Abstract
Purpose
Guidelines in the United States recommend consideration of testing for mutations in the BRCA1 and BRCA2 genes for women diagnosed with breast cancer under age 45. Identification of mutations among survivors has implications for secondary prevention and familial risk reduction. Although only 10% of breast cancers are diagnosed under age 45, there are approximately 2.8 million breast cancer survivors in the United States, such that the young survivor population likely numbers in the hundreds of thousands. However, little is known about genetic testing rates in this population. We assessed trends in BRCA1/2 testing among breast cancer survivors who were under age 45 at diagnosis and were treated from 2005 to 2012.
Methods
Using insurance claims from a national database (MarketScan), we identified incident breast cancer cases among (1) women age ≤ 40 and (2) women age 41-45. We measured BRCA1/2 testing using Kaplan-Meier analysis and Cox proportional hazards models.
Results
Among 26985 patients analyzed, BRCA1/2 testing rates increased with each year of diagnosis from 2005 to 2012 (P<0.001). However, among women treated in earlier years, testing rates did not approach those of patients treated later, even after extended follow-up (median time from surgery to testing among patients treated in 2005, not reached; median time to testing among patients treated in 2012, 0.2 months for women ≤ 40 and 1.0 month for women age 41-45). Women age 41-45 had lower rates than women ≤ 40 throughout the analysis period (P<0.001 for each year).
Conclusions
BRCA1/2 testing rates among young women with incident breast cancer increased substantially in the last decade. However, most survivors treated in earlier years have never been tested. Our results demonstrate a need to better incorporate genetic counseling into survivorship and primary care for this population.
INTRODUCTION
Mutations in the BRCA1 and BRCA2 genes are associated with under 10% of breast cancer cases [1, 2]. However, germline mutations in BRCA1 or BRCA2 confer a substantially increased risk of breast cancer, with a cumulative incidence by age 70 of 44-78% among BRCA1 mutation carriers and 31%-56% among BRCA2 mutation carriers [1, 3].
In 2001, the National Comprehensive Cancer Network (NCCN) recommended consideration of genetic testing for patients with a history of breast cancer diagnosed at age ≤ 40, and for patients with clinical or family histories otherwise suggestive of the hereditary breast and ovarian cancer syndrome [4]. By 2005, consideration of testing was additionally recommended for patients between age 40 and 50, if deemed clinically appropriate [5]. The 2009 guidelines increased the upper limit for age at diagnosis for which testing should be generally considered from 40 to 45 years [6]. Other testing criteria for women include triple negative breast cancer diagnosed at age ≤ 60, any epithelial ovarian cancer, and pancreatic cancer in the setting of a concerning family history [7]. In one study of patients with incident breast cancer diagnosed from 2004-2007, 30% of women age ≤ 40 had BRCA1/2 testing, and black and Hispanic women were less likely to have testing than white women [8]. However, testing rates began to increase substantially for women diagnosed at the end of that study period, and it is not known to what degree that trend has impacted survivors with more remote diagnoses.
There are currently 2.8 million survivors of breast cancer living in the United States [9], so although just 10% of cases of breast cancer are diagnosed at age ≤ 45 [10], the number of survivors from that age group is likely in the hundreds of thousands. Within the oncology community, there is increasing interest in genetic testing and especially in novel gene panel testing in breast cancer [11–14], but long-term survivors of breast cancer have fewer visits to an oncologist with each passing year [15]. Consideration of even basic, standard-of-care genetic testing may therefore not be routinely incorporated into the care of young survivors who were not tested at the time of diagnosis. Nevertheless, identification of BRCA1 and BRCA2 mutations within the survivor population has implications for prevention of ovarian cancer and a second primary breast cancer [16], as well as for genetic testing and risk reduction within families.
In this study, we used insurance claims data to assess rates of BRCA1/2 testing within a cohort of privately insured young women with incident breast cancer treated in the United States from 2005-2012. Since the 2009 NCCN guidelines were the first to explicitly recommend consideration of testing for all women diagnosed under age 45, we separately analyzed women diagnosed at age ≤ 40 and women diagnosed at age 41 to 45. Our specific aim was to assess for differences in genetic testing rates among survivors according to year of diagnosis.
METHODS
We identified patients with incident breast cancer diagnosed from 2005-2012 using the MarketScan database [17]. We specifically analyzed rates of BRCA1/2 testing among (1) women age ≤ 40 at diagnosis and (2) women age 41-45 at diagnosis.
MarketScan [17] is a proprietary database consisting of a convenience sample of paid medical claims for patients with employment-based health insurance. This dataset contains health insurance claims data for individuals in the United States with primary or Medicare supplemental coverage. The data are de-identified and meet Health Insurance Portability and Accountability (HIPAA) confidentiality requirements [17, 18]. For this analysis, we used data from the Commercial Claims and Encounters and the Medicare Supplemental and Coordination of Benefits databases. We applied a modified version of the Nattinger algorithm [19–21] to identify incident breast cancer cases. Briefly, potential cases were identified based upon a breast cancer ICD-9 code (174.x, malignant neoplasm of the female breast). The algorithm further identified patients who additionally had a procedure code for mastectomy, lumpectomy, or axillary lymph node dissection. Patients who met these criteria were included if they had at least two outpatient claims on different dates with a primary diagnosis of breast cancer, as well as either (1) a mastectomy claim, or (2) a lumpectomy or partial mastectomy claim followed by at least one radiation therapy claim. They could also be included if they had a surgical claim plus at least two claims with a primary breast cancer diagnosis, but did not have both a claim for another type of cancer and a claim for secondary cancer of the breast (ICD-9 codes 198.81 or 198.2). Patients with a claim under a breast cancer diagnosis or for a breast cancer procedure within the preceding three years, indicating prevalent rather than incident cases, were excluded. To capture claims for BRCA1/2 testing that occurred close in time to the diagnosis of the index cancer, we included patients diagnosed from 2005 to 2012 who had continuous coverage through the 6 months prior to the month of their index cancer-directed procedure and during the month of that procedure. We did not otherwise require a continuous coverage period after the index cancer-directed procedure, but rather censored patients in our analyses on the date they no longer had continuous documented coverage. Patients with documented BRCA testing claims prior to 6 months before diagnosis were excluded. The year of diagnosis was defined as the year in which there was a claim for the index breast cancer surgery.
We identified BRCA1/2 testing claims using mutation-specific HCPCS procedure codes S3818-S3823, as previously described [8]. These codes were discontinued in 2012, and Medicare then introduced new specific HCPCS codes 81211-81217 for BRCA testing [22, 23]; we also included claims filed under the new codes. A primary aim of our analysis was to identify potential underuse of testing, so we sought to capture as many potential BRCA mutation testing claims as possible. In our primary analysis, we therefore also included claims filed under stackable CPT codes 83890-83909, 83912, 83914, or 88271, which until 2012 could be used to bill for molecular biological techniques that might have been used for BRCA1/2 testing. Since these codes were not specific for BRCA testing, we included them only when filed under an ICD-9 code for a personal history of breast cancer (174.x-175.x, 233.0, V10.03), genetic counseling and testing (V26.3x), or other genetic screening (V82.79). We also conducted a sensitivity analysis that included only the BRCA1/2 mutation-specific procedure codes. Since HCPCS procedure codes S3818-S3823 would not have been covered by Medicare, and Medicare patients may therefore not have had such claims submitted, we performed a second sensitivity analysis excluding patients with Medicare supplemental coverage.
We assessed rates of BRCA1/2 mutation testing, and timing of testing, using Kaplan-Meier analyses. To capture BRCA1/2 testing claims that followed identification of cancer but predated cancer surgery, we defined the index date for these analyses as six months (180 days) prior to surgery. Patients were censored on the date they were no longer included in the database due to changes in insurance coverage, or at the end of the follow-up period (December 31, 2013). Time to testing was compared among years of diagnosis within patient cohorts via the log-rank test. Confidence bands in our figures were generated via the Hall-Wellner method [24]. We conducted multivariable analyses using Cox proportional hazards models. Two-sided P values less than 0.05 were considered statistically significant. Analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC).
RESULTS
We identified 35388 patients with incident breast cancer (15149 women age ≤ 40 and 20239 women age 41-45) who had surgery from 2005-2012. Of those patients, we excluded 3584 women age ≤ 40 and 4223 women age 41-45 who did not have continuous documented insurance coverage for the month of their index breast cancer surgery and the 180 days prior to surgery. We also excluded 365 women age ≤ 40 and 231 women age 41-45 who had documentation of BRCA1/2 testing prior to a 180 day period before surgery. Our analysis cohort therefore included 26985 patients (Table 1). Continuous insurance coverage data for patients treated in each year are listed in Supplemental Table 1.
Table 1.
Patient characteristics
| Women age ≤ 40 N (%) |
Women age 41–45 N (%) |
|
|---|---|---|
| Total | 11200 (100) | 15785 (100) |
| Age at diagnosis | ||
| ≤ 25 | 210 (2) | |
| 26–30 | 831 (7) | |
| 31–35 | 2776 (25) | |
| 36–40 | 7383 (66) | |
| 41–45 | 15785 (100) | |
| Index surgery claim | ||
| Lumpectomy | 5967 (53) | 10177 (64) |
| Mastectomy | 4461 (40) | 4878 (31) |
| Other* | 772 (7) | 730 (5) |
| Insurance plan type† | ||
| PPO | 6887 (61) | 9673 (61) |
| CDHP | 411 (4) | 600 (4) |
| Comprehensive | 145 (1) | 282 (2) |
| EPO | 178 (2) | 250 (2) |
| HDHP | 213 (2) | 274 (2) |
| HMO | 1828 (16) | 2507 (16) |
| POS | 943 (8) | 1300 (8) |
| POS with capitation | 104 (0.9) | 142 (0.9) |
| Missing/unknown | 491 (4) | 757 (5) |
| Region | ||
| Northeast | 1729 (15) | 2673 (17) |
| South Central | 2579 (23) | 3677 (23) |
| South | 4774 (43) | 6455 (41) |
| West | 1868 (17) | 2642 (17) |
| Missing/Unknown | 250 (2) | 338 (2) |
Other surgery type includes patients whose index surgery claim was for regional node dissection only, or whose index claim contained both a mastectomy and a lumpectomy procedure code.
Insurance plan type: PPO, preferred provider organization; CDHP, consumer-driven health plan (PPO combined with a health reimbursement arrangement); Comprehensive (coverage handled by one policy with deductible and coinsurance, no incentive for use of particular providers); EPO, exclusive provider organization (all care managed by a primary care physician with referrals required, payment non-capitated); HDHP (high deductible health plan combined with health savings account); HMO, health maintenance organization; POS, point-of-service (primary care physician manages care; patients incentivized to use particular providers).
Patients treated in later years were more likely to have genetic testing; nevertheless, despite these increases, patients treated in earlier years had lower plateaus in their testing rates with time (Table 2; Figure 1; log-rank P<0.001 for women age ≤ 40 at diagnosis and for women age 41-45 at diagnosis). Among women age ≤ 40 treated in 2012, 72.9% (95% CI, 70.7-75.1%) had genetic testing within one year after breast cancer surgery, and the median time from surgery to testing was 0.2 months. Similarly, among women age 41-45 treated in 2012, 65.3% (95% CI, 63.3%-67.3%) had genetic testing by one year after surgery, and the median time from surgery to testing was 1.0 month. However, among women treated in 2005 and followed over time, less than half had testing as of December 31, 2013 (median not reached for women age ≤ 40 at diagnosis or women age 41-45 at diagnosis; Table 2). Among women age ≤ 40 treated in 2005, 4.9% (95% CI, 3.7%-6.5%) had a claim for genetic testing by the date of surgery, but among women age ≤ 40 treated in 2012, 47.5% (95% CI, 45.1%-49.9%) had a testing claim by their surgery date.
Table 2.
Rates of BRCA1/2 mutation testing
| Year of breast surgery | N | Outcomes of Kaplan-Meier analysis (%) | Kaplan-Meier estimates of BRCA1/2 testing rates* | |||||
|---|---|---|---|---|---|---|---|---|
| Censored before 12/31/13 | Follow up to 12/31/13 | BRCA1/2 testing | Median time to testing (Months after surgery) | By surgery date % (95% CI)† | By one year after surgery % (95% CI)† | By five years after surgery % (95% CI)† | ||
| 2005 | ||||||||
| Women age 40 and under | 914 | 63.0 | 10.9 | 26.0 | Not reached | 4.9 (3.7–6.5) | 19.6 (17.0–22.5) | 35.3 (31.1–39.9) |
| Women age 41–45 | 1375 | 63.6 | 15.9 | 20.5 | Not reached | 2.2 (1.5–3.1) | 13.1 (11.4–15.2) | 25.0 (22.1–28.2) |
| 2006 | ||||||||
| Women age 40 and under | 888 | 45.3 | 11.6 | 43.1 | 66.4 | 6.6 (5.2–8.5) | 32.9 (29.9–36.3) | 48.3 (44.5–52.3) |
| Women age 41–45 | 1323 | 54.7 | 17.6 | 27.7 | Not reached | 4.8 (3.7–6.1) | 19.2 (17.1–21.5) | 30.6 (27.8–33.7) |
| 2007 | ||||||||
| Women age 40 and under | 1141 | 40.2 | 11.2 | 48.6 | 40.6 | 12.1 (10.3–14.1) | 39.8 (36.9–42.7) | 53.1 (49.8–56.5) |
| Women age 41–45 | 1648 | 48.1 | 16.0 | 35.9 | Not reached | 7.3 (6.2–8.7) | 26.5 (24.4–28.8) | 41.8 (39.0–44.7) |
| 2008 | ||||||||
| Women age 40 and under | 1544 | 35.7 | 10.3 | 54.0 | 13.9 | 19.5 (17.7–21.6) | 48.5 (46.0–51.1) | 61.2 (58.2–64.2) |
| Women age 41–45 | 2167 | 42.4 | 15.4 | 42.2 | 67.7 | 11.4 (10.1–12.8) | 35.4 (33.4–37.5) | 48.4 (45.9–51.0) |
| 2009 | ||||||||
| Women age 40 and under | 1767 | 30.2 | 10.3 | 59.5 | 4.5 | 28.6 (26.6–30.8) | 57.3 (54.9–59.7) | N/A |
| Women age 41–45 | 2571 | 37.9 | 16.0 | 46.2 | 42.1 | 17.2 (15.8–18.7) | 43.1 (41.1–45.1) | N/A |
| 2010 | ||||||||
| Women age 40 and under | 1700 | 23.3 | 12.4 | 64.4 | 2.1 | 34.1 (31.9–36.4) | 61.5 (59.2–63.9) | N/A |
| Women age 41–45 | 2158 | 28.0 | 19.1 | 52.8 | 15.0 | 22.7 (21.0–24.5) | 48.4 (46.2–50.5) | N/A |
| 2011 | ||||||||
| Women age 40 and under | 1572 | 18.1 | 13.8 | 68.1 | 0.8 | 38.7 (36.3–41.1) | 66.4 (64.0–68.7) | N/A |
| Women age 41–45 | 2234 | 22.7 | 18.8 | 58.6 | 4.5 | 29.7 (27.9–31.7) | 56.1 (54.1–58.3) | N/A |
| 2012 | ||||||||
| Women age 40 and under | 1674 | 12.8 | 14.2 | 73.0 | 0.2 | 47.5 (45.1–49.9) | 72.9 (70.7–75.1) | N/A |
| Women age 41–45 | 2309 | 15.2 | 20.0 | 64.8 | 1.0 | 38.3 (36.3–40.3) | 65.3 (63.3–67.3) | N/A |
The beginning of the Kaplan-Meier ascertainment period was defined as six months before the date of breast cancer surgery
95% CI=95% pointwise confidence interval for the Kaplan-Meier estimate
Figure 1. Cumulative rates of BRCA1/2 testing claims*.
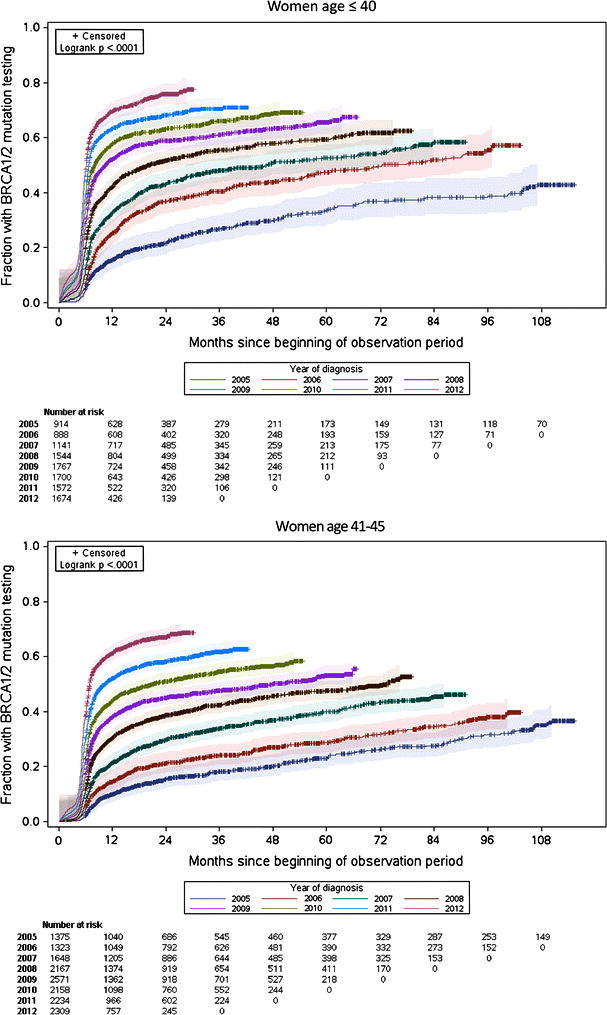
*Each graph contains Kaplan-Meier failure curves, where events were defined as BRCA1/2 testing claims. The year of diagnosis was defined as the year in which breast cancer surgery occurred; the ascertainment period began 180 days before the date of breast cancer surgery. Patients were censored on the date they no longer had continuous insurance coverage recorded within the MarketScan database. The shaded areas represent 95% Hall-Wellner confidence bands for each curve.
In a multivariable Cox proportional hazards model, women age ≤ 40 consistently had a higher likelihood of testing than women age 41-45 (P<0.001 for the contrast between the cohorts in each year from 2005-2012). There was slight variation in testing rates according to geographic region and insurance plan type (Table 3).
Table 3.
Multivariable Cox proportional hazards model for BRCA1/2 testing*
| HR (95% CI) | P | |
|---|---|---|
| Diagnosis year/cohort | <0.001 | |
| 2005 | ||
| Women age ≤ 40 | Reference | |
| Women age 41–45 | 0.71 (0.60–0.84) | |
| 2006 | ||
| Women age ≤ 40 | 1.70 (1.44–2.00) | |
| Women age 41–45 | 0.97 (0.82–1.14) | |
| 2007 | ||
| Women age ≤ 40 | 2.11 (1.81–2.46) | |
| Women age 41–45 | 1.39 (1.19–1.62) | |
| 2008 | ||
| Women age ≤ 40 | 2.71 (2.34–3.13) | |
| Women age 41–45 | 1.83 (1.59–2.12) | |
| 2009 | ||
| Women age ≤ 40 | 3.42 (2.97–3.94) | |
| Women age 41–45 | 2.23 (1.94–2.57) | |
| 2010 | ||
| Women age ≤ 40 | 4.03 (3.50–4.65) | |
| Women age 41–45 | 2.75 (2.39–3.17) | |
| 2011 | ||
| Women age ≤ 40 | 4.77 (4.14–5.50) | |
| Women age 41–45 | 3.48 (3.03–4.00) | |
| 2012 | ||
| Women age ≤ 40 | 6.05 (5.26–6.97) | |
| Women age 41–45 | 4.63 (4.03–5.33) | |
| Insurance plan type† | <0.001 | |
| PPO | Reference | |
| CDHP | 1.14 (1.05–1.24) | |
| Comprehensive | 1.01 (0.87–1.17) | |
| EPO | 1.05 (0.93–1.19) | |
| HDHP | 1.26 (1.13–1.40) | |
| HMO | 0.86 (0.82–0.91) | |
| POS | 1.03 (0.97–1.10) | |
| POS with capitation | 1.17 (0.98–1.41) | |
| Missing/unknown | 0.90 (0.83–0.98) | |
| Region | <0.001 | |
| South | Reference | |
| Northeast | 1.15 (1.09–1.21) | |
| South Central | 1.18 (1.13–1.23) | |
| West | 1.06 (1.00–1.11) | |
| Missing/unknown | 1.18 (1.06–1.32) |
The outcome was the first BRCA1/2 testing claim recorded, using a Cox proportional hazards model that included the three independent variables listed in this table.
Insurance plan type: PPO, preferred provider organization; CDHP, consumer-driven health plan (PPO combined with a health reimbursement arrangement); EPO, exclusive provider organization (all care managed by a primary care physician with referrals required, payment non-capitated); HDHP (high deductible health plan combined with health savings account); HMO, health maintenance organization; POS, point-of-service (primary care physician manages care; patients incentivized to use particular providers).
In a sensitivity analysis restricted to claims specific to BRCA 1/2 mutation testing (excluding non-specific genetic testing claims for molecular techniques), estimated testing rates were lower, but the patterns of change over time were similar (Supplemental Table 2; Supplemental Figure 1). We also conducted a second sensitivity analysis excluding patients with Medicare supplemental coverage, since Medicare would not have reimbursed HCPCS ‘S’ codes under which most of the BRCA1/2 mutation testing claims were billed. The patterns of change remained similar (Supplemental Table 3; Supplemental Figure 2).
DISCUSSION
In a cohort of young women with employer-based or Medicare supplemental insurance and breast cancer treated from 2005-2012, we found that a large proportion of young breast cancer survivors have not undergone testing for mutations in the BRCA1 and BRCA2 genes. Rates of testing increased substantially for patients treated in later years, but testing rates for patients treated in earlier years never approached those of patients treated later, even after extended follow-up. In the United States, approximately 7% of cases of breast cancer occur before age 40 [25], and approximately 10% of cases occur before age 45 [10]. During our study period alone, there were therefore approximately 192,000 cases of breast cancer in this age group. We found that less than half of patients diagnosed in 2005 had genetic testing by the end of 2013. At a time when there is increasing interest in issues around expanded panel genetic testing for newly diagnosed patients with breast cancer [11–14], our results indicate that there is a substantial population of survivors who have never undergone established, standard-of-care genetic testing.
We found an increase in testing rates with each successive year at diagnosis, during a period when genetic testing was becoming more readily available, likely indicating that most young patients with breast cancer who are offered genetic testing are interested in pursuing it. The lower plateau in testing rates among young women diagnosed in earlier years, even after extended follow-up, is therefore especially notable. Although some studies indicate that patients with BRCA1/2-associated breast cancers have a worse prognosis than those with sporadic disease [26, 27], others have demonstrated that the two groups have similar outcomes [28–32]. Furthermore, the large majority of patients diagnosed with breast cancer will be long-term survivors [33]. Some of these survivors, such as the cohort of women in our analysis who were age 41-45 at diagnosis, would meet current criteria for genetic testing, but may not have met criteria when they were diagnosed. Other survivors may have been diagnosed when genetic counseling and testing were less widely available, or prior to the protections extended by the Genetic Information Nondiscrimination Act of 2008, which prohibited discrimination in the workplace or health insurance marketplace on the basis of a genetic predisposition to disease. Our results indicate that providers should consider offering genetic testing to this population, given the possibility that doing so may further mitigate cancer risks, both for patients and their families [16]. Information regarding hereditary risk factors and genetic testing results is a recommended component of survivorship care planning for current patients [34]. However, patients with remote diagnoses may be unaware of advances in genetic testing. Optimizing this process will require engagement of primary care providers, since with more time since diagnosis, long-term survivors of breast cancer have more visits with their primary care physicians and fewer visits with their oncologists [15].
Strengths of our analysis included its basis in a large database of privately insured patients, which provided a nationwide sample [17] with which to assess rates of genetic testing in young women with breast cancer. Nevertheless, there are limitations. We studied subgroups of breast cancer patients with indications for genetic testing based on age alone, who could therefore be identified from insurance claims data using a previously validated algorithm [19–21]. This is not a complete list of indications for BRCA1/2 testing; for example, individuals are also eligible if they have consistent family history patterns, ovarian cancer, or triple negative breast cancer diagnosed at age ≤ 60, or if they are male [7]. We also studied a privately insured population, which may limit the generalizability of our results. Still, rates of genetic testing were likely higher in the population we studied than in patients who were uninsured or covered by Medicaid or Medicare without supplemental coverage, or who had less obvious or more recently identified indications for testing. In that case, the rates of genetic testing we ascertained may, in fact, represent an upper limit relative to those in the general population. This would further reinforce the need to consider genetic testing for survivors to whom it has not previously been offered.
In addition, follow-up in our analysis was based on continuous insurance coverage within a plan included in the MarketScan database. Given a median length of follow-up of three to four years, many patients diagnosed in earlier years were censored. However, the upper quartile of length of follow-up extended to 7-8 years for patients diagnosed in 2005-2006, which still allowed us to assess rates of genetic testing over an extended period of time for patients with long-term data. Although our data cannot inform this question directly, there is no obvious reason to suspect that censoring would lead to a systematic underestimation of BRCA1/2 testing rates. Indeed, patients who were censored may actually have been less likely to have BRCA1/2 testing due to competing health risks. In that case, our low measured testing rates for patients diagnosed in earlier years might again represent an upper limit estimate of actual population rates among cancer survivors.
This analysis was based on paid insurance claims, and we therefore could not assess how often providers discussed the possibility of genetic testing with these patients, or how often patients were referred for genetic counseling. We also could not assess how often patients considered genetic testing but decided not to have it done, chose to pay privately for testing rather than submit an insurance claim, or had a claim for genetic testing denied without subsequently submitting a claim that was paid.
Finally, our case-finding algorithm may not have captured all breast cancer patients who had metastatic disease at diagnosis, and therefore did not undergo surgery [20]. However, only approximately 4% of female patients with breast cancer have distant metastatic disease at diagnosis [35]. Furthermore, approximately half of patients diagnosed with stage IV disease undergo surgery for their primary tumor [36] and may therefore have been captured by our algorithm, so the proportion of patients excluded for this reason was likely small.
In conclusion, within a cohort of young women treated for breast cancer from 2005 to 2012, for whom current guidelines recommend consideration of BRCA1/2 testing, rates of testing increased with later years of diagnosis. Still, survivors treated in earlier years and followed over time never approached the testing rates of those diagnosed in later years. There are approximately 2.8 million survivors of breast cancer in the United States [9], and approximately 10% of new cases are diagnosed at age ≤ 45 [10], such that there are likely hundreds of thousands of current survivors from this population. Our results point to a need to optimize access to genetic counseling among eligible survivors, and to incorporate it into survivorship and primary care for patients with a history of successful treatment for early-stage disease. Further research should be conducted into strategies for increasing awareness of advances in genetic testing among cancer survivors and their physicians, and into assessment of the clinical impact and cost of such efforts.
Supplementary Material
Acknowledgments
Funding:
This work was supported by the Duncan Family Institute, the Baker Institute for Health and Biosciences, and the Cancer Prevention Research Institute of Texas (Grant RP140020.)
Footnotes
Conflict of Interest:
Kenneth L. Kehl declares that he has no conflict of interest. Chan Shen declares that she has no conflict of interest. Jennifer K. Litton declares that she has no conflict of interest. Banu Arun declares that she has no conflict of interest. Sharon H. Giordano declares that she has no conflict of interest.
Ethical approval:
This article does not contain any studies with human participants or animals performed by any of the authors. The MarketScan insurance claims data were fully deidentified prior to analysis, and the Institutional Review Board at the University of Texas MD Anderson Cancer Center exempted this study from review.
Erratum:
In the original publication of this article, the first sentence of the last paragraph of the second page was published erroneously as, “We identified BRCA1/2 testing claims using mutation-specific HCPCS codes S1818-S1823, as previously described [8].”
The correct sentence should read as, “We identified BRCA1/2 testing claims using mutation-specific procedure codes S3818-S3823, as previously described [8].”
The text of the manuscript submitted to PubMed Central has been corrected. The authors regret this error.
References
- 1.Foulkes WD. Inherited susceptibility to common cancers. N Engl J Med. 2008;359:2143–2153. doi: 10.1056/NEJMra0802968. [DOI] [PubMed] [Google Scholar]
- 2.Pharoah PDP, Antoniou A, Bobrow M, et al. Polygenic susceptibility to breast cancer and implications for prevention. Nat Genet. 2002;31:33–36. doi: 10.1038/ng853. [DOI] [PubMed] [Google Scholar]
- 3.Antoniou A, Pharoah PDP, Narod S, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Genetic/Familial High-Risk Screening: Breast. 2001 Version 1. [Google Scholar]
- 5.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian. 2005 Version 1. [Google Scholar]
- 6.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian. 2009 Version 1. [Google Scholar]
- 7.National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast and Ovarian. 2014 Version 2. [Google Scholar]
- 8.Levy DE, Byfield SD, Comstock CB, et al. Underutilization of BRCA1/2 testing to guide breast cancer treatment: black and Hispanic women particularly at risk. Genet Med. 2011;13:349–55. doi: 10.1097/GIM.0b013e3182091ba4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Cancer Society. Breast Cancer Key Statistics. 2015 http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics. Accessed 29 Nov 2015.
- 10.American Cancer Society. Estimated New Cases for the Four Major Cancers by Sex and Age Group. 2015 http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044511.pdf. Accessed 29 Nov 2015.
- 11.Easton DF, Pharoah PDP, Antoniou AC, et al. Gene-panel sequencing and the prediction of breast-cancer risk. N Engl J Med. 2015;372:2243–57. doi: 10.1056/NEJMsr1501341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phimister EG. Curating the way to better determinants of genetic risk. N Engl J Med. 2015;372:2227–8. doi: 10.1056/NEJMe1506276. [DOI] [PubMed] [Google Scholar]
- 13.Tung N, Battelli C, Allen B, et al. Frequency of mutations in individuals with breast cancer referred for BRCA1 and BRCA2 testing using next-generation sequencing with a 25-gene panel. Cancer. 2015;121:25–33. doi: 10.1002/cncr.29010. [DOI] [PubMed] [Google Scholar]
- 14.Desmond A, Kurian AW, Gabree M, et al. Clinical Actionability of Multigene Panel Testing for Hereditary Breast and Ovarian Cancer Risk Assessment. JAMA Oncol. 2015;1:943–51. doi: 10.1001/jamaoncol.2015.2690. [DOI] [PubMed] [Google Scholar]
- 15.Snyder CF, Frick KD, Peairs KS, et al. Comparing care for breast cancer survivors to non-cancer controls: A five-year longitudinal study. J Gen Intern Med. 2009;24:469–474. doi: 10.1007/s11606-009-0903-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon JS, Gutierrez-Barrera AM, Young D, et al. Expanding the criteria for BRCA mutation testing in breast cancer survivors. J Clin Oncol. 2010;28:4214–4220. doi: 10.1200/JCO.2010.28.0719. [DOI] [PubMed] [Google Scholar]
- 17.Hansen L, Chang S. White Paper: Health Research Data for the Real World: The Thomson Reuters Marketscan Databases. 2011:32. [Google Scholar]
- 18.Barcenas CH, Niu J, Zhang N, et al. Risk of Hospitalization According to Chemotherapy Regimen in Early-Stage Breast Cancer. J Clin Oncol. 2014;32:2010–2017. doi: 10.1200/JCO.2013.49.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nattinger AB, Laud PW, Bajorunaite R, et al. An algorithm for the use of Medicare claims data to identify women with incident breast cancer. Health Serv Res. 2004;39:1733–1749. doi: 10.1111/j.1475-6773.2004.00315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giordano SH, Lin YL, Kuo YF, et al. Decline in the use of anthracyclines for breast cancer. J Clin Oncol. 2012;30:2232–2239. doi: 10.1200/JCO.2011.40.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold HT, Do HT. Evaluation of three algorithms to identify incident breast cancer in Medicare claims data. Health Serv Res. 2007;42:2056–2069. doi: 10.1111/j.1475-6773.2007.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Medicare & Medicaid Services (CMS) CMS Manual System, Pub 100-04, Medicare Claims Processing, Transmittal 2639, Change Request 8162. Effective Date April 1, 2012. https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/Downloads/R2639CP.pdf. Accessed 15 Feb 2015.
- 23.Centers for Medicare & Medicaid Services (CMS) CMS Manual System, Pub 100-04 Medicare Claims Processing. Transmittal 2423, Change Request 7751. Effective Date: April 1, 2012. http://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/downloads/R2423CP.pdf. Accessed 15 Feb 2015.
- 24.Hall WJ, Wellner JA. Confidence Bands for a Survival Curve from Censored Data. Biometrika. 1980;67:133–143. [Google Scholar]
- 25.Anders CK, Johnson R, Litton J, et al. Breast Cancer Before Age 40 Years. Semin Oncol. 2009;36:237–249. doi: 10.1053/j.seminoncol.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jóhannsson OT, Ranstam J, Borg A, Olsson H. Survival of BRCA1 breast and ovarian cancer patients: a population-based study from southern Sweden. J Clin Oncol. 1998;16:397–404. doi: 10.1200/JCO.1998.16.2.397. [DOI] [PubMed] [Google Scholar]
- 27.Brekelmans CTM, Seynaeve C, Menke-Pluymers M, et al. Survival and prognostic factors in BRCA1-associated breast cancer. Ann Oncol. 2006;17:391–400. doi: 10.1093/annonc/mdj095. [DOI] [PubMed] [Google Scholar]
- 28.Verhoog LC, Brekelmans CTM, Seynaeve C, et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet. 1998;351:316–321. doi: 10.1016/S0140-6736(97)07065-7. [DOI] [PubMed] [Google Scholar]
- 29.Rennert G, Bisland-Naggan S, Barnett-Griness O, et al. Clinical outcomes of breast cancer in carriers of BRCA1 and BRCA2 mutations. N Engl J Med. 2007;357:115–123. doi: 10.1056/NEJMoa070608. [DOI] [PubMed] [Google Scholar]
- 30.El-Tamer M, Russo D, Troxel A, et al. Survival and recurrence after breast cancer in BRCA1/2 mutation carriers. Ann Surg Oncol. 2004;11:157–164. doi: 10.1245/ASO.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Bordeleau L, Panchal S, Goodwin P. Prognosis of BRCA-associated breast cancer: A summary of evidence. Breast Cancer Res Treat. 2010;119:13–24. doi: 10.1007/s10549-009-0566-z. [DOI] [PubMed] [Google Scholar]
- 32.Bayraktar S, Gutierrez-Barrera AM, Liu D, et al. Outcome of triple-negative breast cancer in patients with or without deleterious BRCA mutations. Breast Cancer Res Treat. 2011;130:145–153. doi: 10.1007/s10549-011-1711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2011. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2011/, based on November 2013 SEER data submission, posted to the SEER web site, April 2014. [Google Scholar]
- 34.McCabe MS, Bhatia S, Oeffinger KC, et al. American society of clinical oncology statement: Achieving high-quality cancer survivorship care. J Clin Oncol. 2013;31:631–640. doi: 10.1200/JCO.2012.46.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gnerlich JL, Deshpande AD, Jeffe DB, et al. Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol. 2011;18:1837–1844. doi: 10.1245/s10434-010-1468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gnerlich J, Jeffe DB, Deshpande AD, et al. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988-2003 SEER data. Ann Surg Oncol. 2007;14:2187–94. doi: 10.1245/s10434-007-9438-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


