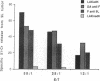
Abstract
Recombinant interleukin 2 (rIL-2) and various effector cell populations were used for adoptive immunotherapy in the Fischer strain 9L rat gliosarcoma model. The in vivo cytotoxicities of nonspecifically activated lymphocytes and specifically activated cytotoxic T lymphocytes (CTLs) were assessed in a modified in vivo neutralization (Winn) assay. Effector cells (10(6)) and 9L tumor cells (10(5] were combined with 10(4) units of rIL-2 and stereotactically implanted into the right frontal centrum semiovale of the Fischer (F344) rat. At 7 and 14 days, additional effector cells (10(6] and rIL-2 (10(4) units) were administered through the same burr hole. Nonspecifically activated splenocytes were lymphokine-activated killer (LAK) cells, both plastic-adherent and nonadherent, whereas specifically activated CTLs were either syngeneic (genetically identical) or allogeneic (genetically dissimilar). Syngeneic CTLs were T lymphocytes from Fischer rats primed in vivo with 9L cells and restimulated in vitro. Allogeneic CTLs were generated by exposing DA rat lymphocytes either to irradiated Fischer lymph node cells or to 9L Fisher tumor cells in vitro. Control groups included rats bearing 9L tumor who were untreated, those who received peripheral (i.p. or s.c.) administration of rIL-2, or those who received syngeneic unstimulated T lymphocytes and rIL-2. For a set of animals given the same inoculum of 9L tumor, significantly improved survival was shown for groups treated with nonadherent or adherent LAK cells (P less than or equal to 0.0003), syngeneic CTLs (P = 0.0327), or allogeneic CTLs (P = 0.0025) over untreated control animals by using Mantel-Haenzel nonparametric logrank equations. Only treatment with allogeneic CTLs prevented tumor take.
Full text
PDF


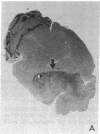
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright A. L., Gill T. J., 3rd, Geyer S. J. Immunogenetic control of brain tumor growth in rats. Cancer Res. 1977 Aug;37(8 Pt 1):2512–2521. [PubMed] [Google Scholar]
- Bellgrau D. Induction of cytotoxic T cell precursors in vivo. Role of T helper cells. J Exp Med. 1983 May 1;157(5):1505–1515. doi: 10.1084/jem.157.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrau D., Wilson D. B. Immunological studies of T-cell receptors. I. Specifically induced resistance to graft-versus-host disease in rats mediated by host T-cell immunity to alloreactive parental T cells. J Exp Med. 1978 Jul 1;148(1):103–114. doi: 10.1084/jem.148.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgrau D., Zöller M. Cytotoxic T lymphocyte responses to spontaneous tumors: immunogenicity dependent on the recognition of processed tumor antigens. J Immunol. 1983 May;130(5):2005–2007. [PubMed] [Google Scholar]
- Bhondeley M. K., Mehra R. D., Mehra N. K., Mohapatra A. K., Tandon P. N., Roy S., Bijlani V. Imbalances in T cell subpopulations in human gliomas. J Neurosurg. 1988 Apr;68(4):589–593. doi: 10.3171/jns.1988.68.4.0589. [DOI] [PubMed] [Google Scholar]
- Chiu K. M., Harris J. E., Kroin J. S., Slayton W., Braun D. P. The immunological response of Wistar rats to the intracranially implanted C-6 glioma cell line. J Neurooncol. 1983;1(4):365–372. doi: 10.1007/BF00165720. [DOI] [PubMed] [Google Scholar]
- Crafts D., Wilson C. B. Animal models of brain tumors. Natl Cancer Inst Monogr. 1977 Dec;46:11–17. [PubMed] [Google Scholar]
- Fontana A., Hengartner H., de Tribolet N., Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–1844. [PubMed] [Google Scholar]
- Gately M. K., Glaser M., Dick S. J., Mettetal R. W., Jr, Kornblith P. L. In vitro studies on the cell-mediated immune response to human brain tumors. I. Requirement for third-party stimulator lymphocytes in the induction of cell-mediated cytotoxic responses to allogeneic cultured gliomas. J Natl Cancer Inst. 1982 Dec;69(6):1245–1254. [PubMed] [Google Scholar]
- Geyer S. J., Landay A. Immunogenetic and immunologic aspects of gliosarcoma growth in rats. Lab Invest. 1983 Oct;49(4):436–444. [PubMed] [Google Scholar]
- Henkart P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu Rev Immunol. 1985;3:31–58. doi: 10.1146/annurev.iy.03.040185.000335. [DOI] [PubMed] [Google Scholar]
- Hook G. R., Greenwood M. A., Barba D., Ikejiri B., Chen S. N., Oldfield E. H., Weber R. J., Muul L. M. Morphology of interleukin-2-stimulated human peripheral blood mononuclear effector cells killing glioma-derived tumor cells in vitro. J Natl Cancer Inst. 1988 Apr 6;80(3):171–177. doi: 10.1093/jnci/80.3.171. [DOI] [PubMed] [Google Scholar]
- Ingram M., Shelden C. H., Jacques S., Skillen R. G., Bradley W. G., Techy G. B., Freshwater D. B., Abts R. M., Rand R. W. Preliminary clinical trial of immunotherapy for malignant glioma. J Biol Response Mod. 1987 Oct;6(5):489–498. [PubMed] [Google Scholar]
- Jacobs S. K., Wilson D. J., Melin G., Parham C. W., Holcomb B., Kornblith P. L., Grimm E. A. Interleukin-2 and lymphokine activated killer (LAK) cells in the treatment of malignant glioma: clinical and experimental studies. Neurol Res. 1986 Jun;8(2):81–87. doi: 10.1080/01616412.1986.11739735. [DOI] [PubMed] [Google Scholar]
- Kruse C. A., Mitchell D. H., Lillehei K. O., Johnson S. D., McCleary E. L., Moore G. E., Waldrop S., Mierau G. W. Interleukin-2-activated lymphocytes from brain tumor patients. A comparison of two preparations generated in vitro. Cancer. 1989 Oct 15;64(8):1629–1637. doi: 10.1002/1097-0142(19891015)64:8<1629::aid-cncr2820640813>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Merchant R. E., Grant A. J., Merchant L. H., Young H. F. Adoptive immunotherapy for recurrent glioblastoma multiforme using lymphokine activated killer cells and recombinant interleukin-2. Cancer. 1988 Aug 15;62(4):665–671. doi: 10.1002/1097-0142(19880815)62:4<665::aid-cncr2820620403>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Mulvin D. W., Kruse C. A., Mitchell D. H., Marcell T., James G. T., Johnston M. R. Lymphokine-activated killer cells with interleukin-2: dose toxicity and localization in isolated perfused rat lungs. Mol Biother. 1990 Mar;2(1):38–43. [PubMed] [Google Scholar]
- Takai N., Tanaka R., Yoshida S., Hara N., Saito T. In vivo and in vitro effect of adoptive immunotherapy of experimental murine brain tumors using lymphokine-activated killer cells. Cancer Res. 1988 Apr 15;48(8):2047–2052. [PubMed] [Google Scholar]
- Vujanovic N. L., Herberman R. B., Maghazachi A. A., Hiserodt J. C. Lymphokine-activated killer cells in rats. III. A simple method for the purification of large granular lymphocytes and their rapid expansion and conversion into lymphokine-activated killer cells. J Exp Med. 1988 Jan 1;167(1):15–29. doi: 10.1084/jem.167.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki T., Handa H., Yamashita J., Watanabe Y., Namba Y., Hanaoka M. Specific adoptive immunotherapy with tumor-specific cytotoxic T-lymphocyte clone for murine malignant gliomas. Cancer Res. 1984 May;44(5):1776–1783. [PubMed] [Google Scholar]