaped incision was made 1 cm from the base of the tail, and the skin flaps were folded back to expose the inguinal mammary glands. One plug was inserted into each of the #4-5 and #9-10 inguinal mammary fat pads. The wounds were closed with wound clips, and mice were rehydrated with 0.9% NaCl. Mice were monitored daily for 7 to 10 days until wound clips were removed. Mice were then monitored twice weekly over for the next 3 weeks until tumors reached 1.5 cm in size, the maximum tumor size allowable. Mice were sacrificed 4 weeks (28 days) posttransplantation.
Osmotic Pump Implantation in Mice
Osmotic pumps were purchased from ALZET (Model 2004), with a manufacture pump rate of 0.23 μl per hour over 4 weeks. Osmotic pumps were filled with 1 mg/ml monoclonal mouse anti-human CCL2 antibody (R&D system, MAB279) or mouse IgG1 isotype control antibody (R&D System, MAB002) according to manufacturer's instructions. The filled pumps were equilibrated for 48 hours by incubation in 0.9% saline at 37°C. On the day of implantation, mice were anesthetized with 2% isoflurane. A 1-cm incision was made in the right dorsum, and one pump containing IgG or anti-CCL2 was inserted in each mouse (n = 5 per group). Pumps were implanted immediately after orthotopic transplantation of tumor cells. The wound was closed by wound clips. Wound clips were removed 7-10 days after surgery.
Pump Rate Analysis
Osmotic pump activity was characterized according to manufacturer protocol. Briefly, the pump was filled with 0.1% w/v trypan blue (Sigma), placed in 15 ml 0.9% saline solution in 50 ml canonical tube, and incubated at 37°C. At days 2, 3, 4, 7,10, 14, 21, and 28, the trypan blue in the saline solution was measured at OD590 [28]. After each measurement, the pump was transferred to a new fresh tube containing 15 ml 0.9% saline solution.
Blood Collection
Mice were anesthetized using 2% isoflurane. Using a 25-gauge needle, blood samples (50 μl/mouse/time point) were collected on the day before surgery (day 0), week 2, and week 4 posttransplantation through the submandibular vein. To prepare blood samples for ELISA, 0.5 M ethylenediaminetetraacetic acid was added to blood samples at 10% of total volume. Blood samples were centrifuged at 2000×g for 15 minutes at 4°C, and the supernatant containing plasma proteins was collected for analysis.
Preparation of Tumor Tissues for Interstitial Fluid Analysis
Interstitial fluid was collected from tumor tissues using a procedures previously described [29]. Forty-milligram to 100-mg samples from primary tumor tissues were weighed and homogenized with a pellet pestle in phosphate-buffered saline (PBS) added at a ratio of 3 μl:1 mg. The supernatant was collected after two rounds of centrifugation at 16,000×g for 15 minutes at 4°C.
ELISA of CCL2 Antibody Levels
To prepare plates for ELISA analysis of CCL2 antibodies, 96-well high-protein binding plates were incubated with 100 μl/well with 10 ng/ml recombinant human CCL2 (Peprotech, #300-04) diluted in PBS overnight. Plates were washed with PBS/0.05% Tween-20, and then wells were blocked with PBS containing 10% BSA for 2 hours. Wells were coated with CCL2 antibodies as standards, which were diluted to final concentrations of 10 μg/ml, 1 μg/ml, 500 ng/ml, 100 ng/ml, 10 ng/ml, and 1 ng/ml in PBS/2% BSA. As a negative control, wells were coated with IgG1 isotype control at a final concentration of 1 μg/ml. Wells were incubated with 100 μl of plasma or tumor interstitial fluid samples diluted 1:100 in PBS/2% BSA. Samples were incubated for 2 hours at room temperature, washed with PBS/0.05% Tween-20, and then incubated with 0.5 μg/ml biotinylated goat anti-mouse detection antibody (Vector Laboratories, #VA-9200) for 2 hours. Samples were then incubated with streptavidin conjugated to horse radish peroxidase (Vector Laboratories, #900-K31) for 30 minutes. Reactions were catalyzed with TMB substrate (Thermo Scientific, #34028), stopped with 2 N HCl, and read at OD450. CCL2 antibody levels were normalized to wells incubated with 2% BSA nonspecific binding control.
CCL2 ELISA
Plasma samples were diluted 1:4 in PBS containing 0.1% BSA and 0.05% Tween-20. Tumor interstitial fluid samples were diluted 1:20. Samples were assayed using mouse CCL2 ELISA kit (Peprotech, #900-K59) or human CCL2 ELISA kit (Peprotech, # 900-K31) according to manufacturer protocol.
To generate conditioned medium, MCF10CA1d or hCAF-2300 cells were seeded at 10,000 cells per well in triplicate in a 24-well plate. Cells were then incubated with 500 μl of Dulbecco's modified Eagle medium/10% FBS in the presence or absence of control IgG or CCL2 antibody (1 or 10 μg/ml) for 24 hours. The medium containing IgG or anti-CCL2 was collected and assayed for CCL2 levels by ELISA (Peprotech, #900-K31). To analyze for CCL2 levels post–antibody treatment, the cells were washed once with PBS and reincubated in serum free medium without IgG or antibody treatment for an additional 24 hours. The samples were collected and assayed for CCL2 levels by ELISA.
Tissue Embedding and Histology
Tumor samples were fixed in 10% neutral buffed formalin overnight and then dehydrated in a series of: 70%, 90%, and 100% ethanols for 30 minutes each. Tissues were further dehydrated in isopropanol for 1 hour, 50:50 isopropanol:wax at 60°C for 1 hour, and in wax overnight at 60°C. Tissues were mounted on cassettes and sectioned into 5-μm thin slices onto glass slides. For hematoxylin and eosin (H&E) stain, slides were dewaxed in two changes of xylenes at 5 minutes each and rehydrated in a series of ethanols (100%, 90%, 70%, 50%) at 3 minutes each. Slides were stained with Mayer's hematoxylin for 2 minutes and eosin for 1.5 minutes, dehydrated, and then mounted with Cytoseal under glass coverslips.
Co-Immunofluoresence and Immunohistochemistry
For co-immunofluorescent staining, dewaxed slides were subject to antigen retrieval using 10 mM sodium citrate buffer pH 6.0 for 20 minutes in pressure cooker under low pressure setting for antigen retrieval. Samples were blocked for endogenous mouse IgG using the M.O.M kit (Vector Lab., #BMK-2202) and then incubated with mouse anti-cytokeratin 5 (CK5) at a 1:50 dilution (Thermo Fisher Scientific, #MA5–12596) and with rat anti-F4/80 (Abcam, #ab6640) at a 1:100 dilution in PBS 3% FBS. After overnight staining at 4°C, slides were washed three times in PBS containing 0.05% Tween-20 and incubated with Alexa Fluor 568 goat anti-rat dilution (Invitrogen, #A11077) and Alexa Fluor 488 goat anti-mouse at a 1:100 dilution (Invitrogen, #A11001) for 2 hours. Slides were counterstained with 4′,6-diamidino-2-phenylindole at a 1:500 dilution and then mounted in PBS containing 50% glycerol. Eight high-power images for each slide were acquired using the FL Auto EVOS imaging system at 20× magnification. For image analysis, F4/80 positive cells per image were quantified using Image J particles analysis with threshold set above background. For compartmental analysis, the stroma at the tumor periphery was defined as the area bordering the tumor that was negative for CK5 staining. The tumor core was defined as areas positive for CK5 expression. F4/80 cells in each of the compartments were quantified and normalized to area size.
For immunohistochemistry staining, dewaxed slides were subject to antigen retrieval, treated in sodium citrate buffer, and blocked for endogenous peroxidase activity in deionized water (80%):methanol (10%):hydrogen peroxide (10%) for 10 minutes. After blocking for 1 hour in PBS/5% FBS, slides were incubated with rabbit anti–von Willebrand factor 8 (vWF8) at a 1:100 dilution (Chemicon International, #AB7356) overnight. Slides were washed in PBS 3 times for 10 minutes each, incubated with biotinylated anti-rabbit secondary antibody at a 1:500 dilution, and then incubated with peroxidase conjugated to streptavidin (Vector Lab., #PK-6100) for 30 minutes. Protein expression was detected using 3,3′-diaminobenzidine substrate (Vector Lab., #SK-4100). Slides were counterstained with Mayer's hematoxylin for 2 minutes, dehydrated, and mounted with Cytoseal (Thermo Fisher, #348976). Eight images per samples were acquired under 10× magnification using the FL Auto EVOS imaging system. Expression was quantified by Image J software using procedures previously described [30].
Lung Whole Mount Staining
Lung metastasis was analyzed by whole mount staining as described previously [15]. Briefly, lung tissues were fixed in neutral buffered formalin overnight at 4°C and dehydrated on 70%, 95%, and 100% ethanol for 1 hour each. Lung tissues were cleared in xylene overnight; rehydrated through a series of 100%, 95%, and 70% ethanols; and counterstained with hematoxylin for 5 minutes. Tissues were destained in 1% HCl and incubated with tap water for 10 to 20 minutes. Micrometastases were counted using a Motic AE31 inverted microscope at 20× magnification.
Statistical Analysis
Statistical analysis was performed using Graphpad software. Two-tailed Student’s t test was used for two-group comparisons. One-way ANOVA with Bonferonni post hoc comparison was used for multiple-group comparisons. Statistical significance was determined by P < .05. *P < .05, **P < .01, ***P < .001, and ns = not significant (P > .05).
Results
Antibody Neutralization of Cell Migration Induced by Human Recombinant CCL2
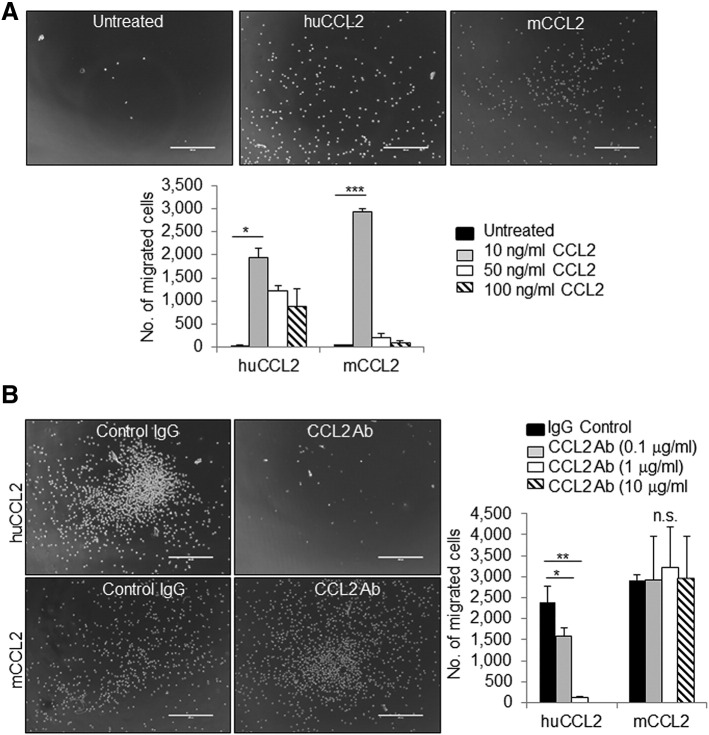
Multiple studies have utilized murine CCL2-neutralizing antibodies to demonstrate that targeting CCL2 activity inhibits mammary tumor progression [15], [16], [17], [18]. Murine CCL2 exhibits a 70% protein homology to human CCL2, indicating high degree of conservation [9]. The difference in sequence homology could affect receptor binding and activity. While the goal is to develop clinically active neutralizing antibodies for patients, i.e., antibodies that target human tissues, there have been few studies characterizing the effectiveness of human-specific CCL2-neutralizing antibodies on cancer progression in mouse models relative to endogenous and exogenous CCL2 levels. To address this issue, we obtained human-specific CCL2-neutralizing antibodies from a commercial source (R&D systems) and first analyzed the specificity of neutralization of murine and human CCL2. THP-1 human monocytes were cultured in vitro, stimulated with increasing dosages of murine or human CCL2, and analyzed for Transwell migration. Both murine CCL2 and human CCL2 were found to increase migration of THP-1 cells at 10 ng/ml, indicating cross-reactivity between mouse CCL2 and human CCR receptors. Chemotaxis decreased at 50 and 100 ng/ml, possibly reflecting desensitization of chemokine receptors at excess ligand concentrations. Human CCL2 induced migration with a wider range of concentration compared to murine CCL2 (Figure 1A), indicating higher sensitivity of human cells to human CCL2 compared to murine CCL2. Treatment of THP-1 cells with 1 μg/ml CCL2-neutralizing antibodies inhibited migration induced by human CCL2 but not mouse CCL2 (Figure 1B), indicating that CCL2-neutralizing antibodies specifically block human CCL2.
Figure 1.
Neutralizing antibodies inhibit THP1 cell migration induced by recombinant human CCL2 protein. (A) THP1 cells were stimulated with increasing concentrations of human (hCCL2) or mouse (mCCL2) recombinant CCL2 protein and analyzed for Transwell migration after 2 hours. Representative images of cells migrated into lower chamber are shown. (B) THP1 cells were stimulated with 10 ng/ml hCCL2 or mCCL2 in the presence or absence of increasing doses of CCL2-neutralizing antibodies or control IgG and analyzed for Transwell migration. Representative images of cells treated with CCL2 and 1 μg/ml control IgG or CCL2-neutralizing antibodies. Statistical analysis was performed using one-way ANOVA test with Bonferonni post hoc comparison. Statistical significance was determined by *P < .05, ***P < .01, and ns = not significant. Mean ± SEM is shown. Scale bar = 400 μm.
CCL2 Antibody Penetration of Primary MCF10CA1d Breast Tumor Xenografts
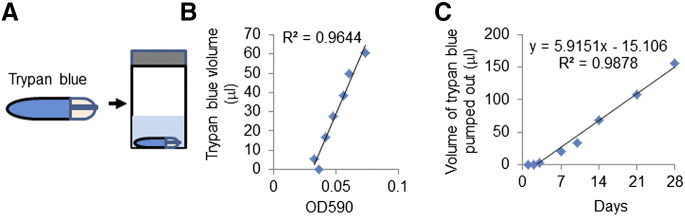
As the majority of studies involving CCL2 antibody delivery in vivo involved interval injections, we asked whether continuous delivery of CCL2 antibodies would increase therapeutic effectiveness. Osmotic pump delivery of CCL2 antibodies could stabilize drug levels and avoid dosage fluctuations caused by interval drug injections [31], [32], [33]. We obtained mini–osmotic pumps (Alzet) capable of continuous drug delivery for 4 weeks in tumor-bearing mice. To characterize their long-term function, osmotic pumps were filled with trypan blue dye placed in 50-ml conical tube containing sterile saline solution in cell culture incubator (Figure 2A). Pump activity was monitored over 4 weeks through sampling of saline and absorbance measurement of trypan blue (Figure 2B). After 3 days of incubation, the pump rate was found to be stable over 4 weeks, with an average rate of 5.9 μl per day (Figure 2C). This rate was close to the expected pump rate of 5.52 μl per day.
Figure 2.
Characterization of osmotic pump rate in vitro. (A) Diagram of how osmotic pump activity was tested in vitro using trypan blue dye. Osmotic pumps were filled with trypan blue dye and placed in 50-ml conical tubes containing saline solution. Activity was determined by measuring the optical density (OD590) of trypan blue in saline over 4 weeks. (B) The levels of trypan blue effluxed into saline solution were plotted against OD590. (C) Volume of trypan blue pumped out was plotted over time.
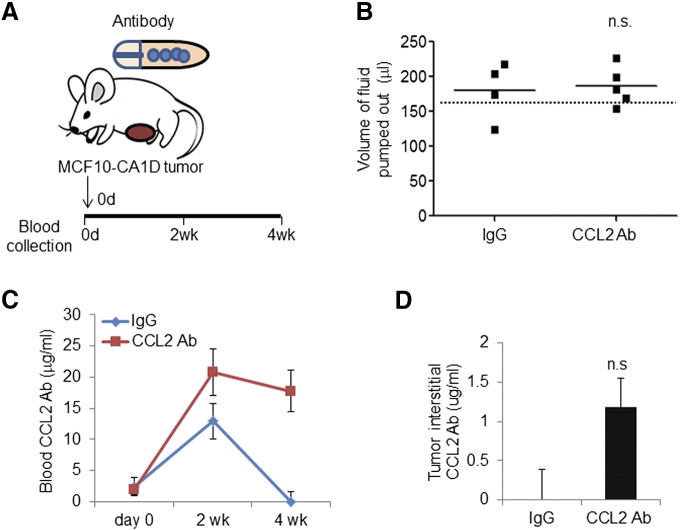
In previous studies, we had shown that breast stromal fibroblasts and cancer cells expressed high levels of CCL2, and CCL2 expression in basal-like breast cancers correlated with poor patient prognosis in basal-like breast cancers [15], [27]. Therefore, we analyzed the effectiveness of osmotic pump delivery of CCL2-neutralizing antibodies on tumor growth and progression using the MCF10CA1d model of basal-like breast cancer [24], [25]. MCF10CA1d breast cancer cells were co-grafted with human breast cancer–associated fibroblasts in the mammary glands of nude mice. Osmotic pumps with an expected delivery of rate of 0.3 mg/kg/day were filled with 1 mg/ml control IgG or anti-CCL2 and then implanted subcutaneously immediately after cellular transplantation (Figure 3A).
Figure 3.
Osmotic pump delivery of CCL2-neutralizing antibodies in the MCF10CA1d breast tumor xenograft model. (A) Diagram of pump implantation. Mice orthotopically transplanted with fibroblasts and MCF10CA1d breast cancer cells were implanted with osmotic pumps containing CCL2-neutralizing antibodies or isotype control IgG1 for 4 weeks (N = 5/group). (B) The volume of fluid pumped out was determined by subtracting the residual volume from initial volume after 4 weeks in vivo implantation. Dotted line indicates the expected volume pumped out (155 μl). (C) Measurement CCL2 antibody concentration in blood by ELISA. (D) ELISA analysis of tumor interstitial CCL2 antibody concentration in harvested tumor samples after 4 weeks of implantation. Statistical analysis was performed using two-tailed Student’s t test. Statistical significance was determined by P < .05. ns = not significant. Mean ± SEM is shown.
Mice were treated for 4 weeks until the control tumor reached 1.5 cm in size, the maximum allowable tumor size. We determined the efficiency of osmotic pump activity by measuring the volume of IgG and anti-CCL2 delivered. The residual volumes in the pumps were subtracted from the starting volume of 234 ± 2 μl. In the anti-CCL2–treated group, osmotic pumps delivered 186 ± 13 μl compared to 180 ± 20 μl of control IgG delivered, indicating consistent pump activity between groups (Figure 3B). To determine the levels of anti-CCL2 in blood circulation, anti-CCL2 levels were measured by ELISA from blood sampled at day 0, the day before surgery, and 2 weeks and 4 weeks postsurgery. Anti-CCL2 levels were undetectable before implantation, but 21 μg/ml was detected at week 2 and 18 μg/ml at week 4, indicating systemic delivery over time (Figure 3C). To determine whether anti-CCL2 penetrated the primary tumor, antibody levels in the tumor interstitial fluid were measured by ELISA. Approximately 1.1 μg/ml of anti-CCL2 was detected within the tumor, relative to control IgG–treated mice (Figure 3D). Placement of the tumor in either the #4-5 or #9-10 inguinal mammary gland did not affect antibody penetration or CCL2 levels (Supplemental Figure 1). In summary, these data indicate that osmotic pump delivery of CCL2-neutralizing antibodies led to stable antibody levels and penetrated tumor tissues.
Increased CCL2 levels in Tumor Bearing Mice Treated with CCL2-Neutralizing Antibodies
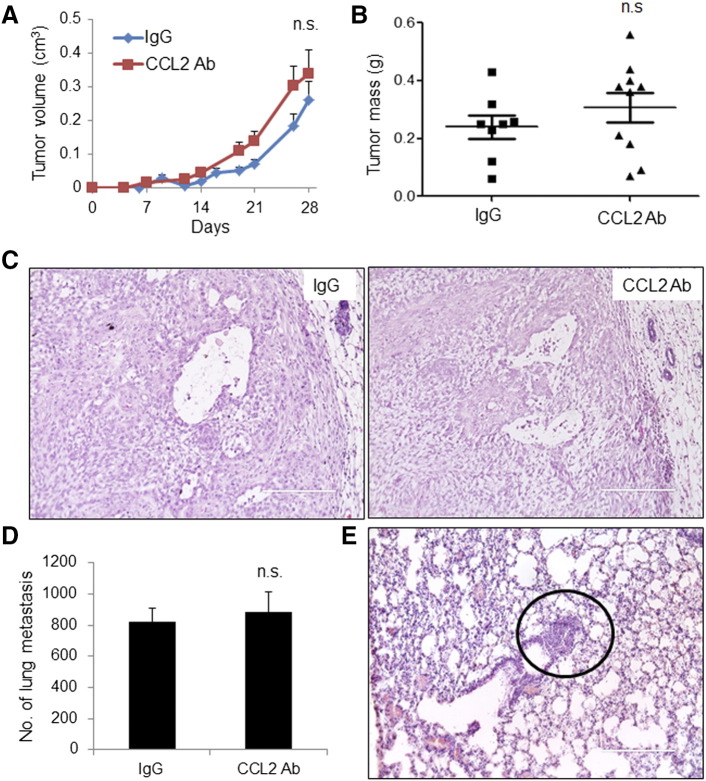
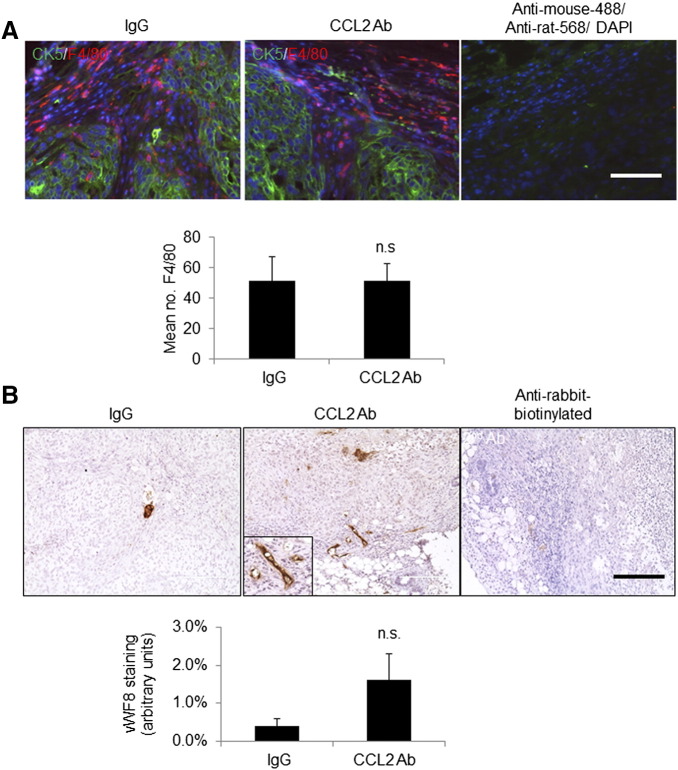
To determine the effect of anti-CCL2 on tumor growth and invasion, mice were measured for tumor growth on a weekly basis for up to 4 weeks. Compared to control IgG–treated mice, anti-CCL2–treated tumors did not show significant changes in tumor growth over time or tumor mass 4 weeks posttreatment (Figure 4, A and B). By H&E stain, primary tumors from IgG- and anti-CCL2–treated mice appeared to be high-grade tumors, exhibiting extensive necrosis and tumor invasion into the fat pad (Figure 4C). By whole mount staining and H&E stain of lung tissues, there were no significant differences in lung metastasis between IgG- and anti-CCL2–treated mice (Figure 4, D and E). We examined for possible changes in tumor angiogenesis and macrophage recruitment by immunostaining of primary tumors. Sections were co-immunofluorescent stained for antibodies to F4/80, a macrophage marker, with human-specific CK5 to distinguish MCF10CA1d breast cancer cells. Macrophages were primarily detected at the edge of the tumor, with few macrophages within the tumor tissue (Figure 5A). There were no differences in total levels of macrophages regardless of localization between IgG- and anti-CCL2–treated mice (Figure 5A, Supplemental Figure 2A). By vWF8 staining, there were no significant differences in tumor angiogenesis between IgG- and anti-CCL2–treated mice (Figure 5B, Supplemental Figure 2B).
Figure 4.
CCL2-neutralizing antibodies do not significantly affect progression of MCF10A1D breast tumor xenografts. IgG control– or CCL2 antibody–treated mice were analyzed for (A) tumor growth over time; (B) tumor mass at 28 days; (C) malignancy as assessed by H&E stain of primary tumor tissues; and (D) lung metastasis, which was assessed by lung whole mount staining. (E) Lung metastasis was validated by H&E stain. Representative metastatic lesion is circled. Statistical analysis was performed using two-tailed Student’s t test. Statistical significance was determined by P < .05. ns = not significant. Mean ± SEM is shown. Scale bar = 200 μm.
Figure 5.
Delivery of CCL2-neutralizing antibodies did not significantly affect macrophage infiltration or tumor angiogenesis. (A) Primary tumor tissues were co-immunofluorescent stained for CK5 (green) and F4/80 (red). Secondary antibody controls overlaid with 4′,6-diamidino-2-phenylindole are shown. (B) Primary tumor tissues were immunostained for vWF8. Magnified insert shows positive staining. Staining was quantified by Image J. Statistical analysis was performed using two-tailed Student’s t test. Statistical significance was determined by P < .05. ns = not significant. Mean ± SEM is shown. Scale bar = 200 μm.
Increased CCL2 levels in Tumor Bearing Mice Treated with CCL2-Neutralizing Antibodies
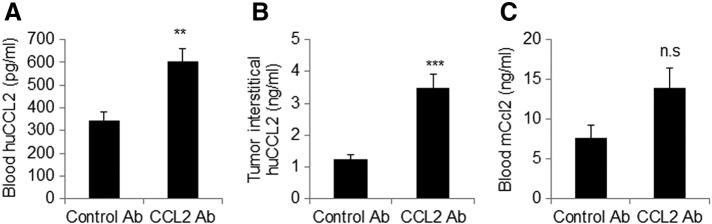
Given the lack of therapeutic efficacy with neutralizing antibodies, we examined for CCL2 expression levels in tumor-bearing mice. CCL2 levels were measured from blood samples and tumor interstitial fluid by ELISA. Mice treated with CCL2 antibodies showed significantly higher levels of human CCL2 in blood samples, with a mean concentration of 603 ng/ml compared to 346 ng/ml in IgG-treated mice (Figure 6A). Anti-CCL2 treatment also increased expression of human CCL2 in the tumor interstitial fluid, with a mean concentration of 3.5 ng/ml compared to 1.2 ng/ml in the control IgG group (Figure 6B). Furthermore, blood samples from anti-CCL2–treated mice showed a modest but not statistically significant increase in murine CCL2 expression (Figure 6C).
Figure 6.
Delivery of CCL2-neutralizing antibodies to tumor-bearing mice increased the levels of human CCL2 in blood and in primary tumor tissues. Tumor-bearing mice were treated with control IgG or CCL2-neutralizing antibodies. Four weeks posttransplantation, ELISAs were performed to measure the levels of (A) human CCL2 in blood, (B) human CCL2 in tumor interstitial fluid, and (C) murine CCL2 in blood. Statistical analysis was performed using two-tailed Student’s t test. Statistical significance was determined by P < .05. **P < .01, ***P < .001, ns = not significant.
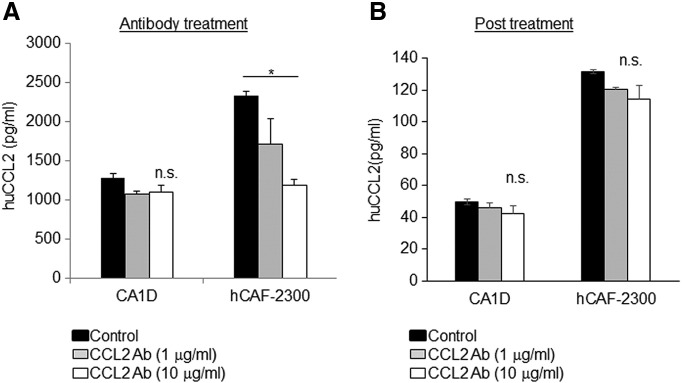
We addressed the possibility that anti-CCL2 treatment led to compensatory upregulation of CCL2 in breast cancer cells and fibroblasts, thereby increasing the levels found in circulation and in tumor tissues. MCF10CA1d breast cancer cells and fibroblasts were cultured in the presence or absence of anti-CCL2 or control IgG and measured for CCL2 secretion by ELISA. CCL2 expression in fibroblasts was twice as high compared to MCA10CA1D cancer cells. Increasing the concentration of anti-CCL2 to10 μg/ml reduced the levels of CCL2 in fibroblasts from 2300 to 1000 pg/ml and resulted in a small but not statistically significant decrease in CCL2 levels in breast cancer cells to 1000 pg/ml (Figure 7A). The presence of neutralizing antibodies inhibited detection of recombinant CCL2 (Supplemental Figure 3), indicating that the ELISAs detected free CCL2 secreted from cells but not CCL2 bound to neutralizing antibodies. To further determine how the presence of anti-CCL2 affected CCL2 levels in cultured cells, the antibody containing medium was removed, and cells were incubated in fresh medium for 24 hours to generate conditioned medium. When the conditioned medium was examined for CCL2 by ELISA, there were no significant differences in CCL2 levels in fibroblasts or breast cancer cells compared to IgG treatment (Figure 7B). In summary, these data indicate that neutralizing antibodies are capable of binding and sequestering free CCL2, but a continuous presence of CCL2 antibodies is required to maintain this sequestration.
Figure 7.
CCL2 levels in cultured cells are dependent on the presence of neutralizing antibodies. Cultured MCF10CA1d breast cancer cells or hCAF-2300 fibroblasts were treated with control IgG or CCL2-neutralizing antibodies for 24 hours. (A) Medium was analyzed for CCL2 expression by ELISA. (B). Cells were washed to remove antibodies and incubated in serum free condition medium for an additional 24 hours. CCL2 levels in conditioned medium were analyzed by ELISA. Statistical analysis was performed using one-way ANOVA test with Bonferonni post hoc comparison. Statistical significance was determined by P < .05. *P < .05, ns = not significant.
Discussion
CCL2 is a therapeutic target of interest for the treatment of cancer, and the effectiveness of CCL2 targeting alone or in combination with other agents is currently being determined in multiple clinical trials [20], [21], [22]. However, human-specific CCL2 antibodies have not been well characterized in preclinical studies. Furthermore, the primary method for targeting CCL2 has involved bolus injections of neutralizing antibodies. In these studies, we tested whether continuous delivery of human-specific CCL2-neutralizing antibodies in animals bearing breast tumor xenografts would be an effective therapeutic strategy. Our studies showed that stable delivery of CCL2-neutralizing antibodies over 4 weeks results in little therapeutic efficacy and increased CCL2 levels over time.
Previous studies have demonstrated that drugs delivered via osmotic pumps enhance therapeutic efficacy compared to bolus injections [32], [34], [35], [36]. For example, osmotic pump delivery of carboplatin to mouse models of ovarian cancer inhibited tumor growth more effectively than intraperitoneal injection and resulted in fewer toxic side effects [36]. In another study, osmotic pump delivery of interleukin-13 drug conjugates significantly slowed tumor growth and increased survival in animals with pancreatic cancer [35]. These therapeutic effects are in part due to stable drug delivery modulated by osmotic pumps [32]. In previous studies, we found that interval injections of CCL2 antibodies in mice resulted in a one-third decline of antibody levels within 24 hours [15]. Here, we found that osmotic pump delivery of CCL2-neutralizing antibodies resulted in stable levels of antibody of approximately 18 to 20 μg/ml in circulation over several weeks, while 10 μg/ml was shown to be inhibitory in vitro. However, osmotic pump delivery of CCL2-neutralizing antibodies did not significantly affect primary tumor growth or invasiveness. One possible factor limiting the effectiveness of the neutralizing antibody is tissue penetration. Approximately 1.1 μg/ml of antibodies was detected in tumor tissues compared to 18-20 μg/ml in blood circulation. These data indicate that significantly lower levels of antibodies penetrated tumor tissues. Difficulties in drug penetration have been reported in animal models and in patients, and have been attributed to a number of factors including leaky vasculature that does not extend into the tumor, tumor interstitial pressure, and a basement membrane that provides a barrier to drug entry [37]. It would be of interest in the future to further investigate the mechanisms preventing antibody uptake in order to enhance therapeutic efficacy.
In order to test the effectiveness of human-specific CCL2 therapeutic antibodies in preclinical models involving human and mouse cells, it was necessary to determine how the antibody would cross-react with other species. Previous studies have shown that murine-specific CCL2 antibodies inhibited tumor growth and metastasis transplanted with murine mammary or prostate carcinoma cells [15], [38]. One study showed that murine-specific CCL2-neutralizing antibodies inhibited the growth of MDA-MB-231 breast tumor xenografts and macrophage recruitment in mice [39]. The limited effectiveness of human-specific CCL2 antibodies observed in our studies could be due in part to binding specificity. Human CCL2 and mouse CCL2 share a 70% homology in amino acids [9], enabling human cells to respond to recombinant protein from both species. While both murine and human CCL2 stimulated human cells in vitro, the neutralizing antibodies inhibited human but not murine CCL2–induced cell migration. Antibody treatment of tumor-bearing mice increased the levels of human CCL2 and also murine CCL2 in circulation, although the increase in murine CCL2 was not statistically significant. CCL2 is expressed by a variety of murine cell types found within the primary breast tumor, including endothelial cells and bone marrow–derived cells [9]. It is possible that the combined increase in human and murine CCL2 abrogated the effects of CCL2 antibody neutralization.
The CCL2 antibody concentrations detected in blood circulation and in tumor tissues were associated with increased human CCL2 levels and slight increase in murine CCL2 levels, indicating a physiological effect of CCL2 antibody delivery. These phenotypes are consistent with previous studies characterizing the effects of antibody targeting of ligands. Delivery of the CCL2-neutralizing antibody CNTO0800 resulted in elevated CCL2 levels in patients during clinical trials [21], [22]. Mathematical modeling studies indicated that antibody binding prevented clearance of CCL2 and that a rapid disassociation of antibodies from the ligand contributed to the increased levels of free CCL2 [23]. In our studies, we found a two- to three-fold increase in CCL2 levels with antibody treatment in animals. Treatment of cultured cells with CCL2 antibodies reduced the levels of free CCL2 in fibroblasts and slightly decreased levels in breast cancer cells. However, 1000 pg/ml of CCL2 was still detectable even with 10 μg/ml of antibody present. This may potentially be due to the equilibrium between bound and unbound CCL2. After removal of antibodies, we found that the CCL2 expression levels in cultured cells were similar to control treatment, ruling out compensatory CCL2 production. Thus, the increased level of CCL2 observed in vivo could be potentially due to the enhanced stability of CCL2 by antibody binding, and a constant equilibrium between binding and dissociation of CCL2-antibody complexes. It would be of interest in the future to conduct studies on the pharmacokinetics of CCL2 antibodies in animal models of breast cancer.
In these studies, we delivered CCL2-neutralizing antibodies at a dosage of approximately 0.3 mg/kg/day. This dosage was based on previous studies reporting effectiveness of CCL2-neutralizing antibodies when delivered at dosages ranging from 0.4 to 2.9 mg/kg/day [15], [16], [19]. The slightly lower dosage was due to limitations in pump rate from an osmotic pump capable of drug delivery over 4 weeks. It is possible that a higher concentration of CCL2 antibodies might induce an anticancer effect or might further elevate CCL2 levels, contributing to a rebound effect that promotes tumor progression. These studies would require a higher pump rate with shorter pump time, requiring more frequent pump replacement in the MCF10CA1d tumor model and increasing physical stress to the animals. From a clinical perspective, this would require long-term continuous delivery of high dosages of anti-CCL2 to prevent a possible rebound effect caused by decreased CCL2 antibody levels.
Conclusions
In summary, our studies report limitations in the use of human-specific CCL2-neutralizing antibodies for the treatment of breast cancer in mouse models. Previous studies have shown that knockdown of CCL2 in breast tumor xenografts effectively inhibited tumor growth and metastasis and significantly reduced CCL2 levels [27], [40]. These studies suggest that CCL2 remains a viable therapeutic target in anticancer treatment. Strategies to inhibit CCL2 expression, rather than CCL2 activity only, may be a more effective approach to target CCL2. Alternatively, CCR2 inhibitors, which are in the preclinical pipeline [14], may overcome limitations caused by CCL2 targeting. Furthermore, the development of more physiologically relevant preclinical models may in turn improve the design of anticancer drugs.
Conflicts of Interest
None.
Funding Source
This work was supported by funds from the American Cancer Society (RSG-13-182-01-CSM) and by the National Institutes of Health (R01CA172764) to N. Cheng.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.tranon.2017.06.009.
Contributor Information
Min Yao, Email: myao2@kumc.edu.
Curtis Smart, Email: smartcurtis93@yahoo.com.
Qingting Hu, Email: qhu@kumc.edu.
Nikki Cheng, Email: ncheng@kumc.edu.
Appendix A. Supplementary data
Supplemental Figure 1. Location of breast tumor xenografts is not a factor in CCL2 antibody uptake or CCL2 levels.
Supplemental Figure 2. Quantification of F4/80 macrophage infiltration in MCF10CA1d primary tumor tissues.
Supplemental Figure 3. Human recombinant CCL2 bound to neutralizing antibodies can not be detected by ELISA.
References
- 1.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Ann Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 2.Palomino DC, Marti LC. Chemokines and immunity. Einstein (Sao Paulo) 2015;13:469–473. doi: 10.1590/S1679-45082015RB3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao M, Brummer G, Acevedo D, Cheng N. Cytokine regulation of metastasis and tumorigenicity. Adv Cancer Res. 2016;132:265–367. doi: 10.1016/bs.acr.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Laing KJ, Secombes CJ. Chemokines. Dev Comp Immunol. 2004;28:443–460. doi: 10.1016/j.dci.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 6.Rees PA, Greaves NS, Baguneid M, Bayat A. Chemokines in wound healing and as potential therapeutic targets for reducing cutaneous scarring. Adv Wound Care. 2015;4:687–703. doi: 10.1089/wound.2014.0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ridiandries A, Tan JT, Bursill CA. The role of CC-chemokines in the regulation of angiogenesis. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou D, Huang C, Lin Z, Zhan S, Kong L, Fang C, Li J. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cell Sign. 2014;26:192–197. doi: 10.1016/j.cellsig.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hembruff SL, Cheng N. Chemokine signaling in cancer: implications on the tumor microenvironment and therapeutic targeting. Cancer Ther. 2009;7:254–267. [PMC free article] [PubMed] [Google Scholar]
- 11.Soria G, Ben-Baruch A. The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer Lett. 2008;267:271–285. doi: 10.1016/j.canlet.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Zhang J, Patel L, Pienta KJ. CC chemokine ligand 2 (CCL2) promotes prostate cancer tumorigenesis and metastasis. Cytokine Growth Factor Rev. 2010;21:41–48. doi: 10.1016/j.cytogfr.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12:278–287. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 14.Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA, Yano M. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–662. doi: 10.1016/S1470-2045(16)00078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hembruff SL, Jokar I, Yang L, Cheng N. Loss of transforming growth factor-beta signaling in mammary fibroblasts enhances CCL2 secretion to promote mammary tumor progression through macrophage-dependent and -independent mechanisms. Neoplasia. 2010;12:425–433. doi: 10.1593/neo.10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, Wojno K, Snyder LA, Yan L, Pienta KJ. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res. 2007;67:9417–9424. doi: 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 18.Zhu X, Fujita M, Snyder LA, Okada H. Systemic delivery of neutralizing antibody targeting CCL2 for glioma therapy. J Neurooncol. 2011;104:83–92. doi: 10.1007/s11060-010-0473-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonapace L, Coissieux MM, Wyckoff J, Mertz KD, Varga Z, Junt T, Bentires-Alj M. Cessation of CCL2 inhibition accelerates breast cancer metastasis by promoting angiogenesis. Nature. 2014;515:130–133. doi: 10.1038/nature13862. [DOI] [PubMed] [Google Scholar]
- 20.Brana I, Calles A, LoRusso PM, Yee LK, Puchalski TA, Seetharam S, Zhong B, de Boer CJ, Tabernero J, Calvo E. Carlumab, an anti–C-C chemokine ligand 2 monoclonal antibody, in combination with four chemotherapy regimens for the treatment of patients with solid tumors: an open-label, multicenter phase 1b study. Target Oncol. 2015;10:111–123. doi: 10.1007/s11523-014-0320-2. [DOI] [PubMed] [Google Scholar]
- 21.Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ, Li S, Seetharam S, Puchalski TA, Takimoto C. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Invest New Drugs. 2013;31:760–768. doi: 10.1007/s10637-012-9869-8. [DOI] [PubMed] [Google Scholar]
- 22.Sandhu SK, Papadopoulos K, Fong PC, Patnaik A, Messiou C, Olmos D, Wang G, Tromp BJ, Puchalski TA, Balkwill F. A first-in-human, first-in-class, phase I study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 in patients with solid tumors. Cancer Chemother Pharmacol. 2013;71:1041–1050. doi: 10.1007/s00280-013-2099-8. [DOI] [PubMed] [Google Scholar]
- 23.Fetterly GJ, Aras U, Meholick PD, Takimoto C, Seetharam S, McIntosh T, de Bono JS, Sandhu SK, Tolcher A, Davis HM. Utilizing pharmacokinetics/pharmacodynamics modeling to simultaneously examine free CCL2, total CCL2 and carlumab (CNTO 888) concentration time data. J Clin Pharmacol. 2013;53:1020–1027. doi: 10.1002/jcph.140. [DOI] [PubMed] [Google Scholar]
- 24.Santner SJ, Dawson PJ, Tait L, Soule HD, Eliason J, Mohamed AN, Wolman SR, Heppner GH, Miller FR. Malignant MCF10CA1 cell lines derived from premalignant human breast epithelial MCF10AT cells. Breast Cancer Res Treat. 2001;65:101–110. doi: 10.1023/a:1006461422273. [DOI] [PubMed] [Google Scholar]
- 25.Strickland LB, Dawson PJ, Santner SJ, Miller FR. Progression of premalignant MCF10AT generates heterogeneous malignant variants with characteristic histologic types and immunohistochemical markers. Breast Cancer Res Treat. 2000;64:235–240. doi: 10.1023/a:1026562720218. [DOI] [PubMed] [Google Scholar]
- 26.Fang WB, Mafuvadze B, Yao M, Zou A, Portsche M, Cheng N. TGF-beta negatively regulates CXCL1 chemokine expression in mammary fibroblasts through enhancement of Smad2/3 and suppression of HGF/c-Met signaling mechanisms. PLoS One. 2015;10:e0135063. doi: 10.1371/journal.pone.0135063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fang WB, Yao M, Brummer G, Acevedo D, Alhakamy N, Berkland C, Cheng N. Targeted gene silencing of CCL2 inhibits triple negative breast cancer progression by blocking cancer stem cell renewal and M2 macrophage recruitment. Oncotarget. 2016 doi: 10.18632/oncotarget.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uliasz TF, Hewett SJ. A microtiter trypan blue absorbance assay for the quantitative determination of excitotoxic neuronal injury in cell culture. J Neurosci Methods. 2000;100:157–163. doi: 10.1016/s0165-0270(00)00248-x. [DOI] [PubMed] [Google Scholar]
- 29.Haslene-Hox H, Oveland E, Berg KC, Kolmannskog O, Woie K, Salvesen HB, Tenstad O, Wiig H. A new method for isolation of interstitial fluid from human solid tumors applied to proteomic analysis of ovarian carcinoma tissue. PLoS One. 2011;6:e19217. doi: 10.1371/journal.pone.0019217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yao M, Yu E, Staggs V, Fan F, Cheng N. Elevated expression of chemokine C-C ligand 2 in stroma is associated with recurrent basal-like breast cancers. Mod Pathol. 2016 doi: 10.1038/modpathol.2016.78. [DOI] [PubMed] [Google Scholar]
- 31.Rosen H, Abribat T. The rise and rise of drug delivery. Nat Rev Drug Discov. 2005;4:381–385. doi: 10.1038/nrd1721. [DOI] [PubMed] [Google Scholar]
- 32.Patra CN, Swain S, Sruti J, Patro AP, Panigrahi KC, Beg S, Rao ME. Osmotic drug delivery systems: basics and design approaches. Recent Pat Drug Deliv Formul. 2013;7:150–161. doi: 10.2174/1872211311307020007. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Pan H, Ye T, Liu D, Li Q, Chen F, Yang X, Pan W. Recent aspects of osmotic pump systems: functionalization, Clinical use and Advanced Imaging Technology. Curr Drug Metab. 2016;17:279–291. doi: 10.2174/1389200216666151015115706. [DOI] [PubMed] [Google Scholar]
- 34.Vassileva V, Allen CJ, Piquette-Miller M. Effects of sustained and intermittent paclitaxel therapy on tumor repopulation in ovarian cancer. Mol Cancer Ther. 2008;7:630–637. doi: 10.1158/1535-7163.MCT-07-2117. [DOI] [PubMed] [Google Scholar]
- 35.Shimamura T, Fujisawa T, Husain SR, Joshi B, Puri RK. Interleukin 13 mediates signal transduction through interleukin 13 receptor alpha2 in pancreatic ductal adenocarcinoma: role of IL-13 Pseudomonas exotoxin in pancreatic cancer therapy. Clin Cancer Res. 2010;16:577–586. doi: 10.1158/1078-0432.CCR-09-2015. [DOI] [PubMed] [Google Scholar]
- 36.Zhidkov N, De Souza R, Ghassemi AH, Allen C, Piquette-Miller M. Continuous intraperitoneal carboplatin delivery for the treatment of late-stage ovarian cancer. Mol Pharm. 2013;10:3315–3322. doi: 10.1021/mp400345h. [DOI] [PubMed] [Google Scholar]
- 37.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6:583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 38.Mizutani K, Sud S, McGregor NA, Martinovski G, Rice BT, Craig MJ, Varsos ZS, Roca H, Pienta KJ. The chemokine CCL2 increases prostate tumor growth and bone metastasis through macrophage and osteoclast recruitment. Neoplasia. 2009;11:1235–1242. doi: 10.1593/neo.09988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujimoto H, Sangai T, Ishii G, Ikehara A, Nagashima T, Miyazaki M, Ochiai A. Stromal MCP-1 in mammary tumors induces tumor-associated macrophage infiltration and contributes to tumor progression. Int J Cancer. 2009;125:1276–1284. doi: 10.1002/ijc.24378. [DOI] [PubMed] [Google Scholar]
- 40.Fang WB, Yao M, Jokar I, Alhakamy N, Berkland C, Chen J, Brantley-Sieders D, Cheng N. The CCL2 chemokine is a negative regulator of autophagy and necrosis in luminal B breast cancer cells. Breast Cancer Res Treat. 2015;150:309–320. doi: 10.1007/s10549-015-3324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Location of breast tumor xenografts is not a factor in CCL2 antibody uptake or CCL2 levels.
Supplemental Figure 2. Quantification of F4/80 macrophage infiltration in MCF10CA1d primary tumor tissues.
Supplemental Figure 3. Human recombinant CCL2 bound to neutralizing antibodies can not be detected by ELISA.