Abstract
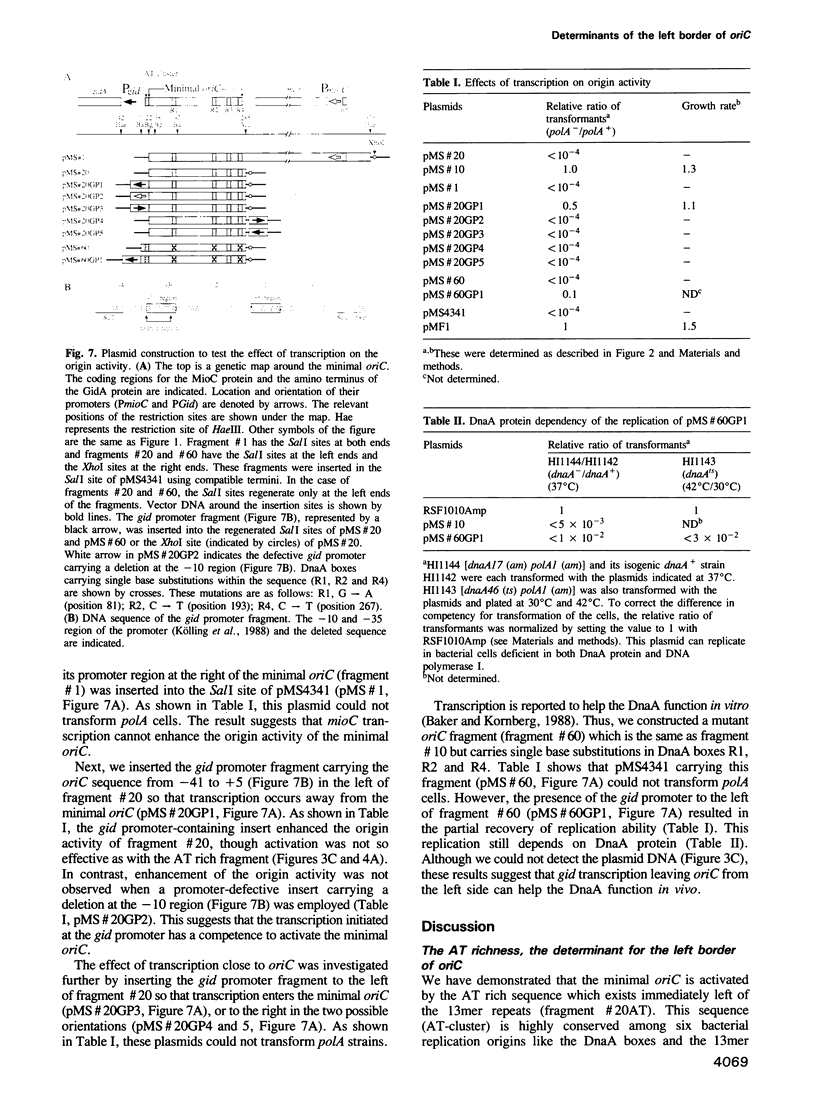
We have identified novel, cis-acting elements which enhance in vivo the replication activity of plasmids carrying the minimal oriC of Escherichia coli. These are (i) the AT rich sequence ('AT-cluster') which exists immediately left of the 13mer repeats and (ii) the gid transcriptional unit. The 'AT-cluster' was functionally replaced by an unrelated AT rich sequence. This was also the case for the left and middle 13mers; they were substituted by the AT rich fragment from mini-F plasmid. The left 13mer was replaced by the AT rich sequence which did not show the 'reduced helical stability' known as the important character of the 13mer region. In contrast to these results, the right 13mer sequence was strictly required. As to the effect of the transcription from the gid promoter, the minimal oriC was activated only when the transcription was directed away from the left side of it. mioC transcription proceeding toward the oriC had no effect on the activation. Mutations in the DnaA boxes were partially suppressed by gid transcription leaving oriC from the left side. From these results, we propose that the AT richness is a determinant to identify the left border of oriC. It is presumed that gid transcription introduces negative superhelicity at the AT rich region and facilitates DnaA dependent duplex opening.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M. M., Izakowska M., Bagdasarian M. Suppression of the DnaA phenotype by mutations in the rpoB cistron of ribonucleic acid polymerase in Salmonella typhimurium and Escherichia coli. J Bacteriol. 1977 May;130(2):577–582. doi: 10.1128/jb.130.2.577-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. A., Kornberg A. Transcriptional activation of initiation of replication from the E. coli chromosomal origin: an RNA-DNA hybrid near oriC. Cell. 1988 Oct 7;55(1):113–123. doi: 10.1016/0092-8674(88)90014-1. [DOI] [PubMed] [Google Scholar]
- Bramhill D., Kornberg A. Duplex opening by dnaA protein at novel sequences in initiation of replication at the origin of the E. coli chromosome. Cell. 1988 Mar 11;52(5):743–755. doi: 10.1016/0092-8674(88)90412-6. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., Jonczyk P. Essential role of the gyrB gene product in the transcriptional event coupled to dnaA-dependent initiation of Escherichia coli chromosome replication. Mol Gen Genet. 1981;183(1):134–138. doi: 10.1007/BF00270151. [DOI] [PubMed] [Google Scholar]
- Filutowicz M., Jonczyk P. The gyrB gene product functions in both initiation and chain polymerization of Escherichia coli chromosome replication: suppression of the initiation deficiency in gyrB-ts mutants by a class of rpoB mutations. Mol Gen Genet. 1983;191(2):282–287. doi: 10.1007/BF00334827. [DOI] [PubMed] [Google Scholar]
- Filutowicz M. Requirement of DNA gyrase for the initiation of chromosome replication in Escherichia coli K-12. Mol Gen Genet. 1980 Jan;177(2):301–309. doi: 10.1007/BF00267443. [DOI] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Hirano M., Shigesada K., Imai M. Construction and characterization of plasmid and lambda phage vector systems for study of transcriptional control in Escherichia coli. Gene. 1987;57(1):89–99. doi: 10.1016/0378-1119(87)90180-6. [DOI] [PubMed] [Google Scholar]
- Kowalski D., Eddy M. J. The DNA unwinding element: a novel, cis-acting component that facilitates opening of the Escherichia coli replication origin. EMBO J. 1989 Dec 20;8(13):4335–4344. doi: 10.1002/j.1460-2075.1989.tb08620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kölling R., Gielow A., Seufert W., Kücherer C., Messer W. AsnC, a multifunctional regulator of genes located around the replication origin of Escherichia coli, oriC. Mol Gen Genet. 1988 Apr;212(1):99–104. doi: 10.1007/BF00322450. [DOI] [PubMed] [Google Scholar]
- Leonard A. C., Whitford W. G., Helmstetter C. E. Involvement of DNA superhelicity in minichromosome maintenance in Escherichia coli. J Bacteriol. 1985 Feb;161(2):687–695. doi: 10.1128/jb.161.2.687-695.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7024–7027. doi: 10.1073/pnas.84.20.7024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lother H., Kölling R., Kücherer C., Schauzu M. dnaA protein-regulated transcription: effects on the in vitro replication of Escherichia coli minichromosomes. EMBO J. 1985 Feb;4(2):555–560. doi: 10.1002/j.1460-2075.1985.tb03664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louarn J., Bouché J. P., Patte J., Louarn J. M. Genetic inactivation of topoisomerase I suppresses a defect in initiation of chromosome replication in Escherichia coli. Mol Gen Genet. 1984;195(1-2):170–174. doi: 10.1007/BF00332741. [DOI] [PubMed] [Google Scholar]
- Løbner-Olesen A., Atlung T., Rasmussen K. V. Stability and replication control of Escherichia coli minichromosomes. J Bacteriol. 1987 Jun;169(6):2835–2842. doi: 10.1128/jb.169.6.2835-2842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui M., Oka A., Takanami M., Yasuda S., Hirota Y. Sites of dnaA protein-binding in the replication origin of the Escherichia coli K-12 chromosome. J Mol Biol. 1985 Aug 5;184(3):529–533. doi: 10.1016/0022-2836(85)90299-2. [DOI] [PubMed] [Google Scholar]
- Meijer M., Beck E., Hansen F. G., Bergmans H. E., Messer W., von Meyenburg K., Schaller H. Nucleotide sequence of the origin of replication of the Escherichia coli K-12 chromosome. Proc Natl Acad Sci U S A. 1979 Feb;76(2):580–584. doi: 10.1073/pnas.76.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Ohmori H., Yura T., Nagata T. Requirement of the Escherichia coli dnaA gene function for ori-2-dependent mini-F plasmid replication. J Bacteriol. 1987 Apr;169(4):1724–1730. doi: 10.1128/jb.169.4.1724-1730.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murotsu T., Tsutsui H., Matsubara K. Identification of the minimal essential region for the replication origin of miniF plasmid. Mol Gen Genet. 1984;196(2):373–378. doi: 10.1007/BF00328075. [DOI] [PubMed] [Google Scholar]
- Nagata T., Murakami Y., Imai M. Requirement of the Escherichia coli dnaA gene function for integrative suppression of dnaA mutations by plasmid R 100-1. Mol Gen Genet. 1988 Jul;213(1):163–165. doi: 10.1007/BF00333414. [DOI] [PubMed] [Google Scholar]
- Nozaki N., Okazaki T., Ogawa T. In vitro transcription of the origin region of replication of the Escherichia coli chromosome. J Biol Chem. 1988 Oct 5;263(28):14176–14183. [PubMed] [Google Scholar]
- Oka A., Sasaki H., Sugimoto K., Takanami M. Sequence organization of replication origin of the Escherichia coli K-12 chromosome. J Mol Biol. 1984 Jul 15;176(4):443–458. doi: 10.1016/0022-2836(84)90171-2. [DOI] [PubMed] [Google Scholar]
- Oka A., Sugimoto K., Takanami M., Hirota Y. Replication origin of the Escherichia coli K-12 chromosome: the size and structure of the minimum DNA segment carrying the information for autonomous replication. Mol Gen Genet. 1980 Apr;178(1):9–20. doi: 10.1007/BF00267207. [DOI] [PubMed] [Google Scholar]
- Samitt C. E., Hansen F. G., Miller J. F., Schaechter M. In vivo studies of DnaA binding to the origin of replication of Escherichia coli. EMBO J. 1989 Mar;8(3):989–993. doi: 10.1002/j.1460-2075.1989.tb03462.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauzu M. A., Kücherer C., Kölling R., Messer W., Lother H. Transcripts within the replication origin, oriC, of Escherichia coli. Nucleic Acids Res. 1987 Mar 25;15(6):2479–2497. doi: 10.1093/nar/15.6.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnos M., Zahn K., Inman R. B., Blattner F. R. Initiation protein induced helix destabilization at the lambda origin: a prepriming step in DNA replication. Cell. 1988 Feb 12;52(3):385–395. doi: 10.1016/s0092-8674(88)80031-x. [DOI] [PubMed] [Google Scholar]
- Scholz P., Haring V., Wittmann-Liebold B., Ashman K., Bagdasarian M., Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene. 1989 Feb 20;75(2):271–288. doi: 10.1016/0378-1119(89)90273-4. [DOI] [PubMed] [Google Scholar]
- Stuitje A. R., de Wind N., van der Spek J. C., Pors T. H., Meijer M. Dissection of promoter sequences involved in transcriptional activation of the Escherichia coli replication origin. Nucleic Acids Res. 1986 Mar 11;14(5):2333–2344. doi: 10.1093/nar/14.5.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Oka A., Sugisaki H., Takanami M., Nishimura A., Yasuda Y., Hirota Y. Nucleotide sequence of Escherichia coli K-12 replication origin. Proc Natl Acad Sci U S A. 1979 Feb;76(2):575–579. doi: 10.1073/pnas.76.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Hiraga S. Negative control of oriC plasmid replication by transcription of the oriC region. Mol Gen Genet. 1985;200(1):21–26. doi: 10.1007/BF00383307. [DOI] [PubMed] [Google Scholar]
- Tsao Y. P., Wu H. Y., Liu L. F. Transcription-driven supercoiling of DNA: direct biochemical evidence from in vitro studies. Cell. 1989 Jan 13;56(1):111–118. doi: 10.1016/0092-8674(89)90989-6. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. Production of single-stranded plasmid DNA. Methods Enzymol. 1987;153:3–11. doi: 10.1016/0076-6879(87)53044-0. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Shyy S. H., Wang J. C., Liu L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell. 1988 May 6;53(3):433–440. doi: 10.1016/0092-8674(88)90163-8. [DOI] [PubMed] [Google Scholar]
- Yamaki H., Ohtsubo E., Nagai K., Maeda Y. The oriC unwinding by dam methylation in Escherichia coli. Nucleic Acids Res. 1988 Jun 10;16(11):5067–5073. doi: 10.1093/nar/16.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung B. Y., Kornberg A. The dnaA initiator protein binds separate domains in the replication origin of Escherichia coli. J Biol Chem. 1989 Apr 15;264(11):6146–6150. [PubMed] [Google Scholar]
- Zyskind J. W., Cleary J. M., Brusilow W. S., Harding N. E., Smith D. W. Chromosomal replication origin from the marine bacterium Vibrio harveyi functions in Escherichia coli: oriC consensus sequence. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1164–1168. doi: 10.1073/pnas.80.5.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]




