Abstract
We recently reported a linear association between higher systolic blood pressure (SBP) and risk of mortality in hemodialysis (HD) patients when SBP is measured outside of the dialysis unit (“out-of-dialysis-unit-SBP”) despite there being a U-shaped association between SBP measured in the dialysis unit (“dialysis-unit-SBP”) with risk of mortality. Here we explored the relationship between SBP with cardiovascular (CVD) events, which has important treatment implications but has not been well-elucidated. Among 383 HD participants enrolled in the prospective Chronic Renal Insufficiency Cohort (CRIC) Study, multivariable splines and Cox models were used to study the association between SBP and adjudicated CVD events (heart failure, myocardial infarction, ischemic stroke, peripheral artery disease), controlling for differences in demographics, CVD risk factors and dialysis parameters. Dialysis-unit-SBP and out-of-dialysis-unit-SBP were modestly correlated (r=0.34, p<0.001). We noted a U-shaped association of dialysis-unit-SBP and risk of CVD events, with the nadir risk between 140–170 mmHg. In contrast, there was a linear stepwise association between out-of-dialysis-unit-SBP with risk of CVD events. Participants with out-of-dialysis-unit-SBP ≥ 128 mmHg (top two quartiles) had greater than two-fold increased risk of CVD events compared with those with out-of-dialysis-unit-SBP ≤112 mmHg (bottom quartile) (3rd SBP quartile: adjusted hazard ratio [aHR] 2.08 [1.12, 3.87] and 4th SBP quartile: aHR 2.76 [1.42, 5.33]). In conclusion, among HD patients, although there is a U-shaped (“paradoxical”) association of dialysis-unit-SBP and risk of CVD, there is a linear association of out-of-dialysis-unit-SBP with risk of CVD. Out-of-dialysis-unit BP provides key information and may be an important therapeutic target.
Keywords: CVD, dialysis, blood pressure, hemodialysis, heart failure
INTRODUCTION
Among patients on maintenance hemodialysis (HD), prior observational studies have consistently noted a U-shaped association between level of systolic blood pressure (SBP) measured in the dialysis unit and risk of all-cause mortality.1–12 HD patients with SBP less than 140 mmHg measured prior to the dialysis treatment (pre-dialysis SBP) experience higher risk of mortality than those with SBP above 140 mmHg.3, 4, 12, 13 Moreover, patients with pre-dialysis SBP of 150–179 mmHg appear to be at similar, if not lower, adjusted risk for all-cause mortality compared to those with pre-dialysis SBP of 140–149 mmHg, even accounting for case-mix.3, 14 In the absence of robust randomized controlled trial data, these “reverse epidemiology” and “paradoxical BP” observational data have led to uncertainty among practitioners on how to manage BP in HD patients.3, 4, 13, 15
Recently, we confirmed the “paradoxical” U-shaped association between dialysis-unit-SBP and risk of all-cause mortality in a multi-center cohort of incident HD patients, but reported that there was a linear stepwise independent association between higher level of SBP measured outside of the dialysis unit at one sitting in a single day and higher risk of mortality,12 an association similar to that observed in the general population. However, whether these same associations also apply for CVD, which remains the leading cause of morbidity and mortality in HD patients, has not been well described. A better understanding of the association of BP with CVD events would guide treatment in this high-risk patient population, particularly in the absence of clinical trials.
In this study, we examined the association between SBP measured in the dialysis unit (measured prior to starting dialysis) and outside of the dialysis unit (at a research study visit) and risk of CVD events. We also examined the association of other BP components: diastolic BP (DBP) and pulse pressure (PP) with CVD events. We hypothesized that there would be a linear association with risk of CVD events with BP measured outside of the dialysis unit and a U-shaped association with BP measured at the dialysis unit.
METHODS
Study Population
We studied participants of the Chronic Renal Insufficiency Cohort (CRIC) Study. The CRIC Study is a NIH-sponsored multi-center prospective observational cohort16–18 study which initially enrolled 3,939 participants age 21 to 74 years with chronic kidney disease (CKD) with estimated glomerular filtration rate (eGFR) 20–70 ml/min/1.73m2 by the MDRD equation between 2003 and 2008.19 Exclusion criteria included New York Heart Association Class III or IV heart failure and severe liver disease. Study participants have been followed annually through in-person visits and interim six-month telephone calls. A subset of enrolled CRIC participants have had progression of their CKD and have initiated HD. Informed consent was obtained from each participating site.
We studied 377 CRIC participants who initiated chronic HD by March 31, 2013, and had measures of both dialysis-unit and out-of-dialysis-unit-BP available. Consistent with our prior study,12, 20 we included only participants who had at least one CRIC study visit when their eGFR was <30 ml/min/1.73 m2 prior to starting HD.
Predictors
We examined three BP components: SBP (primary exposure) as well as DBP and PP (calculated from SBP minus DBP) (secondary exposures), all in mmHg and modeled in quartiles.
Dialysis-unit-blood pressure
For CRIC participants who started maintenance HD, study personnel obtained records from each patient’s dialysis unit approximately 6 months after HD initiation and abstracted information on BP measurements recorded at the start of each HD session. The mean of the BP measurements obtained from these dialysis unit records obtained over 1 week was used to define “dialysis-unit-BP” in our study.12
Out-of-dialysis-unit-blood pressure
We used mean BP obtained at the first in-person CRIC research study visit after initiation of maintenance HD. BP was measured by centrally trained staff using a standardized method.12, 21 Per the CRIC protocol, BP measurement is performed in a quiet, standardized setting. Participants abstain from caffeine, smoking, and exercise at least one-half hour prior to and until completion of the BP measurement. The Tycos Classic Hand Aneroid sphygmomanometer is the standard equipment for all BP measurements at CRIC clinical visits. The mean of three seated resting BP readings was used to define out-of-dialysis-unit-BP.
Outcomes
Our primary outcome was time to adjudicated CVD events which occurred after ascertainment of both dialysis-unit and out-of-dialysis-unit-BP. CVD events included heart failure (HF), myocardial infarction (MI), ischemic stroke, and peripheral artery disease (PAD) events identified through March 31, 2013.22 Participants were censored at death or end of study period. Deaths were identified from report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and the Social Security Death Master File.
Study participants were queried every 6 months during alternating in-person and telephone visits about whether they were hospitalized, experienced a possible CVD event, or underwent a selected set of diagnostic tests/procedures. International Classification of Diseases, Ninth Revision (ICD-9) discharge codes were obtained for all hospitalizations and relevant medical records were retrieved for review by at least 2 physicians to ascertain events of HF, MI, and stroke. Trained study staff reviewed medical records classified with ICD-9 codes that suggest a PAD event.23, 24
HF events were determined based on clinical symptoms, radiographic evidence of pulmonary edema, physical examination of the heart and lungs, central venous hemodynamic monitoring data, and echocardiographic imaging in hospitalized patients based on the Framingham and ALLHAT (Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial) criteria.25, 26 Diagnosis of probable or definite MI was based on symptoms consistent with acute ischemia, cardiac biomarker levels, and electrocardiograms as recommended by a consensus statement on the universal definition of MI.27 Two neurologists reviewed all hospitalizations suggestive of stroke. Outcomes included both probable and definite ischemic stroke. The latter was determined based on autopsy findings or sudden onset of neurologic symptoms supported with computed tomography or magnetic resonance imaging demonstration of infarction in a territory where an injury or infarction would be expected to create those symptoms. The former was defined as sudden or rapid onset of 1 major or 2 minor neurologic signs or symptoms lasting for more than 24 hours or until the patient died with no evidence of hemorrhage or infarction on computed tomography or magnetic resonance imaging performed within 24 hours of the onset of symptoms.28 Ascertainment of PAD was based on nurse-abstracted hospital records indicating that amputation, bypass procedure, angioplasty, or surgical/vascular procedure for abdominal aortic aneurysm or non-coronary arteries took place.23 Multiple events during the same hospitalization were only counted as one event (since we used a composite outcome).
Covariates
History of CVD was determined by self-report (at baseline) and occurrence of an adjudicated CVD event during CRIC follow-up (i.e. occurred between enrollment into CRIC and ascertainment of BP in this study). Medication use was ascertained by self-reported. For analyses based on out-of-dialysis-unit-BP, covariates were obtained from the same CRIC study visit as the out-of-dialysis-unit-BP measurement. For the analyses based on dialysis-unit-BP, covariates were obtained from the closest study visit prior to the dialysis-unit-BP measurement. Selected measurements taken during routine clinical care abstracted from dialysis-unit records included: dose of dialysis (Kt/V), serum albumin and hemoglobin level, mean intra-dialytic weight gain (IDWG) over one week.12
Statistical methods
We compared characteristics across quartiles of out-of-dialysis-unit-SBP using ANOVA tests for continuous variables and chi-squared tests for categorical variables. The start time for each patient for all time-to-event analyses was the latter of the date of dialysis-unit-BP measurement or the date of the out-of-dialysis-unit-BP measurement. Participants were censored if they disenrolled from CRIC, died or at the end of follow-up (on March 31, 2013). We examined the association of each BP component measured in the dialysis-unit and out-of-dialysis-unit with risk of adjudicated CVD events. We first explored the association between SBP and CVD events using adjusted penalized smoothing splines with N0.2 evenly spaced knots (at the quintiles of the marginal distribution of the independent variable) among the inner 99% distribution of SBP in Cox models.29–31 This allowed us to display the relationship of SBP and CVD without making assumptions about the shape of the relationship.12 We then performed multivariable Cox proportional hazard modeling SBP in quartiles. We first adjusted for demographics; next we adjusted for CVD risk factors (tobacco use, body mass index [BMI], diabetes, history of CVD); and finally we additionally adjusted for dialysis related variables (Kt/V, serum albumin and hemoglobin level).12
In secondary analyses, we repeated our models examining dialysis-unit and out-of- dialysis-unit-DBP and PP measurements as predictors of CVD events.
In a sensitivity analysis we adjusted for the number of self-reported BP medication classes prescribed. In a second sensitivity analysis, we excluded PAD as part of our composite outcome since PAD is not always considered a “hard” outcome in CVD trials.
RESULTS
Study participants
Among 377 eligible participants that initiated maintenance HD during follow-up, those with higher levels of out-of-dialysis-unit-SBP were more likely to have higher DBP and were more likely to be current smokers (Table 1). For out-of-dialysis-unit-BP measures, 82.5% of were performed on the right arm and 17.5% on the left arm. The mean (±SD) of each of the three SBP readings was the following: SBP #1 132 (±36), SBP #2 131 (±25), and SBP #3 131 (±26) mm Hg. The mean (SD) of each of the three DBP readings was the following: DBP #1 67 (±14), DBP #2 66 (±13) and DBP #3 66 (±14) mm Hg.
Table 1.
Characteristics of study participants by quartiles of out-of-dialysis-unit-systolic blood pressure (SBP) (N=377)
| Characteristic | Q1 (SBP 70–112 mmHg) N=93 |
Q2 (SBP 113–127 mmHg) N=95 |
Q3 (SBP 128–145 mmHg) N=93 |
Q4 (SBP 146–238 mmHg) N=96 |
P value |
|---|---|---|---|---|---|
| Age mean ±SD (years) | 59±12 | 59±11 | 61±10 | 61±11 | 0.2 |
| Female (%) | 40 | 43 | 41 | 42 | 1.0 |
| Race/ethnicity (%) | 0.1 | ||||
| Non-Hispanic White | 18 | 21 | 20 | 7 | |
| Non-Hispanic Black | 63 | 63 | 67 | 75 | |
| Hispanic | 13 | 13 | 13 | 15 | |
| Other | 6 | 3 | 0 | 3 | |
| Systolic blood pressure, mean ±SD (mmHg) | 102±8 | 121±4 | 137±5 | 165±18 | <0.0001 |
| Diastolic blood pressure, mean ±SD (mmHg) | 57± 9 | 64±11 | 69±13 | 74±14 | <0.0001 |
| Pulse pressure, mean ±SD (mmHg) | 44±11 | 56±12 | 68±14 | 91±18 | <0.0001 |
| Body Mass Index, mean ±SD (kg/m2) | 31± 8 | 32± 8 | 30± 8 | 30± 7 | 0.1 |
| Hypertension (%) | 99 | 97 | 99 | 100 | 0.3 |
| Diabetes (%) | 65 | 68 | 76 | 72 | 0.3 |
| Current Smoker (%) | 4 | 14 | 14 | 18 | 0.04 |
| History of CVD (%) | 55 | 60 | 60 | 66 | 0.5 |
| Dialysis vintage (days), median (IQR) | 206 (115, 314) | 205 (113, 319) | 232 (123, 340) | 205 (107, 299) | 0.7 |
| Kt/V, mean ±SD | 1.58± 0.36 | 1.56± 0.34 | 1.53± 0.34 | 1.53± 0.30 | 0.8 |
| Serum albumin, mean ±SD (mg/dL) | 3.9± 0.8 | 3.8± 0.4 | 3.8± 0.4 | 3.8± 0.4 | 0.2 |
| Hemoglobin, mean ±SD (g/L) | 11.7± 1.3 | 11.6± 1.9 | 11.7± 1.3 | 12.0± 2.7 | 0.6 |
Correlation between dialysis-unit-BP and out-of-dialysis-unit-BP
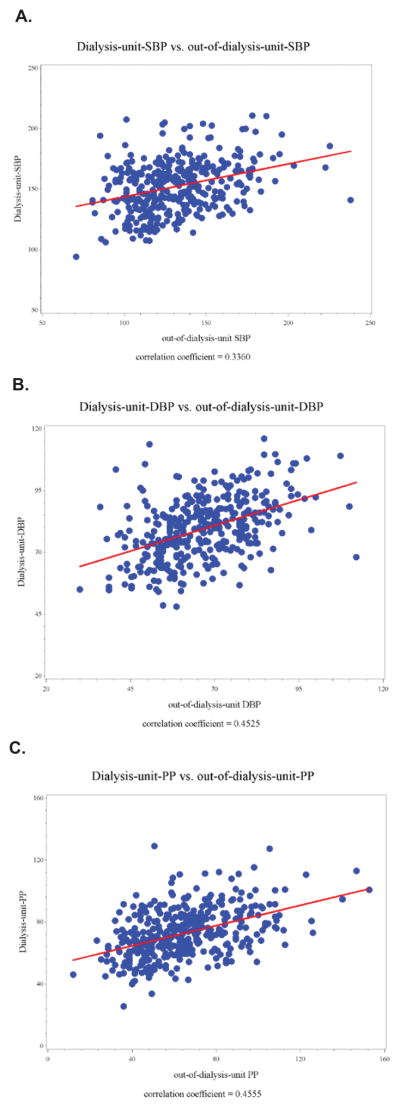
The median time between dialysis-unit-BP and out-of-dialysis-unit-BP measures was 101 [IQR 36, 195] days. Overall there was a modest correlation between level of dialysis-unit-SBP and out-of-dialysis-unit-SBP (correlation coefficient=0.34, p<0.001) (Figure 1a). Only 39% (148/377) of participants were matched by categories of dialysis-unit-SBP and out-of-dialysis-unit-SBP (Table 2). The correlation between dialysis-unit measures of DBP and PP with out-of-dialysis-unit measures were also modest (Figures 1b and 1c).
Figure 1.
Figure 1a. Correlation between dialysis-unit-systolic blood pressure (SBP) and out-of-dialysis-unit-SBP (N=377)
Figure 1b. Correlation between dialysis-unit-diastolic blood pressure (DBP) and out-of-dialysis-unit-DBP (N=377)
Figure 1c. Correlation between dialysis-unit-pulse pressure (PP) and out-of-dialysis-unit-PP (N=377)
Table 2.
Correlation between categories of dialysis-unit-systolic blood pressure (SBP) and out-of-dialysis-unit-SBP (N=377)
| Dialysis-unit-SBP | Out-of-dialysis-unit-SBP | ||
|---|---|---|---|
| <120 mmHg | 120–140 mmHg | >140 mmHg | |
| <120 mmHg | N=19 | N=2 | N=1 |
| 120–140 mmHg | N=40 | N=26 | N=20 |
| >140 mmHg | N=74 | N=92 | N=103 |
Dialysis-unit-SBP and risk of CVD events
There were a total of 113 first CVD events observed over a mean (±SD) follow-up time of 2.4 (±1.71) years. The types of CVD events were as follows: 59 HF events, 19 MI events, 8 strokes, 18 PAD events, 7 with MI and HF during the same hospitalization, 1 with MI and PAD during the same hospitalization and 1 with MI, stroke and PAD during the same hospitalization.
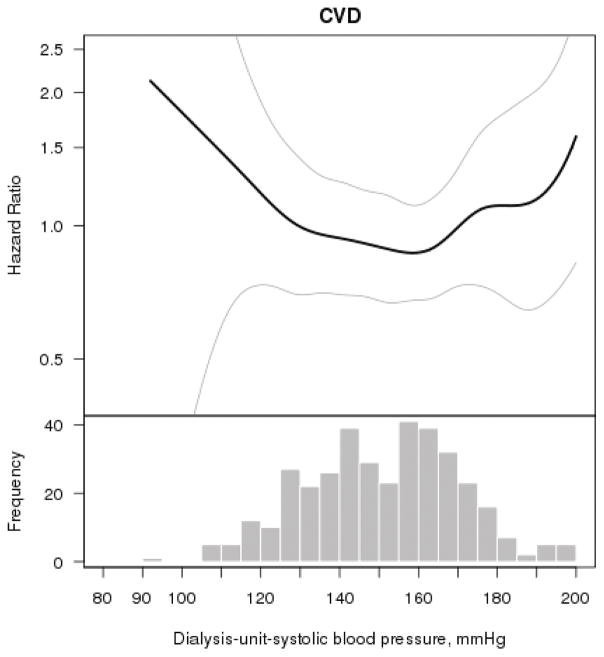
Multivariable splines demonstrated a U-shaped association of dialysis-unit-SBP and risk of CVD events, with the nadir being between 150–170 mmHg (Figure 2). Compared with the 2nd quartile, there was not a statistically significant association between the lowest or highest quartiles of dialysis-unit-SBP with risk of CVD events in unadjusted or multivariable models (Table 3).
Figure 2. Multivariable association of dialysis-unit-systolic blood pressure with cardiovascular events (N=377).
The smooth spline estimates the hazard ratio of cardiovascular events, according to systolic blood pressure (SBP) (mmHg) measured in the dialysis unit among CRIC participants. All analyses are adjusted for age, gender, race/ethnicity, tobacco use, body mass index, diabetes, history of cardiovascular disease, Kt/V, serum albumin and hemoglobin level. Dotted lines represent 95% confidence intervals. Below each spline is the histogram of the distribution of systolic blood pressure to indicate the range of the majority of the data.
Table 3.
Association between systolic blood pressure (SBP) and risk of cardiovascular (CVD) events (N=377)
| Blood pressure measure | CVD Events | Unadjusted | Model 1: Adjusted for age, sex and race/ethnicity | Model 2: Adjusted for patient characteristics* | Model 3: Adjusted for patient characteristics + dialysis variables† | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dialysis-unit-SBP (mmHg) | Number of events | Rate (per 100py) | HR (95% CI) | p value | HR (95%CI) | p value | HR (95%CI) | p value | HR (95%CI) | p value |
| Q1 (94–137) | 26 | 11.98 | 1.00 (0.59, 1.72) | 1.0 | 0.93 (0.54, 1.60) | 0.8 | 0.86 (0.49, 1.51) | 0.6 | 0.75 (0.44, 1.39) | 0.4 |
| Q2 (138–152) | 27 | 11.85 | Ref | Ref | Ref | Ref | ||||
| Q3 (153 -165) | 26 | 10.53 | 0.89 (0.52, 1.53) | 0.7 | 0.93 (0.54, 1.59) | 0.8 | 0.81 (0.47, 1.41) | 0.5 | 0.65 (0.36, 1.18) | 0.2 |
| Q4 (166–211) | 34 | 16.24 | 1.37 (0.82, 2.27) | 0.2 | 1.45 (0.87, 2.41) | 0.2 | 1.40 (0.83, 2.35) | 0.2 | 1.06 (0.60, 1.85) | 0.9 |
| Out-of-dialysis-unit-SBP (mmHg) | Number of events | Rate (per 100py) | HR (95% CI) | p value | HR (95%CI) | p value | HR (95%CI) | p value | HR (95%CI) | p value |
| Q1 (70–112) | 21 | 8.11 | Ref | Ref | Ref | Ref | ||||
| Q2 (113–127) | 25 | 10.79 | 1.32 (0.74, 2.35) | 0.4 | 1.27 (0.71, 2.28) | 0.4 | 1.20 (0.66, 2.17) | 0.6 | 1.33 (0.71, 2.50) | 0.4 |
| Q3 (128–145) | 33 | 15.13 | 1.84 (1.07, 3.19) | 0.03 | 1.69 (0.98, 2.93) | 0.06 | 1.77 (1.01, 3.11) | 0.047 | 2.14 (1.17, 3.90) | 0.01 |
| Q4 (146–238) | 34 | 17.68 | 2.14 (1.24, 3.69) | 0.006 | 2.24 (1.29, 3.89) | 0.004 | 2.44 (1.38, 4.29) | 0.002 | 2.90 (1.55, 5.42) | <0.001 |
patient characteristics include: age, gender, race/ethnicity, tobacco use, BMI, diabetes, history of cardiovascular disease (covariates taken from same visit/closest prior visit of SBP reading)
dialysis variables: Kt/V, serum albumin and hemoglobin level
Out-of-dialysis-unit-SBP and risk of CVD events
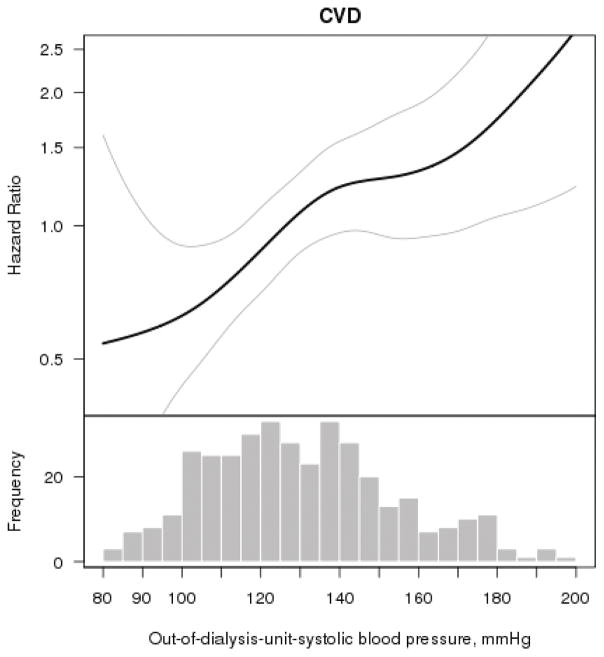
In contrast, multivariable splines showed a linear direct association of out-of-dialysis-unit-SBP with CVD events (Figure 3). Unadjusted rates (per 100 person-years) of CVD events increased across increasing quartiles of out-of-dialysis-unit-SBP (Table 3). There was a graded association between higher out-of-dialysis-unit-SBP and risk of CVD in unadjusted models as well as in models adjusting for patient demographics characteristics and comorbidity (Table 3). Participants with out-of-dialysis-unit-SBP ≥128 mmHg had greater than two-fold increased risk of CVD events compared with those with out-of-dialysis-unit-SBP ≤112 mmHg (Table 3).
Figure 3. Multivariable association of out-of-dialysis-unit-systolic blood pressure with cardiovascular events (N=377).
The smooth spline estimates the hazard ratio of cardiovascular events, according to systolic blood pressure SBP) (mmHg) measured in the outside the dialysis unit (at a CRIC study visit) among CRIC participants All analyses are adjusted for age, gender, race/ethnicity, tobacco use, body mass index, diabetes, history of cardiovascular disease, Kt/V, serum albumin and hemoglobin level. Dotted lines represent 95% confidence intervals. Below each spline is the histogram of the distribution of systolic blood pressure to indicate the range of the majority of the data.
Sensitivity analyses
In a sensitivity analysis, similar associations of dialysis-unit and out-of-dialysis-unit-SBP with CVD events were observed after additional adjustment for number of BP medication classes (Table S1).
In a second sensitivity analysis, we did not include PAD as part of our composite outcome and repeat our models. With this exclusion, results were similar to the main analysis (Table S2).
Dialysis-unit and out-of-dialysis-unit-DBP and risk of CVD events
Crude rates of CVD events were highest in those in the lowest and highest quartiles of dialysis-unit-DBP (Table S3). Multivariable splines suggested a U-shaped association between dialysis-unit-DBP and risk of CVD which was not observed with out-of-dialysis-unit-DBP (Figures S1a and S1b). In adjusted models examining quartiles of DBP, there was a statistically significant association between low and high dialysis-unit-DBP with CVD events (i.e. a U-shape). No association was noted with quartiles of out-of-dialysis-unit-DBP in either unadjusted or adjusted analyses (Table S1).
Dialysis-unit and out-of-dialysis-unit-PP and risk of CVD events
Multivariable splines suggested a J-shaped association between dialysis-unit-PP and CVD risk (Figure S2a). There was no statistically significant association between quartiles of dialysis-unit-PP with risk of CVD in either unadjusted or adjusted analyses (Table S2). In contrast there was a strong linear association of out-of-dialysis-unit-PP with risk of CVD events (Figure S2b), with greater than 2-fold increased risk of CVD events for participants in the top vs. bottom quartile of out-of-dialysis-unit-PP (Table S4).
DISCUSSION
In this multi-center prospective research cohort of maintenance HD patients, we found a U-shaped association between dialysis-unit-SBP and risk of CVD events, with the lowest risk of CVD events at the range of 150–170 mmHg. However, among these same participants, there was a strong linear and positive association between out-of-dialysis-unit-SBP and risk of CVD events.12 Our results add important observational data on BP in HD patients, particularly given the relative paucity of clinical trials in this patient population. Unlike most of the prior literature on BP and outcomes in HD patients which have focused all-cause mortality, we studied CVD events, which has important implications in the care of these patients.
The small body of prior literature regarding BP and CVD in HD patients includes two papers which reported no association of dialysis-unit-SBP with risk of CVD events32, 33 and another which reported a J-shaped association.34 Some of these studies were limited by not having out-of-dialysis-BP measures.34 The study by Alborzi et al studied 150 HD patients at a single center and reported that while there was no association of pre-dialysis-SBP with CVD death, there was a significant association of home BP with CVD death.33 Home BP was ascertained using several weeks of home BP recordings; which contrasts with our study, which relied on SBP readings from a single visit. Furthermore, Alborzi et al did not examine CVD events (only CVD death) and included primarily African-American participants. The same research group led by Agarwal found that out-of-dialysis-unit-SBP among HD patients was a stronger correlate than dialysis-unit-SBP with subclinical CVD --- as assessed by left ventricular hypertrophy--- but did not ascertain clinical CVD events.35 Out-of-dialysis-unit-BP was assessed here via 44-hour interdialytic ambulatory BP monitoring (ABPM) or average of 3 daily home measurements obtained over 1 week.35 Thus, our study makes a unique contribution by linking higher out-of-dialysis-unit-BP measured at one sitting with higher risk of clinically important CVD events.
These data add to the body of evidence that out-of-dialysis-unit-SBP should be measured and potentially targeted for treatment in HD patients to improve outcomes.1, 36 Shifting treatment SBP target from dialysis-unit to out-of-dialysis-unit represents a potential way to address the current therapeutic dilemma faced by practitioners who care for HD patients. These challenges have been brought on in part by the “paradoxical BP” and “reverse epidemiology” literature which suggests lowering of SBP to <140 mmHg would be associated with harm (or no benefit) in HD patients.1, 3–6, 12, 13, 15
Our data suggest that it is not necessary to perform ABPM (or multiple home BP measurements) in order to gather important prognostic information. This opens up the possibility that BP measured at a single clinical encounter--for example, when the patient is in the office of an internist or cardiologist--may help guide treatment to improve outcomes in HD patients. However, since most clinical counter office blood pressures are a single measurement and do not adhere to standardized protocols, further studies are needed.
Currently in clinical practice, many primary care providers, cardiologists and other specialists often defer treatment of blood pressure to the nephrologist; yet non-nephrologists actually observe out-of- dialysis-unit-BP readings whereas most nephrologists typically have access only to dialysis-unit-BP readings. Notably, we found significant disparities in participants who would meet criteria for BP treatment depending on which BP measure was used. Only 39% (103/269) of participants (Table 2) with dialysis-unit-SBP >140 mmHg had out-of-dialysis-unit-SBP also >140 mmHg. Relying only on measurement of dialysis-unit-SBP may be leading to over-treatment of BP, contributing to intra-dialytic hypotension and other adverse consequences, such as myocardial stunning. These data should also be considered in the design of future clinical trials of BP control in HD patients, which have traditionally only targeted dialysis-unit-BP.37–39
In terms of the other BP components, we also observed a U-shaped association between dialysis-unit-DBP and risk of CVD events. This finding is consistent with prior reports of dialysis-unit-DBP and mortality.40–42 Lower DBP may result from increased arterial stiffening and may lead to decreased coronary perfusion and left ventricular hypertrophy, thus contributing to greater risk of CVD events. Since there was no association of out-of-dialysis-unit-DBP with CVD events; neither dialysis-unit-DBP nor out-of-dialysis-unit-DBP appear to be appropriate BP treatment targets.
For PP, there have been prior reports that higher dialysis-unit-PP in HD patients was associated with greater risk of all-cause mortality43,44 and of CVD.32 Our study adds to this prior body of literature by reporting that out-of-dialysis-unit-PP was more consistently associated with CVD events compared with dialysis-unit-PP. Although currently there are no medications that specifically target PP, a marker of arterial stiffness, a recent clinical trial of HD patients reported that atenolol was superior to lisinopril in improving arterial stiffness.45 Thus, understanding the relationship of various BP components with outcomes among HD patients may help in selection of appropriate BP medications as more therapies emerge.
Previous hypotheses to explain the U-shape association between SBP and adverse outcomes have included: survival bias, competitive risk factors, or neurohormonal state unique to HD patients.3, 14 However, these other explanations seem unlikely since we observed a linear association between higher SBP and risk of CVD when out-of-dialysis-unit-SBP is measured in the same patients. Instead, we hypothesize the inability to mount an elevated BP in response to fluid accumulated between HD sessions—reflected in the dialysis-unit-BP documented at the start of each HD session—is an adverse prognostic marker.12 Dialysis-unit-BP may reflect mostly the transient effect of inter-dialytic volume accumulation, rather than being a good overall indication of BP load as it relates to end-organ damage. Regardless of the exact pathophysiologic mechanism, these data strongly suggest that the focus should be on measuring and treating out-of-dialysis-unit-BP, rather than dialysis-unit-BP. Existing guidelines recommend dialysis-unit-SBP as the target of treatment and recommend a BP goal of <140/90 mmHg recorded at the start of each HD session.46,47–51 However, our data and data from others1, 33, 35 strongly suggest that out-of-dialysis-unit-SBP may be more important when targeting a level of BP for treatment in HD patients.
Our results are consistent with the few randomized controlled trials of BP lowering in patients on HD. In a meta-analysis of 8 such trials of ESRD patients, lowering of dialysis-unit- BP was associated with lower risk of CVD events and CVD mortality.37, 52 Results from these interventional studies are not consistent with the “paradoxical BP” and “reverse epidemiology” literature1, 3–6, 12, 13, 15 which would predict that pharmacological lowering of SBP in range of 140 mmHg would be associated with harm (or no benefit) in HD patients.
Our study had several strengths, including a diverse number of HD participants recruited from multiple sites in the U.S. Out-of-dialysis-unit-BP was measured using a standardized protocol by trained research staff. CVD outcomes were ascertained by rigorous adjudication methods. We were able to capture comorbid conditions uniformly using research grade data. We also recognize several limitations. We quantified correlations of dialysis-unit and out-of-dialysis unit-BP readings which were not taken simultaneously. However, our time-to-event analysis was based from the later of the two measures for all analyses. We do not have reliable information on the timing of out-of-dialysis-unit-BP measurements relative to HD treatment sessions although we believe the majority of CRIC research study visits did not take place on the same day as a scheduled HD treatment. Details of changes in BP during the HD sessions (such as nadir SBP) are not available. We did not analyze changes in anti-hypertensive use after the initial study visit after ESRD. We presume there were adjustments in antihypertensive medication over time as these patients were receiving regular clinical care. However, this would not have changed our findings since we are contrasting BP measures in the same study participants, who are thus exposed to the same medications over time. We studied a composite CVD events outcome and had limited power to examine individual types of CVD events due to the overall low number of events. Due to the relatively small sample size, confidence intervals on the U-shaped splines were relatively wide in Figures 2 although the dialysis-unit-SBP clearly did not have a linear association with CVD events in the same manner as the out-of-dialysis-unit-SBP. The CRIC adjudication process adapted validated procedures used in other major CVD studies and did not use ESRD-specific criteria. However, to our knowledge, ESRD-specific CVD adjudication processes have not been developed and validated. For HF events, it may be difficult to delineate with certainty the role of volume overload related to missed dialysis or dietary indiscretion or incorrect dry weight estimation. But prior studies using similar or less rigorous case definitions have shown that the syndrome of heart failure/volume overload in dialysis patients is associated with very poor outcomes53–56 so this is an important clinical entity regardless of its exact pathophysiology. We were unable to study incident CVD events as majority of participants had prevalent CVD (and were taking anti-hypertensive medications). However this largely reflects the HD population which has a large burden of pre-existing CVD. We did not have concurrent 24-hour ABPM in these participants and there were no standardized measurements of BP in the dialysis units.57–60 We only studied those who volunteered to enroll in this prospective cohort study and patients with advanced HF were not enrolled into CRIC, which may limit generalizability.
In conclusion, in this multi-center study of HD patients, there was a U-shaped association between dialysis-unit-SBP and risk of CVD events, with the lowest risk among participants with SBP 150–170 mmHg. Among these same participants, there was a strong linear association between a one-time reading of higher out-of-dialysis-unit-SBP and risk of CVD events. The findings support the argument that targeting BP measured outside of the dialysis unit may not only be more appropriate than targeting BP measured in the dialysis unit12 but may be more feasible that perhaps previously realized since BP readings taken at a single setting are associated with important outcomes (without the need for 44-hour ambulatory or weeklong home BP measurements). Although further study on the biological mechanisms to explain the observed associations is needed, the results from our study may inform clinical management of the HD patient population to improve CVD outcomes as well as help in the design of future clinical trials of BP reduction in HD patients.
Supplementary Material
PERSPECTIVES.
Among a multi-center, diverse cohort of patients on maintenance hemodialysis, there was a strong, positive, linear association between out-of-dialysis-unit-SBP and a U-shaped association between dialysis-unit-SBP and cardiovascular events was observed. Greater effort to obtain out-of-dialysis unit-SBP in hemodialysis patients should be made which may help guide clinical management as well as in the planning of clinical trials of blood pressure control to decrease risk of cardiovascular disease in this high risk patient population.
NOVELTY AND SIGNIFICANCE.
What is new
Low and high blood pressure (e.g. U-shape) measured in the dialysis unit in hemodialysis patients is associated with higher risk of cardiovascular events among patients on dialysis.
When blood pressure is measured outside of the dialysis unit in these same hemodialysis patients, there is a linear association with higher blood pressure and higher risk of cardiovascular events.
What is relevant
Cardiovascular disease remains the leading cause of morbidity and mortality among patients on dialysis.
Hypertension is extremely prevalent in patients on dialysis and there remains uncertainty on how best to manage these high-risk patients.
This study helps inform clinical management of the high-risk dialysis population as well as may guide the design of future clinical trials of blood pressure reduction in kidney disease.
Summary
Greater effort to obtain out-of-dialysis unit blood pressure in hemodialysis patients should be made which may inform clinical practice and future studies to reduce the risk of cardiovascular disease.
Acknowledgments
SOURCES OF FUNDING
This work was supported by the following grants: K23 DK088865 (Bansal), R01 DK70939 (Hsu), K24 DK92291 (Hsu). Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). This work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003 and K01 DK092353 (Anderson), Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane COBRE for Clinical and Translational Research in Cardiometabolic Diseases P20 GM109036, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131.
Footnotes
DISCLOSURES
None of the authors have any conflicts of interests.
References
- 1.Agarwal R. Blood pressure and mortality among hemodialysis patients. Hypertension. 2010;55:762–768. doi: 10.1161/HYPERTENSIONAHA.109.144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovesdy CP, Bleyer AJ, Molnar MZ, Ma JZ, Sim JJ, Cushman WC, Quarles LD, Kalantar-Zadeh K. Blood pressure and mortality in u.S. Veterans with chronic kidney disease: A cohort study. Ann Intern Med. 2013;159:233–242. doi: 10.7326/0003-4819-159-4-201308200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 4.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P. “U” curve association of blood pressure and mortality in hemodialysis patients. Medical directors of dialysis clinic, inc. Kidney Int. 1998;54:561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 5.Robinson BM, Tong L, Zhang J, Wolfe RA, Goodkin DA, Greenwood RN, Kerr PG, Morgenstern H, Li Y, Pisoni RL, Saran R, Tentori F, Akizawa T, Fukuhara S, Port FK. Blood pressure levels and mortality risk among hemodialysis patients in the dialysis outcomes and practice patterns study. Kidney Int. 2012;82:570–580. doi: 10.1038/ki.2012.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33:507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 7.Inrig JK, Patel UD, Toto RD, Szczech LA. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: A secondary analysis of the dialysis morbidity and mortality wave 2 study. Am J Kidney Dis. 2009;54:881–890. doi: 10.1053/j.ajkd.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: The 58th annual fall conference and scientific sessions. Hypertension. 2005;45:811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 9.Li Z, Lacson E, Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, Levin NW. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48:606–615. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Mazzuchi N, Carbonell E, Fernandez-Cean J. Importance of blood pressure control in hemodialysis patient survival. Kidney Int. 2000;58:2147–2154. doi: 10.1111/j.1523-1755.2000.00388.x. [DOI] [PubMed] [Google Scholar]
- 11.Chang TI, Friedman GD, Cheung AK, Greene T, Desai M, Chertow GM. Systolic blood pressure and mortality in prevalent haemodialysis patients in the hemo study. J Hum Hypertens. 2011;25:98–105. doi: 10.1038/jhh.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bansal N, McCulloch CE, Rahman M, et al. Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: The chronic renal insufficiency cohort study. Hypertension. 2015;65:93–100. doi: 10.1161/HYPERTENSIONAHA.114.04334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duranti E, Imperiali P, Sasdelli M. Is hypertension a mortality risk factor in dialysis? Kidney Int Suppl. 1996;55:S173–174. [PubMed] [Google Scholar]
- 14.Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis. 2001;38:1251–1263. doi: 10.1053/ajkd.2001.29222. [DOI] [PubMed] [Google Scholar]
- 15.Cheung AK, Sarnak MJ, Yan G, Dwyer JT, Heyka RJ, Rocco MV, Teehan BP, Levey AS. Atherosclerotic cardiovascular disease risks in chronic hemodialysis patients. Kidney Int. 2000;58:353–362. doi: 10.1046/j.1523-1755.2000.00173.x. [DOI] [PubMed] [Google Scholar]
- 16.Feldman HI, Appel LJ, Chertow GM, et al. The chronic renal insufficiency cohort (cric) study: Design and methods. J Am Soc Nephrol. 2003;14:S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 17.Lash JP, Go AS, Appel LJ, et al. Chronic renal insufficiency cohort (cric) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP Cric Groups, HCS. Ckd in hispanics: Baseline characteristics from the cric (chronic renal insufficiency cohort) and hispanic-cric studies. Am J Kidney Dis. 2011;58:214–227. doi: 10.1053/j.ajkd.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Bansal N, McCulloch CE, Lin F, et al. Different components of blood pressure are associated with increased risk of atherosclerotic cardiovascular disease versus heart failure in advanced chronic kidney disease. Kidney Int. 2016;90:1348–1356. doi: 10.1016/j.kint.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muntner P, Anderson A, Charleston J, Chen Z, Ford V, Makos G, O’Connor A, Perumal K, Rahman M, Steigerwalt S, Teal V, Townsend R, Weir M, Wright JT., Jr Hypertension awareness, treatment, and control in adults with ckd: Results from the chronic renal insufficiency cohort (cric) study. Am J Kidney Dis. 55:441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mills KT, Chen J, Yang W, et al. Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA. 2016;315:2200–2210. doi: 10.1001/jama.2016.4447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu KD, Yang W, Go AS, et al. Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in ckd: Results from the chronic renal insufficiency cohort (cric) study. Am J Kidney Dis. 2015;65:267–274. doi: 10.1053/j.ajkd.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scialla JJ, Xie H, Rahman M, et al. Fibroblast growth factor-23 and cardiovascular events in ckd. J Am Soc Nephrol. 2014;25:349–360. doi: 10.1681/ASN.2013050465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: The framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 26.Einhorn PT, Davis BR, Massie BM, Cushman WC, Piller LB, Simpson LM, Levy D, Nwachuku CE, Black HR. The antihypertensive and lipid lowering treatment to prevent heart attack trial (allhat) heart failure validation study: Diagnosis and prognosis. Am Heart J. 2007;153:42–53. doi: 10.1016/j.ahj.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 27.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397. [DOI] [PubMed] [Google Scholar]
- 28.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the atherosclerosis risk in communities (aric) cohort. Stroke; a journal of cerebral circulation. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 29.Chumlea WC, Guo SS, Kuczmarski RJ, Flegal KM, Johnson CL, Heymsfield SB, Lukaski HC, Friedl K, Hubbard VS. Body composition estimates from nhanes iii bioelectrical impedance data. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26:1596–1609. doi: 10.1038/sj.ijo.0802167. [DOI] [PubMed] [Google Scholar]
- 30.Eilers PHC, Marx BD. Flexible smoothing with b-splines and penalties. 1996:89–121. [Google Scholar]
- 31.Harrell FJ. Regression modeling strategies: With applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 32.Ishimitsu T, Nakano N, Sudo Y, Akashiba A, Takahashi T, Ohta S, Minami J, Matsuoka H. Predictive significance of blood pressure values for the incidence of cardiovascular events in chronic hemodialysis patients. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31:1703–1709. doi: 10.1291/hypres.31.1703. [DOI] [PubMed] [Google Scholar]
- 33.Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2:1228–1234. doi: 10.2215/CJN.02250507. [DOI] [PubMed] [Google Scholar]
- 34.Shafi T, Zager PG, Sozio SM, Grams ME, Jaar BG, Christenson RH, Boulware LE, Parekh RS, Powe NR, Coresh J. Troponin i and nt-probnp and the association of systolic blood pressure with outcomes in incident hemodialysis patients: The choices for healthy outcomes in caring for esrd (choice) study. Am J Kidney Dis. 2014;64:443–451. doi: 10.1053/j.ajkd.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C. Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension. 2006;47:62–68. doi: 10.1161/01.HYP.0000196279.29758.f4. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal R, Andersen MJ. Prognostic importance of ambulatory blood pressure recordings in patients with chronic kidney disease. Kidney Int. 2006;69:1175–1180. doi: 10.1038/sj.ki.5000247. [DOI] [PubMed] [Google Scholar]
- 37.Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, Gallagher M, Roberts MA, Cass A, Neal B, Perkovic V. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: A systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373:1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tepel M, Hopfenmueller W, Scholze A, Maier A, Zidek W. Effect of amlodipine on cardiovascular events in hypertensive haemodialysis patients. Nephrol Dial Transplant. 2008;23:3605–3612. doi: 10.1093/ndt/gfn304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gul A, Miskulin D, Gassman J, Harford A, Horowitz B, Chen J, Paine S, Bedrick E, Kusek JW, Unruh M, Zager P. Design of the blood pressure in dialysis pilot study. Am J Med Sci. 2013 doi: 10.1097/MAJ.0b013e31827daee5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwal R. Hypertension and survival in chronic hemodialysis patients--past lessons and future opportunities. Kidney Int. 2005;67:1–13. doi: 10.1111/j.1523-1755.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 41.Foley RN, Herzog CA, Collins AJ United States Renal Data S. Blood pressure and long-term mortality in united states hemodialysis patients: Usrds waves 3 and 4 study. Kidney Int. 2002;62:1784–1790. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 42.Guerin AP, Pannier B, Marchais SJ, London GM. Cardiovascular disease in the dialysis population: Prognostic significance of arterial disorders. Curr Opin Nephrol Hypertens. 2006;15:105–110. doi: 10.1097/01.mnh.0000203186.11772.21. [DOI] [PubMed] [Google Scholar]
- 43.Klassen PS, Lowrie EG, Reddan DN, DeLong ER, Coladonato JA, Szczech LA, Lazarus JM, Owen WF., Jr Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA. 2002;287:1548–1555. doi: 10.1001/jama.287.12.1548. [DOI] [PubMed] [Google Scholar]
- 44.Tozawa M, Iseki K, Iseki C, Takishita S. Pulse pressure and risk of total mortality and cardiovascular events in patients on chronic hemodialysis. Kidney Int. 2002;61:717–726. doi: 10.1046/j.1523-1755.2002.00173.x. [DOI] [PubMed] [Google Scholar]
- 45.Georgianos PI, Agarwal R. Effect of lisinopril and atenolol on aortic stiffness in patients on hemodialysis. Clin J Am Soc Nephrol. 2015;10:639–645. doi: 10.2215/CJN.09981014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.http://www.kidney.org/professionals/KDOQI/guidelines_cvd/guide12.htm.
- 47.Bolton K, Beddhu S, Campese VM, et al. K/doqi clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis. 2005;45:S7–S153. [PubMed] [Google Scholar]
- 48.Harper J, Nicholas J, Webb L, Casula A, Williams AJ. Uk renal registry 12th annual report (december 2009): Chapter 11 blood pressure profile of prevalent patients receiving dialysis in the uk in 2008: National and centre-specific analyses. Nephron Clin Pract. 2010;115:C239–C260. doi: 10.1159/000301234. [DOI] [PubMed] [Google Scholar]
- 49.Jindal K, Chan CT, Deziel C, Hirsch D, Soroka SD, Tonelli M, Culleton BF. Hemodialysis clinical practice guidelines for the canadian society of nephrology. J Am Soc Nephrol. 2006;17:S1–27. doi: 10.1681/ASN.2005121372. [DOI] [PubMed] [Google Scholar]
- 50.Roberts MA, Pilmore HL, Tonkin AM, Garg AX, Pascoe EM, Badve SV, Cass A, Ierino FL, Hawley CM. Challenges in blood pressure measurement in patients treated with maintenance hemodialysis. Am J Kidney Dis. 2012;60:463–472. doi: 10.1053/j.ajkd.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 51.Hirakata H, Nitta K, Inaba M, et al. Japanese society for dialysis therapy guidelines for management of cardiovascular diseases in patients on chronic hemodialysis. Ther Apher Dial. 2012;16:387–435. doi: 10.1111/j.1744-9987.2012.01088.x. [DOI] [PubMed] [Google Scholar]
- 52.Agarwal R, Sinha AD. Cardiovascular protection with antihypertensive drugs in dialysis patients: Systematic review and meta-analysis. Hypertension. 2009;53:860–866. doi: 10.1161/HYPERTENSIONAHA.108.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Banerjee D, Ma JZ, Collins AJ, Herzog CA. Long-term survival of incident hemodialysis patients who are hospitalized for congestive heart failure, pulmonary edema, or fluid overload. Clin J Am Soc Nephrol. 2007;2:1186–1190. doi: 10.2215/CJN.01110307. [DOI] [PubMed] [Google Scholar]
- 54.Liang KV, Greene EL, Williams AW, Herzog CA, Hodge DO, Owan TE, Redfield MM. Exploratory study of relationship between hospitalized heart failure patients and chronic renal replacement therapy. Nephrol Dial Transplant. 2009;24:2518–2523. doi: 10.1093/ndt/gfn775. [DOI] [PubMed] [Google Scholar]
- 55.Harnett JD, Foley RN, Kent GM, Barre PE, Murray D, Parfrey PS. Congestive heart failure in dialysis patients: Prevalence, incidence, prognosis and risk factors. Kidney Int. 1995;47:884–890. doi: 10.1038/ki.1995.132. [DOI] [PubMed] [Google Scholar]
- 56.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, Fonarow GC. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119:671–679. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ekart R, Kanic V, Pecovnik Balon B, Bevc S, Hojs R. Prognostic value of 48-hour ambulatory blood pressure measurement and cardiovascular mortality in hemodialysis patients. Kidney & blood pressure research. 2012;35:326–331. doi: 10.1159/000336357. [DOI] [PubMed] [Google Scholar]
- 58.Liu W, Ye H, Tang B, Sun Z, Wen P, Wu W, Bian X, Shen X, Yang J. Comparison of 44-hour and fixed 24-hour ambulatory blood pressure monitoring in dialysis patients. Journal of clinical hypertension (Greenwich, Conn) 2014;16:63–69. doi: 10.1111/jch.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roberts MA, Pilmore HL, Tonkin AM, Garg AX, Pascoe EM, Badve SV, Cass A, Ierino FL, Hawley CM. Challenges in blood pressure measurement in patients treated with maintenance hemodialysis. American Journal of Kidney Diseases. 60:463–472. doi: 10.1053/j.ajkd.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 60.Drawz PE, Abdalla M, Rahman M. Blood pressure measurement: Clinic, home, ambulatory, and beyond. American Journal of Kidney Diseases. 2012;60:449–462. doi: 10.1053/j.ajkd.2012.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.