Abstract
Klebsiella pneumoniae (K. pneumoniae) is an opportunistic pathogen that can adhere to host cells or extracellular matrix via type 1 and type 3 fimbriae. KP1_4563 is a gene encoding a hypothetical protein in K. pneumoniae NTUH-K2044. KP1_4563 is located between the type 1 and type 3 fimbrial gene clusters and is likely associated with fimbrial function given its putative conserved domains of unknown function (DUF1471). Cyclic AMP receptor protein (CRP) regulates virulence-related gene expression and is a crucial transcriptional regulator in many bacteria. The predicted DNA recognition motif of CRP is present in the KP1_4563 promoter region. This study aimed to investigate the function of KP1_4563 in fimbriae and its transcriptional regulation mechanism by CRP. We generated Kp-Δ4563 mutant and complementation strains. We utilized phenotype and adhesion assays to evaluate the role of KP1_4563 in fimbriae. We conducted quantitative RT-PCR (qRT-PCR), LacZ fusion, electrophoretic mobility shift, and DNase I footprinting assays to study the transcriptional regulation of KP1_4563 gene by CRP. We found that KP1_4563 negatively regulates the function of type 3 fimbriae. Compared with NTUH-K2044, the absence of KP1_4563 enhanced the ability of Kp-Δ4563 to adhere to A549 cells. CRP negatively regulates KP1_4563 by directly binding to its promoter region. KP1_4563 plays an important role in type 3 fimbrial function. This novel insight will assist in the development of strategies for preventing K. pneumoniae infection.
Introduction
Klebsiella pneumoniae (K. pneumoniae), a common hospital-acquired and potentially community-acquired pathogen, causes catheter-associated urinary tract infections, pneumonia, bacteremia, surgical wound infections, pyogenic liver abscesses and bacterial meningitis [1–6]. The ability of bacteria to adhere to host structures plays a major role in the development of infections. In Enterobacteriaceae, adhesion is mediated by fimbriae [7–10]. Type 1 and type 3 fimbriae are two commonly expressed and well-characterized fimbriae in K. pneumoniae. Type 1 fimbriae, which are found in the majority of the Enterobacteriaceae family, mediate adhesion to mannose-containing receptors on host cells or in the extracellular matrix [11, 12] and are encoded and regulated by the fim gene cluster. Type 1 fimbriae act as virulence factors in urinary tract infections by mediating adhesion to the uroepithelium; these fimbriae also promote the colonization and biofilm formation of K. pneumoniae on urethral catheters [13–16]. Type 3 fimbriae were first described in the Klebsiella species and are common in Enterobacter, Proteus, Serratia, and Providencia species [17–21]. Type 3 fimbriae, which are encoded and regulated by the mrk gene cluster, adhere to epithelial cells in the respiratory or urinary tracts and to extracellular matrix proteins. Moreover, type 3 fimbriae initiate biofilm formation and are required for biofilm maturation [8, 22–24]. The K. pneumoniae strain NTUH-K2044 (K1: O1) was first isolated from the blood of a Taiwanese liver abscess patient [25]. In this strain, the type 1 and type 3 fimbrial gene clusters are physically linked. The 4.6-kb DNA fragment between the gene clusters fim and mrk comprises five open reading frames (ORFs): KP1_4562 and KP1_4563, which are two hypothetical protein encoding genes; KP1_4564 and KP1_4565, which are pecM and pecS homologues, respectively; and KP1_4566, which is a putative high affinity nickel transporter encoding gene [26, 27]. The function of KP1_4563 is currently unknown, but the KP1_4563 protein has putative conserved domains of unknown function (DUF1471). DUF1471, also known as PF07338, YhcN, or BhsA/McbA, is a basic feature of sequences in the family of conserved proteins in Enterobacteriaceae [28]. Eletsky et.al [29] reported that DUF1471 is involved in the host-pathogen interface. In Salmonella enterica Typhimurium, the DUF1471-containing protein YcfR likely influences surface characteristics that mediate surface attachment and cell aggregation [30]. In Escherichia coli (E. coli), YcfR/BhsA has roles that are related to attachment to the surfaces of vegetables [31]. Based on its putative conserved DUF1471 domains, we suspected that KP1_4563 is associated with adhesion in K. pneumoniae and influences fimbrial function.
The cyclic AMP receptor protein (CRP), also called catabolite gene activator protein (CAP), is an important global regulator. In the form of the CRP–cAMP complex, CRP enhances the ability of the RNA polymerase holoenzyme to bind and initiate the transcription of specific sets of genes [32–34]. The CRP–cAMP complex globally regulates gene expression in E.coli by controlling the initiation of transcription of more than 100 operons [35, 36]. CRP is required for carbon metabolism, and regulates the expression of numerous genes that encode bacterial virulence factors, such as flagella, fimbriae, and exotoxins [37–40]. One of our previous studies showed that CRP is an essential virulence regulator: K. pneumoniae with crp knocked out is less virulent in A549 human lung carcinoma cells and in adult female BALB/c mice compared to parental K. pneumoniae [41]. Several studies have showed that in K. pneumoniae, the CRP–cAMP complex specifically binds to intergenic regions in citC-citS for citrate fermentation and to the promoter proximal region of allS for allantoin utilization [41, 42]. The synthetic palindromic DNA recognition motif of CRP is 5′-AAATGTGATCTAGATCACATTT-3′, and it is well characterized in E.coli [43, 44], The consensus DNA site (underlined above) is the most important site for CRP–DNA complex formation. Sequence analysis identified putative CRP binding sites in the promoter region of KP1_4563, which suggests that the CRP–cAMP complex directly regulates the promoter. This hypothesis was confirmed by experimental evidence in this study.
This study aimed to investigate the function and transcriptional regulation mechanism of KP1_4563. Thus, we conducted phenotype and adhesion assays to identify the effect of KP1_4563 on fimbriae function. Subsequently, to study the transcriptional regulation mechanism of KP1_4563 by CRP, qRT-PCR and LacZ fusion assays were performed to verify the transcription of KP1_4563. Furthermore, electrophoretic mobility shift and DNase I footprinting assays were utilized to analyze the specificity of CRP binding to the promoter proximal region of KP1_4563.
Materials and methods
Bacterial strains, plasmids, primers, and growth conditions
The bacterial strains and plasmids used in this study are listed in Table 1. All the primers used in the present work are listed in Table 2. K. pneumoniae and E. coli were grown in Luria–Bertani (LB) medium or LB medium supplemented with antibiotics at the following concentrations: ampicillin (Ap, 100 μg/ml), kanamycin (Km, 50 μg/ml), and chloramphenicol (Cm, 35 μg/ml). To fully express fimbriae, K. pneumoniae strains were statically cultivated in modified Minka medium for 48 h at 37°C. Continuous cultivation was conducted for three generations at 1:1000 dilution in the same medium [45].
Table 1. Bacterial strains and plasmids used in this study.
| Strains or plasmids | Genotype or description | Reference or source |
|---|---|---|
| K. pneumoniae | ||
| K2044 | K1 serotype | [25] |
| Kp-Δcrp | K2044 with deletion of crp | This study |
| Kp-Δ4563 | K2044 with deletion of KP1_4563 | This study |
| Kpc-Δ4563 | Kpc-Δ4563 complemented with KP1_4563 | This study |
| CCW01 | K2044 ΔlacZ strain | [54] |
| CCW01Δcrp | CCW01 with deletion of crp | This study |
| CCW01/placZ15-p4563 | CCW01 complemented with KP1_4563 | This study |
| CCW01Δcrp/placZ15-p4563 | CCW01Δcrp complemented with KP1_4563 | This study |
| E. coli | ||
| DH5α | Cloning host | [66] |
| BL21 | Express the CRP protein | [67] |
| Plasmids | ||
| pKO3-Km | Kmr, suicide vector | [68] |
| pKO3-Km-p4563 | Kmr, suicide vector for KP1_4563 deletion | This study |
| pBAD33 | Cmr, cloning vector | Laboratory stock |
| pBAD33-p4563 | Cmr, cloning vector containing KP1_4563 | This study |
| placZ15 | Cmr, promoter selection vector, lacZ+ | [54] |
| placZ15-p4563 | Cmr, KP1_4563 promoter fused with lacZ reporter | This study |
Table 2. Oligonucleotide primers used in this study.
| Primers | Sequence (5'-3') |
|---|---|
| Gene deletions | |
| KP1_4563-A | ATAAGAATGCGGCCGCGGCGATGCTGATTTATGC |
| KP1_4563-B | CCCTCTGCAACCATTCGCGTTTGCTTTCGATGGACTT |
| KP1_4563-C | AAGTCCATCGAAAGCAAACGCGAATGGTTGCAGAGGG |
| KP1_4563-D | ATAAGAATGCGGCCGCTCGGGGCGATCAGTATGG |
| Complementation of mutant | |
| KP1_4563-HB-KpnI-F | CGGGGTACCAGGAGGAATTCACCATGCTTTCCACCATAAAA |
| KP1_4563-HB-SalI-R | ACGCGTCGACTTATTTAGACAGCTCGGC |
| qRT-PCR | |
| KP1_4563-RT-F | CGGTATGCTCTCCCTGGTC |
| KP1_4563-RT-R | TATTTAGACAGCTCGGCGGTC |
| LacZ fusion | |
| KP1_4563-LacZ-F | CGCGGATCCCGATGCTGATTTATGCCAC |
| KP1_4563-LacZ-R | GGAAGATCTATACCGGCAGCTGCGAGTAA |
| Protein production | |
| KP1_5071-CRP-P-F | GCGGGATCCATGGTGCTTGGCAAACCG |
| KP1_5071-CRP-P-R | GCGAAGCTTTTAACGGGTGCCGTAGACG |
| EMSA | |
| KP1_4563-EMSA-F | CGATGCTGATTTATGCCAC |
| KP1_4563-EMSA-R | ATACCGGCAGCTGCGAGTAA |
| KP1_16S -EMSA-F | CGGTCTGTCAAGTCGGATGTG |
| KP1_16S -EMSA-R | CGGAAGCCACGCCTCAAG |
| DNase I footprinting | |
| KP1_4563-FP-F | ATGTGATACCCCCTTTCAGAAG |
| KP1_4563-FP-R | ATACCGGCAGCTGCGAGTAA |
Amplification of the KP1_4563 coding region together with AGGAGG, which is a ribosome binding site (underlined) consensus sequence, and AATTCACC (italic), a spacer. Bold letters indicate the respective restriction enzyme site in the primer.
Construction of gene deletion and complementation strains
Mutant Kp-Δ4563 was constructed via a previously described unmarked deletion method [46, 47]. In brief, the upstream and downstream flanking DNA fragments of KP1_4563 were amplified. The two flanking fragments were fused by PCR and then cloned into the temperature-sensitive suicide vector pKO3-Km. The recombinant plasmid was introduced into K2044 by electroporation. Integration (at 30°C) and excision (at 43°C) of the plasmid generated Kp-Δ4563, as confirmed by PCR and DNA sequencing.
To construct the complementation strain, the DNA region that contained the intact KP1_4563 gene was amplified via PCR. The DNA fragment was then cloned into the pBAD33 plasmid. Then, the recombinant plasmid was introduced into Kp-Δ4563 via electroporation. The Kpc-Δ4563 complementation strain was selected on LB agar plates supplemented with chloramphenicol and verified via PCR.
Hemagglutination assays
The expression of type 3 fimbriae was examined via mannose-resistant hemagglutination (MRHA) assays as previously described [17, 48]. Briefly, strains were statically cultivated in modified Minka medium. The third generations of the strains were harvested, washed once with phosphate-buffered saline (PBS), and resuspended at a concentration 1010CFU/ml. Then, 2.5% of fresh human erythrocytes were treated with an equal volume of a 0.003% (wt/vol) tannic acid (Sigma) solution in saline for 10 min at 37°C and were washed twice with PBS. Tanned erythrocytes were mixed with equal volumes of a series of 2-fold dilutions of the bacterial suspension with or without 0.25% mannose (Sigma) in 96-well, U-bottomed microtiter plates. The plates were gently agitated at room temperature for 1 min. Then, the minimum bacterial density (CFU/ml) required to agglutinate erythrocytes was measured. The expression of type 1 fimbriae was specifically detected via the mannose-sensitive agglutination of guinea pig red blood cells (RBCs) assays [49]. As described above, 2.5% guinea pig RBCs were mixed with a series of 2-fold dilutions of bacterial suspension with or without 0.25% mannose in 96-well, U-bottomed microtiter plates. Two controls were included in this experiment: E. coli DH5α (type 1 fimbriae expression) and PBS (negative control). The plates were gently agitated at room temperature for 1 min. Then, the minimum bacterial density (CFU/ml) required to agglutinate erythrocytes was measured. The experiment was repeated at least thrice.
Mannan-binding assay
The quantity of fimbriae from the K2044, Kp-Δ4563, and Kpc-Δ4563 strains was examined by mannan-binding assay as previously described [50]. Mannan (Sigma) derived from Saccharomyces cerevisiae was dissolved in 0.02 M bicarbonate buffer. Then, 100 μl of 20 μg/ml mannan was statically incubated in 96-well, flat-bottomed cell culture plates at 37°C for 1 h. The wells were washed thrice with sterile PBS (pH 7.4) and quenched with 0.1% bovine serum albumin (BSA) at 37°C for 15 min. The WT, Kp-Δ4563 and Kpc-Δ4563 strains were statically cultivated in modified Minka medium under the same conditions described above. The third generations of strains were adjusted to OD540 = 2.0. Then, 100 μl of bacterial solution was added to the wells. The strains were then statically incubated at 37°C for 45 min. After incubation, unattached bacteria were removed by washing the wells thrice with sterile PBS. A total of 150 μl of modified Minka medium was added to per well. The strains were then incubated at 37°C for 4 h with shaking at 200 rpm. Finally, the density of bound bacteria in each well was determined by measuring the OD415 with an Absorbance Microplate Reader (BioTek, USA). Each strain was assayed with six technical replicates in each experiment, and at least three biological replicates were performed.
Bacterial adhesion assays
The adhesion ability of the K2044, Kp-Δ4563, and Kpc-Δ4563 strains was examined by adhesion assays as previously described [51]. Briefly, monolayers of A549 human lung epithelial cell lines (8×105) were infected at a multiplicity of infection (MOI) of 100 in 24-well, flat-bottomed cell culture plates, followed by incubation at 37 °C for 4 h. After incubation, the wells were washed thrice with PBS to remove unattached bacteria. Adherent bacteria were released by the addition of 1 ml of 0.1% Triton per well and were then quantified by plating appropriate dilutions on LB agar plates. Adhesion was expressed as the number of CFU that adhered to the A549 cells. The results were presented as the mean of at least three technical repeat wells in each experiment, and at least three biological replicates were performed.
Quantitative RT-PCR (qRT-PCR)
K2044 and Kp-Δcrp strains were statically cultivated in modified Minka medium at 37°C for 48 h. The third generations of the strains were diluted 1:1000 in 15 ml of fresh medium and grown until OD600 = 1.0. The bacteria were pelleted for RNA extraction. RNA was extracted, and the residual DNA was eliminated with an RNAprep Pure Cell/Bacteria Kit (TIANGEN) in accordance with the manufacturer’s instructions. RNA quality was determined via 1.2% agarose gel electrophoresis, and RNA quantity was determined with a NanoDrop 2000 UV-Vis Spectrophotometer (Thermo Scientific). Equal quantities of RNA were converted to cDNA using the random hexamer primer from a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific). Real-time PCR was performed with a SYBR Premix Ex Taq II Kit (Takara) and LightCycler System. Data were normalized with 16S rRNA as the endogenous reference. The relative expression ratio of a target gene was calculated using a previously described method [52]. Every sample was tested in triplicate in each experiment. Each experiment was repeated at least thrice.
LacZ fusion and β-galactosidase assay
The promoter region of KP1_4563 was amplified from K2044 using the primers listed in Table 2. The DNA fragments were cloned into the BamHI and BglII sites of the placZ15 plasmid [53]. The recombinant placZ15-p4563 plasmid was verified via PCR, and then introduced into K. pneumoniae NTUH-K2044ΔlacZ strain CCW01[54] and the deletion mutant CCW01Δcrp. The bacteria carrying different plasmids were statically cultivated in modified Minka medium. β-galactosidase activity in cellular extracts was tested by using the β-Galactosidase Enzyme Assay System (Promega). Promoter activity was expressed as Miller units. Every sample was tested in triplicate in each experiment, and was at least three biological replicates were performed.
Electrophoresis mobility shift assay (EMSA)
The His–CRP protein was purified as previously described [41, 55]. For the EMSA [56, 57], the putative promoter region fragments of KP1_4563 were labeled at the 5′ end with [γ-32P] ATP and T4 polynucleotide kinase. In the final 10 μl of the reaction volume, the labeled DNA fragment (1000 to 2000 cpm/μl) was incubated with increasing amounts of purified His–CRP protein and 20 mM cAMP at room temperature for 20 min in 5× binding buffer. Three controls were included in the EMSA experiment: 1) cold probe (the same promoter-proximal DNA region unlabeled) as a specific DNA competitor, 2) negative probe (the unlabeled coding region of the 16S rRNA gene) as a non-specific DNA competitor, and 3) unrelated protein as a non-specific protein competitor. After incubation, the samples were analyzed by electrophoresis on 4% native polyacrylamide gels in 0.5× TBE buffer at 80–120 voltage and 4°C. Radioactivity was detected via autoradiography after exposure to Kodak film at -70°C.
DNase I footprinting
For the DNase I footprinting [56, 57], the sense or antisense primer of the putative promoter region of the KP1_4563 gene was labeled with [γ-32P] ATP and T4 polynucleotide kinase. The putative promoter region of KP1_4563 was amplified by PCR with 32P-labeled primers. In the final 10 μl of the reaction volume, the purified PCR products (15,000 to 20,000 cpm/μl) were incubated with increasing amounts of purified His–CRP protein with 20 mM cAMP in 5× binding EMSA buffer. The reaction volume was incubated at room temperature for 30 min. Optimized DNase I (Promega) was then added to the reaction mixture, and it was incubated at room temperature for 30–70s. The reaction was quenched by adding 9 μl of stop solution (200 mM NaCl, 30 mM EDTA, and 1% SDS). Then, the mixture was incubated at room temperature for 1 min. The digested DNA was extracted with phenol/chloroform and analyzed on 6% polyacrylamide gels with 8 M urea. Radioactivity was detected as above. Footprints were identified by comparison with sequence ladders.
Nucleotide sequence accession number
All sequences obtained in this study were deposited in GenBank under the accession number NC_012731.
Statistical analyses
Statistical analyses were conducted with SPSS 22.0 software. P<0.05 was considered statistically significant.
Results
The KP1_4563 gene negatively regulates the function of type 3 fimbriae
We successfully generated Kp-Δ4563 and Kpc-Δ4563 strains. We then utilized phenotype and adhesion assays to investigate the role of KP1_4563 in fimbriae.
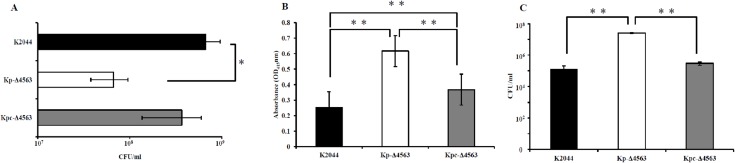
To address the influence of KP1_4563 on type 3 fimbrial function, we performed MRHA, a sensitive functional assay for type 3 fimbriae. The minimum bacterial density (CFU/ml) that was required to agglutinate erythrocytes is showed in Fig 1A. Deleting KP1_4563 from K2044 increased the MRHA activity. The minimum bacterial density of Kp-Δ4563 required to agglutinate erythrocytes was 6.67×107 CFU/ml, which was approximately 10-fold lower than that of K2044 (6.67×108CFU/ml, P<0.05). The MRHA activity weakened when Kp-Δ4563 was complemented with pBAD33-p4563 plasmid, and the minimum bacterial density of Kpc-Δ4563 required to agglutinate erythrocytes was 3.67×108 CFU/ml. Two controls were included in this experiment, E. coli DH5α (type 1 fimbriae expression) and PBS (negative control), which both failed to mediate visible agglutination.
Fig 1. Phenotype and adhesion assays of KP1_4563.
(A) Hemagglutination assays. Mannose-resistant hemagglutination (MRHA) assays were performed with human erythrocytes. The results are expressed as the minimum bacterial density (CFU/ml) required to cause a visible agglutination reaction. Values represent the mean of three independent experiments, and the error bars represent standard deviation. P values were calculated by one-way ANOVA and Tukey HSD post hoc comparisons. (B) Mannan-binding assay. Mean values and standard deviation of six technical replicates are showed. P values were calculated by one-way ANOVA and LSD post hoc comparisons. (C) Bacterial adhesion assays. Data are the means of measurements made in technical triplicates. Error bars represent the standard deviation. P values were calculated by one-way ANOVA and LSD post hoc comparisons. Significant differences are indicated by * for P<0.05 or ** for P<0.01.
The effect of KP1_4563 on the function of type 1 fimbriae was determined via the mannose-sensitive agglutination assays of guinea pig RBCs. The highest bacterial densities (>1×1010CFU/ml, data not showed) of K2044, Kp-Δ4563, and Kpc-Δ4563 examined all failed to induce the visible mannose-sensitive agglutination of guinea pig RBCs. Two controls were included in this experiment, E. coli DH5α, which mediates visible mannose-sensitive agglutination, and PBS, which does not induce agglutination. These results indicated that KP1_4563 is irrelevant to the function of type 1 fimbriae.
Type 1 and type 3 fimbriae both bind to yeast surfaces [49]. A binding assay with mannan derived from Saccharomyces cerevisiae (the receptor compound) was performed to investigate the function of fimbriae. As showed in Fig 1B, compared with K2044, Kp-Δ4563 bound to mannan more strongly. Binding was reduced when Kp-Δ4563 was complemented with the expression-complementary pBAD33-p4563 plasmid. These results indicated that KP1_4563 likely negatively regulates the function of type 3 fimbriae in an unknown manner.
Role of KP1_4563 gene in bacterial adhesion
The ability of K2044, Kp-Δ4563, and Kpc-Δ4563 to adhere to A549 human lung cancer cells were analyzed (Fig 1C). The absence of KP1_4563 dramatically enhanced the adhesion of Kp-Δ4563 to A549 cells compared with that of K2044. Adhesion was weakened when Kp-Δ4563 was complemented with the expression-complementary pBAD33-p4563 plasmid.
CRP negative regulates KP1_4563
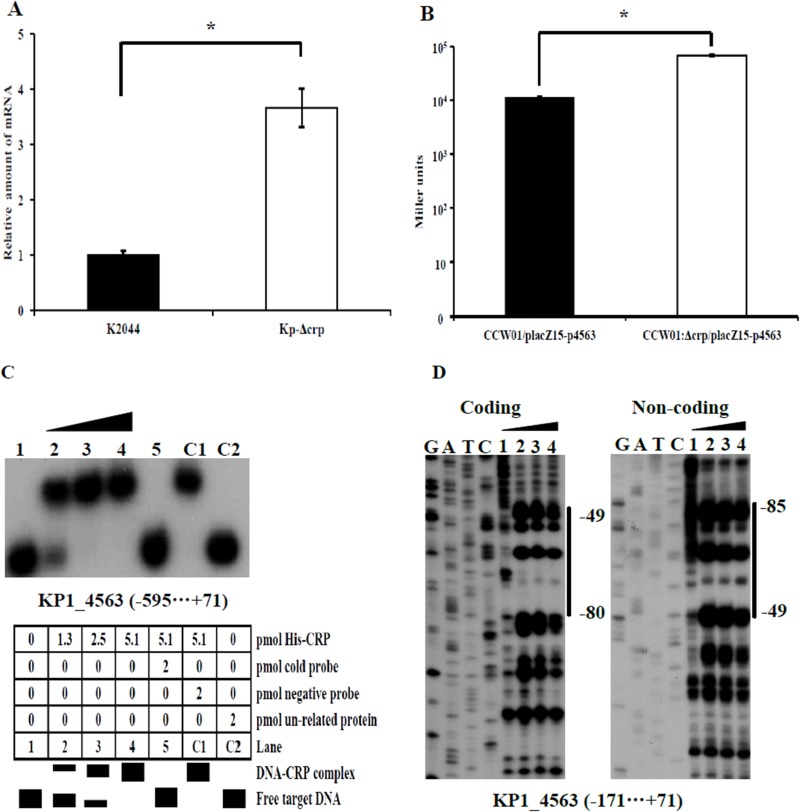
To investigate the regulation of KP1_4563 by CRP, qRT-PCR, LacZ fusion, electrophoretic mobility shift, and DNase I footprinting assays were performed. We monitored the expression of KP1_4563 in K2044 and the Kp-Δcrp mutant via qRT-PCR (Fig 2A). Compared with K2044, the expression levels of KP1_4563 increased 3.7-fold (P<0.05) in the Kp-Δcrp mutant. The results of the LacZ fusion assays showed that the activity of β-galactosidase in CCW01Δcrp/placZ15-p4563 (6.8×104 Miller units) increased approximately 6-fold relative to that of CCW01/placZ15-p4563 (1.14×104 Miller units, P<0.05, Fig 2B). The results of qRT-PCR and the LacZ fusion assays revealed that CRP negatively regulates KP1_4563. EMSA was performed to determine the specificity of CRP binding to the upstream region of the translation start site of KP1_4563. As showed in Fig 2C, purified His–CRP protein bound to the upstream region of KP1_4563 DNA fragments in a dose-dependent manner. Positive EMSA results were observed for KP1_4563, which demonstrated that the CRP–cAMP complex directly bound to the KP1_4563 promoter. The DNase I footprinting showed that His–CRP protected a single DNA region upstream of the KP1_4563 gene in a dose-dependent manner (Fig 2D). The binding site ranged from 49 bp to 85 bp upstream of the KP1_4563 start codon ATG, where the A in the ATG start codon refers to position 1. Therefore, CRP likely negatively regulates the transcription of KP1_4563 by directly bindng to the promoter region.
Fig 2. Transcriptional regulation of KP1_4563 by CRP.
(A) Quantitative RT-PCR (qRT-PCR). Transcriptional expression of KP1_4563 in WT and Kp-Δcrp. The results are expressed as the percentage of WT expression. Data are presented as the mean of at least three technical replicates (mean ± standard deviation). Statistical significance was analyzed by independent samples t- test. Significant difference is indicated by * for P<0.05. (B) LacZ fusion assay. The putative promoter region of KP1_4563 was cloned into the lacZ transcriptional fusion placZ15 plasmid and then introduced into CCW01 or CCW01Δcrp to determine promoter activity. The results are expressed as β-galactosidase activity (Miller units) in the cellular extracts. Statistical significance was analyzed by independent samples t-test. Significant difference is indicated by * for P<0.05. (C) EMSA. The radioactively labeled putative promoter region of KP1_4563 was incubated with increasing amounts of purified His–CRP protein with cAMP and was then subjected to 4% (w/v) native polyacrylamide gels electrophoresis. The interaction between His–CRP and the promoter region of KP1_4563 formed a DNA–CRP complex, which produced a retarded DNA band with decreased mobility. (D) DNase I footprinting. A labeled coding or non-coding DNA fragment was incubated with increasing amounts of His–CRP (lanes 1, 2, 3, and 4 represent 0, 8.5, 16.9, and 25.4 pmol of purified His–CRP protein, respectively) with cAMP and was then subjected to 8 M urea-6% (w/v) polyacrylamide gels electrophoresis. The footprint region is indicated by vertical bars with positions, and the negative numbers indicate the nucleotide positions upstream of the KP1_4563 gene start codon ATG where the A in the ATG start codon refers to position 1.
Discussion
The region between the fim and mrk fimbrial gene clusters is highly conserved in different K. pneumoniae isolates. Sequence analysis of the fimbrial region from K. pneumoniae C132-98, C747, and C4712 has revealed the presence of homologues of the five ORFs described in K. pneumoniae C3091 [26]. KP1_4563 is a hypothetical protein-encoding gene in the fimbrial region. The KP1_4563 protein has putative conserved domains of unknown function (DUF1471). This study describes, for the first time, the role of KP1_4563 in type 3 fimbrial function and elucidates the transcriptional regulation of KP1_4563 by CRP. The function of KP1_4563 reported here will facilitate understanding of the functions of other DUF1471 proteins’ function. Understanding the effect of the KP1_4563 on type 3 fimbrial function will aid in the development of strategies for preventing K. pneumoniae infection.
We investigated fimbrial types in K2044, Kp-Δ4563, and Kpc-Δ4563. The majority of the K. pneumoniae strains express the type 1 and type 3 fimbriae [58–60]. Type 1 fimbrial expression is specifically detected by the mannose-sensitive agglutination of guinea pig RBCs, whereas type 3 fimbrial expression is detected by the agglutination of tannic acid-treated human erythrocytes in a mannose-resistant manner [17, 49]. This study is the first to describe the effects of the KP1_4563 gene on fimbriae. Unexpectedly, K2044, Kp-Δ4563, and Kpc-Δ4563 all failed to mediate the visible mannose-sensitive agglutination of guinea pig RBCs at the highest tested bacterial density (>1×1010CFU/ml, data not showed). These results indicated that KP1_4563 does not influence type 1 fimbrial function. The minimum bacterial density of Kp-Δ4563 required to agglutinate tannic acid-treated human erythrocytes was approximately 10-fold lower than that of K2044. The results of the mannan-binding assay further confirmed the negative regulatory role of KP1_4563 in type 3 fimbriae.
The ability of bacteria to adhere to host structures plays a major role in the development of infections. Given that type 3 fimbriae can adhere to epithelial cells in the respiratory tract, A549 human lung epithelial cell lines were selected as target cells to identify the effect of KP1_4563 on adhesion in K. pneumoniae. As expected, the ability of Kp-Δ4563 to adhere to A549 cells was dramatically enhanced. The results showed that the absence of KP1_4563 increased bacterial adhesion to A549 cells.
We studied the transcriptional regulation mechanism of the KP1_4563 gene by CRP. The results indicated that CRP negatively regulates KP1_4563 by directly binding to the promoter region of KP1_4563 and that KP1_4563 negatively regulates the function of type 3 fimbriae in an unknown manner. Overall, CRP may indirectly and positively regulate the function of type 3 fimbriae. These results corroborate the importance of CRP in regulating virulence-related genes in K. pneumoniae [41].
CRP has been reported to be required for fimbrial production, and the deletion of crp lead to a huge attenuation of the ability to agglutinate yeast cells [61]. Our results clarify that CRP may indirectly and positively regulate the function of type 3 fimbriae by directly regulating the KP1_4563 gene at the molecular level, which might aid in understanding the relationship of CRP and fimbriae. Lin reported that CRP down-regulates type 3 fimbriae expression indirectly through the c-di-GMP signaling pathway [62]. c-di-GMP is a second messenger molecule in bacteria, and the synthesis and decomposition of c-di-GMP is accomplished by diguanylate cyclases with conserved GGDEF domains and phosphodiesterases (PDEs) with EAL domains [63]. The mrkJ gene is immediately adjacent to the mrkABCDF operon that encodes the structural and assembly components of type 3 fimbriae. MrkJ has homology to EAL domain-containing phosphodiesterases (PDEs). Overexpression of mrkJ results in a significant decrease in the intracellular concentration of c-di-GMP and down-regulates type 3 fimbriae expression [64]. Bioinformatic analysis showed that the KP1_4563 protein does not contain conserved GGDEF or EAL domains, which indicates that KP1_4563 negatively regulates the function of type 3 fimbriae without affecting the intracellular concentration of c-di-GMP, but did show that KP1_4563 protein has the putative conserved DUF1471 domains. In E. coli K-12, the YcfR protein has DUF1471 domains, and deleting ycfR caused changes in bacterial cell surface structures and properties by affecting cell surface protein gene expression, which further affects cell aggregation. The remarkable changes in the ycfR mutant may be due to regulation by CRP, as EMSA results showed that CRP binds to the upstream region of the ycfR gene [65]. We suspect that KP1_4563 regulates the function of type 3 fimbriae, which may be associated with changes in bacterial cell surface structures and properties. Further study is needed to elucidate the precise role of KP1_4563 in type 3 fimbrial function.
In conclusion, we found that KP1_4563 negatively regulates type 3 fimbrial function, but does not influence type 1 fimbrial function. Adherence to A549 cells is considerably enhanced in the absence of KP1_4563. Moreover, CRP negatively regulates the transcription of KP1_4563 by directly binding to the upstream KP1_4563 promoter region.
Acknowledgments
We would like to thank Professor Jin-Town Wang from National Taiwan University (Taipei, Taiwan) for providing the K. pneumoniae NTUH-2044 strain and pKO3-Km plasmid, and Professor Hwei-Ling Peng from National Chiao Tung University (Hsin Chu, Taiwan) for providing the K. pneumoniae NTUH-K2044ΔlacZ strain CCW01.
Data Availability
All relevant data are within the paper.
Funding Statement
The research leading to this manuscript has received funding from the National Natural Science Foundation of China (31200064, 31071093 and 31170129).
References
- 1.Barbadoro P, Labricciosa FM, Recanatini C, Gori G, Tirabassi F, Martini E, et al. Catheter-associated urinary tract infection: Role of the setting of catheter insertion. AM J INFECT CONTROL. 2015; 43(7): 707–10. doi: 10.1016/j.ajic.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 2.Ko WC, Paterson DL, Sagnimeni AJ, Hansen DS, Von Gottberg A, Mohapatra S, et al. Community-acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. EMERG INFECT DIS. 2002; 8(2): 160–6. doi: 10.3201/eid0802.010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang CC, Lu CH, Huang CR, Chuang YC, Tsai NW, Chen SF, et al. Culture-proven bacterial meningitis in elderly patients in southern Taiwan: clinical characteristics and prognostic factors. Acta Neurol Taiwan. 2006; 15(2): 84–91. [PubMed] [Google Scholar]
- 4.Luo M, Yang XX, Tan B, Zhou XP, Xia HM, Xue J, et al. Distribution of common pathogens in patients with pyogenic liver abscess in China: a meta-analysis. Eur J Clin Microbiol Infect Dis. 2016; 35(10): 1557–1565. doi: 10.1007/s10096-016-2712-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yadav KK, Awasthi S. The current status of community-acquired pneumonia management and prevention in children under 5 years of age in India: a review. Ther Adv Infect Dis. 2016; 3(3–4): 83–97. doi: 10.1177/2049936116652326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kabbani MS, Ismail SR, Fatima A, Shafi R, Idris JA, Mehmood A, et al. Urinary tract infection in children after cardiac surgery: Incidence, causes, risk factors and outcomes in a single-center study. J Infect Public Health. 2016; 9(5): 600–10. doi: 10.1016/j.jiph.2015.12.017 [DOI] [PubMed] [Google Scholar]
- 7.Favre-Bonte S, Darfeuille-Michaud A, Forestier C. Aggregative adherence of Klebsiella pneumoniae to human intestine-407 cells. INFECT IMMUN. 1995; 63(4): 1318–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarkkanen AM, Virkola R, Clegg S, Korhonen TK. Binding of the type 3 fimbriae of Klebsiella pneumoniae to human endothelial and urinary bladder cells. INFECT IMMUN. 1997; 65(4): 1546–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donnenberg MS, Kaper JB. Enteropathogenic Escherichia coli. INFECT IMMUN. 1992; 60(10): 3953–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hornick DB, Allen BL, Horn MA, Clegg S. Fimbrial types among respiratory isolates belonging to the family Enterobacteriaceae. J CLIN MICROBIOL. 1991; 29(9): 1795–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klemm P, Schembri MA. Bacterial adhesins: function and structure. INT J MED MICROBIOL. 2000; 290(1): 27–35. doi: 10.1016/S1438-4221(00)80102-2 [DOI] [PubMed] [Google Scholar]
- 12.Jones CH, Pinkner JS, Roth R, Heuser J, Nicholes AV, Abraham SN, et al. FimH adhesin of type 1 pili is assembled into a fibrillar tip structure in the Enterobacteriaceae. Proc Natl Acad Sci U S A. 1995; 92(6): 2081–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stahlhut SG, Struve C, Krogfelt KA, Reisner A. Biofilm formation of Klebsiella pneumoniae on urethral catheters requires either type 1 or type 3 fimbriae. FEMS Immunol Med Microbiol. 2012; 65(2): 350–9. doi: 10.1111/j.1574-695X.2012.00965.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Struve C, Bojer M, Krogfelt KA. Characterization of Klebsiella pneumoniae type 1 fimbriae by detection of phase variation during colonization and infection and impact on virulence. INFECT IMMUN. 2008; 76(9): 4055–65. doi: 10.1128/IAI.00494-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kil KS, Darouiche RO, Hull RA, Mansouri MD, Musher DM. Identification of a Klebsiella pneumoniae strain associated with nosocomial urinary tract infection. J CLIN MICROBIOL. 1997; 35(9): 2370–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy CN, Mortensen MS, Krogfelt KA, Clegg S. Role of Klebsiella pneumoniae type 1 and type 3 fimbriae in colonizing silicone tubes implanted into the bladders of mice as a model of catheter-associated urinary tract infections. INFECT IMMUN. 2013; 81(8): 3009–17. doi: 10.1128/IAI.00348-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DUGUID JP. Fimbriae and adhesive properties in Klebsiella strains. J Gen Microbiol. 1959; 21: 271–86. doi: 10.1099/00221287-21-1-271 [DOI] [PubMed] [Google Scholar]
- 18.Adegbola RA, Old DC. Fimbrial haemagglutinins in Enterobacter species. J Gen Microbiol. 1983; 129(7): 2175–80. doi: 10.1099/00221287-129-7-2175 [DOI] [PubMed] [Google Scholar]
- 19.Old DC, Adegbola RA. Haemagglutinins and fimbriae of Morganella, Proteus and Providencia. J MED MICROBIOL. 1982; 15(4): 551–64. doi: 10.1099/00222615-15-4-551 [DOI] [PubMed] [Google Scholar]
- 20.Mobley HL, Chippendale GR. Hemagglutinin, urease, and hemolysin production by Proteus mirabilis from clinical sources. J INFECT DIS. 1990; 161(3): 525–30. [DOI] [PubMed] [Google Scholar]
- 21.Adegbola RA, Old DC. New fimbrial hemagglutinin in Serratia species. INFECT IMMUN. 1982; 38(1): 306–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagnow J, Clegg S. Klebsiella pneumoniae MrkD-mediated biofilm formation on extracellular matrix- and collagen-coated surfaces. MICROBIOLOGY. 2003; 149(Pt 9): 2397–405. doi: 10.1099/mic.0.26434-0 [DOI] [PubMed] [Google Scholar]
- 23.Allen BL, Gerlach GF, Clegg S. Nucleotide sequence and functions of mrk determinants necessary for expression of type 3 fimbriae in Klebsiella pneumoniae. J BACTERIOL. 1991; 173(2): 916–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langstraat J, Bohse M, Clegg S. Type 3 fimbrial shaft (MrkA) of Klebsiella pneumoniae, but not the fimbrial adhesin (MrkD), facilitates biofilm formation. INFECT IMMUN. 2001; 69(9): 5805–12. doi: 10.1128/IAI.69.9.5805-5812.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J EXP MED. 2004; 199(5): 697–705. doi: 10.1084/jem.20030857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Struve C, Bojer M, Krogfelt KA. Identification of a conserved chromosomal region encoding Klebsiella pneumoniae type 1 and type 3 fimbriae and assessment of the role of fimbriae in pathogenicity. INFECT IMMUN. 2009; 77(11): 5016–24. doi: 10.1128/IAI.00585-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang ZC, Liu CJ, Huang YJ, Wang YS, Peng HL. PecS regulates the urate-responsive expression of type 1 fimbriae in Klebsiella pneumoniae CG43. MICROBIOLOGY. 2015; 161(12): 2395–409. doi: 10.1099/mic.0.000185 [DOI] [PubMed] [Google Scholar]
- 28.Rudd KE, Humphery-Smith I, Wasinger VC, Bairoch A. Low molecular weight proteins: a challenge for post-genomic research. ELECTROPHORESIS. 1998; 19(4): 536–44. doi: 10.1002/elps.1150190413 [DOI] [PubMed] [Google Scholar]
- 29.Eletsky A, Michalska K, Houliston S, Zhang Q, Daily MD, Xu X, et al. Structural and functional characterization of DUF1471 domains of Salmonella proteins SrfN, YdgH/SssB, and YahO. PLOS ONE. 2014; 9(7): e101787 doi: 10.1371/journal.pone.0101787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salazar JK, Deng K, Tortorello ML, Brandl MT, Wang H, Zhang W. Genes ycfR, sirA and yigG contribute to the surface attachment of Salmonella enterica Typhimurium and Saintpaul to fresh produce. PLOS ONE. 2013; 8(2): e57272 doi: 10.1371/journal.pone.0057272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng K, Wang S, Rui X, Zhang W, Tortorello ML. Functional analysis of ycfR and ycfQ in Escherichia coli O157:H7 linked to outbreaks of illness associated with fresh produce. Appl Environ Microbiol. 2011; 77(12): 3952–9. doi: 10.1128/AEM.02420-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fic E, Bonarek P, Gorecki A, Kedracka-Krok S, Mikolajczak J, Polit A, et al. cAMP receptor protein from escherichia coli as a model of signal transduction in proteins—a review. J Mol Microbiol Biotechnol. 2009; 17(1): 1–11. doi: 10.1159/000178014 [DOI] [PubMed] [Google Scholar]
- 33.Mukhopadhyay J, Sur R, Parrack P. Functional roles of the two cyclic AMP-dependent forms of cyclic AMP receptor protein from Escherichia coli. FEBS LETT. 1999; 453(1–2): 215–8. [DOI] [PubMed] [Google Scholar]
- 34.Busby S, Ebright RH. Transcription activation by catabolite activator protein (CAP). J MOL BIOL. 1999; 293(2): 199–213. doi: 10.1006/jmbi.1999.3161 [DOI] [PubMed] [Google Scholar]
- 35.Zheng D, Constantinidou C, Hobman JL, Minchin SD. Identification of the CRP regulon using in vitro and in vivo transcriptional profiling. NUCLEIC ACIDS RES. 2004; 32(19): 5874–93. doi: 10.1093/nar/gkh908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Antonio A, Collado-Vides J. Identifying global regulators in transcriptional regulatory networks in bacteria. CURR OPIN MICROBIOL. 2003; 6(5): 482–9. [DOI] [PubMed] [Google Scholar]
- 37.Stella NA, Kalivoda EJ, O'Dee DM, Nau GJ, Shanks RM. Catabolite repression control of flagellum production by Serratia marcescens. RES MICROBIOL. 2008; 159(7–8): 562–8. doi: 10.1016/j.resmic.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skorupski K, Taylor RK. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc Natl Acad Sci U S A. 1997; 94(1): 265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalivoda EJ, Stella NA, O'Dee DM, Nau GJ, Shanks RM. The cyclic AMP-dependent catabolite repression system of Serratia marcescens mediates biofilm formation through regulation of type 1 fimbriae. Appl Environ Microbiol. 2008; 74(11): 3461–70. doi: 10.1128/AEM.02733-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller CM, Aberg A, Straseviciene J, Emody L, Uhlin BE, Balsalobre C. Type 1 fimbriae, a colonization factor of uropathogenic Escherichia coli, are controlled by the metabolic sensor CRP-cAMP. PLOS PATHOG. 2009; 5(2): e1000303 doi: 10.1371/journal.ppat.1000303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xue J, Tan B, Yang S, Luo M, Xia H, Zhang X, et al. Influence of cAMP receptor protein (CRP) on bacterial virulence and transcriptional regulation of allS by CRP in Klebsiella pneumoniae. GENE. 2016; 593(1): 28–33. doi: 10.1016/j.gene.2016.08.006 [DOI] [PubMed] [Google Scholar]
- 42.Meyer M, Dimroth P, Bott M. Catabolite repression of the citrate fermentation genes in Klebsiella pneumoniae: evidence for involvement of the cyclic AMP receptor protein. J BACTERIOL. 2001; 183(18): 5248–56. doi: 10.1128/JB.183.18.5248-5256.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ebright RH, Ebright YW, Gunasekera A. Consensus DNA site for the Escherichia coli catabolite gene activator protein (CAP): CAP exhibits a 450-fold higher affinity for the consensus DNA site than for the E. coli lac DNA site. NUCLEIC ACIDS RES. 1989; 17(24): 10295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gunasekera A, Ebright YW, Ebright RH. DNA sequence determinants for binding of the Escherichia coli catabolite gene activator protein. J BIOL CHEM. 1992; 267(21): 14713–20. [PubMed] [Google Scholar]
- 45.He LY, Jia Y, Lei LC, Yang Y, Han WY. Extraction and identification of type 3 fimbriae of Klebsiella pneumoniae. J MICROBIOL. 2007; 27(4): 15–8. [Google Scholar]
- 46.Hsieh PF, Lin TL, Lee CZ, Tsai SF, Wang JT. Serum-induced iron-acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J INFECT DIS. 2008; 197(12): 1717–27. doi: 10.1086/588383 [DOI] [PubMed] [Google Scholar]
- 47.Hsu CR, Lin TL, Chen YC, Chou HC, Wang JT. The role of Klebsiella pneumoniae rmpA in capsular polysaccharide synthesis and virulence revisited. MICROBIOLOGY. 2011; 157(Pt 12): 3446–57. doi: 10.1099/mic.0.050336-0 [DOI] [PubMed] [Google Scholar]
- 48.Wilksch JJ, Yang J, Clements A, Gabbe JL, Short KR, Cao H, et al. MrkH, a novel c-di-GMP-dependent transcriptional activator, controls Klebsiella pneumoniae biofilm formation by regulating type 3 fimbriae expression. PLOS PATHOG. 2011; 7(8): e1002204 doi: 10.1371/journal.ppat.1002204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stahlhut SG, Struve C, Krogfelt KA. Klebsiella pneumoniae type 3 fimbriae agglutinate yeast in a mannose-resistant manner. J MED MICROBIOL. 2012; 61(Pt 3): 317–22. doi: 10.1099/jmm.0.036350-0 [DOI] [PubMed] [Google Scholar]
- 50.Stahlhut SG, Chattopadhyay S, Kisiela DI, Hvidtfeldt K, Clegg S, Struve C, et al. Structural and population characterization of MrkD, the adhesive subunit of type 3 fimbriae. J BACTERIOL. 2013; 195(24): 5602–13. doi: 10.1128/JB.00753-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Khater F, Balestrino D, Charbonnel N, Dufayard JF, Brisse S, Forestier C. In silico analysis of usher encoding genes in Klebsiella pneumoniae and characterization of their role in adhesion and colonization. PLOS ONE. 2015; 10(3): e116215 doi: 10.1371/journal.pone.0116215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. NAT PROTOC. 2008; 3(6): 1101–8. [DOI] [PubMed] [Google Scholar]
- 53.Lin CT, Huang TY, Liang WC, Peng HL. Homologous response regulators KvgA, KvhA and KvhR regulate the synthesis of capsular polysaccharide in Klebsiella pneumoniae CG43 in a coordinated manner. J BIOCHEM. 2006; 140(3): 429–38. doi: 10.1093/jb/mvj168 [DOI] [PubMed] [Google Scholar]
- 54.Wu CC, Huang YJ, Fung CP, Peng HL. Regulation of the Klebsiella pneumoniae Kpc fimbriae by the site-specific recombinase KpcI. MICROBIOLOGY. 2010; 156(Pt 7): 1983–92. doi: 10.1099/mic.0.038158-0 [DOI] [PubMed] [Google Scholar]
- 55.Zhan L, Han Y, Yang L, Geng J, Li Y, Gao H, et al. The cyclic AMP receptor protein, CRP, is required for both virulence and expression of the minimal CRP regulon in Yersinia pestis biovar microtus. INFECT IMMUN. 2008; 76(11): 5028–37. doi: 10.1128/IAI.00370-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Zhou D, Mao P, Zhang Y, Hou J, Hu Y, et al. Cell density- and quorum sensing-dependent expression of type VI secretion system 2 in Vibrio parahaemolyticus. PLOS ONE. 2013; 8(8): e73363 doi: 10.1371/journal.pone.0073363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li Y, Gao H, Qin L, Li B, Han Y, Guo Z, et al. Identification and characterization of PhoP regulon members in Yersinia pestis biovar Microtus. BMC GENOMICS. 2008; 9: 143 doi: 10.1186/1471-2164-9-143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Livrelli V, De Champs C, Di Martino P, Darfeuille-Michaud A, Forestier C, Joly B. Adhesive properties and antibiotic resistance of Klebsiella, Enterobacter, and Serratia clinical isolates involved in nosocomial infections. J CLIN MICROBIOL. 1996; 34(8): 1963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tarkkanen AM, Allen BL, Williams PH, Kauppi M, Haahtela K, Siitonen A, et al. Fimbriation, capsulation, and iron-scavenging systems of Klebsiella strains associated with human urinary tract infection. INFECT IMMUN. 1992; 60(3): 1187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Podschun R, Sievers D, Fischer A, Ullmann U. Serotypes, hemagglutinins, siderophore synthesis, and serum resistance of Klebsiella isolates causing human urinary tract infections. J INFECT DIS. 1993; 168(6): 1415–21. [DOI] [PubMed] [Google Scholar]
- 61.Ou Q, Fan J, Duan D, Xu L, Wang J, Zhou D, et al. Involvement of cAMP receptor protein in biofilm formation, fimbria production, capsular polysaccharide biosynthesis and lethality in mouse of Klebsiella pneumoniae serotype K1 causing pyogenic liver abscess. J MED MICROBIOL. 2017; 66(1): 1–7. doi: 10.1099/jmm.0.000391 [DOI] [PubMed] [Google Scholar]
- 62.Lin CT, Lin TH, Wu CC, Wan L, Huang CF, Peng HL. CRP-Cyclic AMP Regulates the Expression of Type 3 Fimbriae via Cyclic di-GMP in Klebsiella pneumoniae. PLOS ONE. 2016; 11(9): e162884 doi: 10.1371/journal.pone.0162884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Romling U, Simm R. Prevailing concepts of c-di-GMP signaling. Contrib Microbiol. 2009; 16: 161–81. doi: 10.1159/000219379 [DOI] [PubMed] [Google Scholar]
- 64.Johnson JG, Clegg S. Role of MrkJ, a phosphodiesterase, in type 3 fimbrial expression and biofilm formation in Klebsiella pneumoniae. J BACTERIOL. 2010; 192(15): 3944–50. doi: 10.1128/JB.00304-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang XS, Garcia-Contreras R, Wood TK. YcfR (BhsA) influences Escherichia coli biofilm formation through stress response and surface hydrophobicity. J BACTERIOL. 2007; 189(8): 3051–62. doi: 10.1128/JB.01832-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grant SG, Jessee J, Bloom FR, Hanahan D. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc Natl Acad Sci U S A. 1990; 87(12): 4645–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J MOL BIOL. 1986; 189(1): 113–30. [DOI] [PubMed] [Google Scholar]
- 68.Pan YJ, Fang HC, Yang HC, Lin TL, Hsieh PF, Tsai FC, et al. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J CLIN MICROBIOL. 2008; 46(7): 2231–40. doi: 10.1128/JCM.01716-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.