Abstract
Glycan-binding proteins, which include galectins, are involved at all stages of immunity and inflammation, from initiation through resolution. Galectin-9 (Gal-9) is highly expressed in the liver and has a wide variety of biological functions in innate and adaptive immunity that are instrumental in the maintenance of hepatic homeostasis. In the setting of viral hepatitis, increased expression of Gal-9 drives the expansion of regulatory T cells and contraction of effector T cells, thereby favoring viral persistence. The dichotomous nature of Gal-9 is evident in hepatocellular carcinoma, where loss of expression in hepatocytes promotes tumor growth and metastasis, whereas overexpression by Kupffer cells and endothelial cells inhibits the antitumor immune response. In nonalcoholic fatty liver disease, Gal-9 is involved indirectly in the expansion of protective natural killer T-cell populations. In ischemic liver injury, hepatocyte-derived Gal-9 is both diagnostic and cytoprotective. In drug-induced acute liver failure, plasma levels correlate with outcome. Here, we offer a synthesis of recent and emerging findings on Gal-9 in the regulation of hepatic inflammation. Ongoing studies are warranted to better elucidate the pathophysiology of hepatic immune-mediated diseases and to develop new therapeutic interventions using glycan-binding proteins.
Glycobiology is the study of the structure and function of glycans (i.e., carbohydrates, saccharides, or simple sugars). Because they are dominantly expressed on all eukaryotic and prokaryotic cell surfaces, glycans play central roles in cell–cell and cell–pathogen interactions.(1) Glycan-binding proteins are involved at all stages of immunity and inflammation, from initiation through resolution. In humans, more than 80 glycan-binding proteins have been described that fall into about a dozen structural families, each of which has a conserved carbohydrate recognition domain (CRD).(1) Galectins are a fascinating family of guanosine diphosphates that are secreted into the extracellular environment directly from the cytoplasm independently of the classical endoplasmic reticulum/Golgi trafficking machinery.(2) Galectins are evolutionarily expressed from nematodes to humans.(3) The prototype galectins have one CRD (e.g., galectin-1 [Gal-1]), whereas tandem repeat-type galectins contain two CRDs with differential preferences for carbohydrate binding separated by a linker (e.g., Gal-9), and the chimera-type (Gal-3) contains a single CRD connected to a nonlectin amino-terminal.(4) Galectins are expressed by many immune cells, including macrophages, dendritic cells, B cells, and T cells, as well as endothelial cells and stromal cells in many tissues, including abundant expression in the liver.(5,6) Gal-9 (LGALS9 in human, lgals9 in mouse) was first described as an eosinophil chemoattractant produced by T lymphocytes(7) and demonstrates important pleiotropic immune-regulatory properties.(2,5) Receptors or surface binding partners that have been reported for Gal-9 include T-cell immunoglobulin and mucin domain-containing molecule 3 (TIM-3),(8) glucose transporter 2, glucagon receptor, protein disulfide isomerase, Epstein-Barr virus latent membrane protein-1, immunoglobulin E, and the adhesion molecule cluster of differentiation 44 (CD44), which competes for hyaluronic acid.(9) The paradoxical activities of Gal-9 triggering T-cell death while activating innate immune cells such as dendritic cells to produce tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6; and greater phosphorylation of p38 and AKT) appear largely to be related to the N-terminal and C-terminal CRDs.(10,11) This review summarizes recent findings and describes emerging roles for Gal-9 in hepatic homeostasis and inflammation.
Specific Liver Diseases
VIRAL HEPATITIS
The most striking feature of hepatitis C virus (HCV) infection is its high propensity to establish chronicity, and the transition from acute to chronic infection is characterized by attenuated effector function and expansion of regulatory T cells.(12) We became intrigued with Gal-9 in HCV infection because of the findings that TIM-3 (the best studied natural ligand of Gal-9), a transmembrane protein with a stalk that anchors to an intracellular tail with an SH2 phosphorylation domain, is significantly up-regulated in T cells (particularly virus-specific) of HCV-infected patients, and that TIM-3 blockade could reverse exhaustion and restore CD4+ and CD8+ T-cell function in chronic infection.(13,14) We found that plasma levels of Gal-9 were markedly up-regulated in patients with chronic HCV and that the liver Kupffer cells (KCs) had the highest staining.(15) Moreover, peripherally derived macrophages differentiated with macrophage colony-stimulating factor and stimulated with interferon-gamma (IFN-γ) strongly induce Gal-9 in HCV-infected patients compared to controls, whereas Gal-9 is not induced by lipopolysaccharide, IL-1β, or HCV core protein (Toll-like receptor 2 [TLR2] stimulator).(15) Exosomes released from HCV-infected hepatocytes induced Gal-9 in cultured monocytes, and circulating nonclassical CD16posCD14posHLA-DRpos monocytes have the highest Gal-9 protein levels in chronically infected patients.(16) Stimulation of whole peripheral blood mononuclear cells with recombinant Gal-9 consistently expanded CD4+CD25+FoxP3+ CD127low regulatory T cells (Tregs) in both HCV patients and normal controls, an effect mostly mediated by transforming growth factor beta(15) involving increased phosphorylation of Smad-2/3 and extracellular signal–regulated kinases-1/2.(17) Furthermore, Gal-9 can be produced by Tregs, in particular the CD39pos subset, and can inhibit proliferation and IL-21 production by HCV-specific CD4+ T cells.(18) Gal-9 treatment activates caspase-8, inducing apoptosis of HCV-specific CD8+ T cells (cytotoxic T lymphocytes),(15) which can be reversed with IL-21 derived from T helper 17 (Th17) cells.(18) In mice, Gal-9 suppresses generation of Th17 cells and IL-17 mRNA expression.(19) A paradigm for the roles of Gal-9 in HCV infection is presented in Fig. 1. Data on the role of Gal-9 in hepatitis B virus (HBV) infection are relatively sparse. As with HCV, KCs in HBV-infected liver biopsies express high levels of Gal-9, and serum levels of this protein are significantly increased compared to uninfected controls.(20,21) Moreover, TIM-3 expression is relatively increased on HBV-specific T cells and correlates with impaired effector function (IFN-γ production).(20)
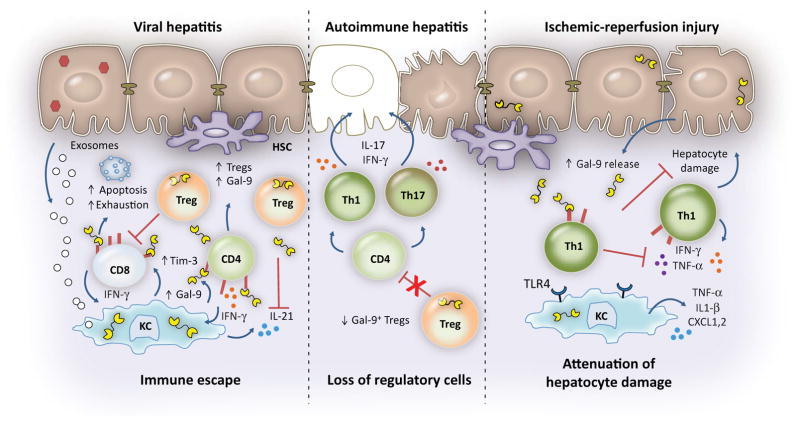
FIG. 1.
Paradigm for Gal-9 in the regulation of immune responses during viral hepatitis infection, AIH, and IRI. Exosomes released from HCV-infected hepatocytes and IFN-γ secreted by the hepatic infiltration of T, NK, and NKT cells induce Gal-9 expression in KCs. Gal-9 binding to Tim-3 induces apoptosis of CD8pos T cells, drives the expansion of Tregs, and suppresses proinflammatory cytokine production, leading to immune escape. Gal-9 expands Tregs (including those that express Gal-9) and leads to contraction of effector CD4+ and CD8+ T cells, including the virus-specific population. Whereas acute, resolving HCV is characterized by strong Th1/Th17 responses and increased levels of IL-21-producing CD4+ T cells and circulating plasma levels of IL-21, persistent infection is associated with increased hepatic and plasma levels of Gal-9; in turn, Gal-9 suppresses IL-21 production, compromising HCV-specific cytotoxic T lymphocyte function by up-regulating exhaustion molecules and becoming more susceptible to apoptosis. Knockdown of Gal-9 in Tregs can rescue IL-21 production by CD4+ T cells. To date, the results for HBV infection (although not as complete) appear similar to those for HCV infection. On the other hand, in AIH, Gal-9pos Tregs exert control over disease activity, and loss of this population leads to uncontrolled effector proinflammatory IFN-γ and IL-17-producing cells that are the main orchestrators of liver damage in AIH and that correlate with disease severity. For the significant proportion of patients with AILD who fail to respond to conventional treatments, Gal-9/Treg-based immunotherapy may represent a novel approach. In the setting of liver IRI, activation of Th1 cells and of macrophages and neutrophil recruitment occur early. Macrophages elaborate inflammatory cytokines and chemokines and up-regulate TLR4 expression. Gal-9, predominantly released from injured hepatocytes, acts as a cytoprotective alarmin. Binding of Gal-9 to Tim-3 on T cells decreases proinflammatory cytokine production, chemokine responses, and hepatocyte damage; blockade of TIM-3 promotes Th1 responses and exacerbates IRI-triggered liver injury but only in the setting of intact TLR4 signaling.(15,16,18,22,27–30) Abbreviations: CXCL, chemokine (C-X-C motif) ligand; HSC, hepatic stellate cell.
AUTOIMMUNE HEPATITIS
Autoimmune hepatitis (AIH) is a progressive inflammatory condition characterized by hypergamma-globulinemia, circulating autoantibodies, and CD4+ effector lymphocyte-mediated liver damage.(22–24) The percentage of TIM-3pos CD4+ T-cell lymphocytes within the effector (CD25neg) population is lower in patients with autoimmune liver disease (AILD; AIH and patients with primary sclerosing cholangitis overlap) and therefore less prone to immune suppression than in healthy controls.(22) Patients with AILD demonstrate lower percentages of circulating Gal-9pos cells within the CD4posCD25pos Treg subset than in healthy controls (Fig. 1). Moreover, the Gal-9 Treg subset in patients with AILD contains more proinflammatory IFN-γpos and IL-17pos cells and fewer transforming growth factor beta–positive and IL-10pos cells.(22) The observation of an inverse correlation between Gal-9pos Tregs and immunoglobulin G levels and titers of autoantibodies, which are the serological markers of the disease, further supports the concept that Gal9pos Tregs exert control over disease activity.(22) A significant proportion of patients with auto-immune liver disease fail to respond to conventional treatments, and these findings may have implications for Treg-based immunotherapy for AILD, including adoptive transfer of autologous, Gal-9 cultured cells or transfection of Tregs with Gal-9 complementary DNA.(22) In this regard, preclinical data in mice with concanavalin A–induced hepatitis,(25) a T cell–dependent model of liver damage, demonstrated disease exacerbation with TIM-3 blockade and improvement with administration of Gal-9.(25) Furthermore, Gal-9 in this model was also shown to inhibit production of the circulating proinflammatory cytokines TNF-α and IL-6. By increasing the Treg to T effector ratio, Gal-9 causes selective apoptosis of concanavalin A–activated CD4+ T cells that further prevents the release of TNF-α, IL-6, and IFN-γ. Thus, inhibition of these important proinflammatory cytokines, which have cytotoxic effects on hepatocytes, represents an alternative mechanism for explaining the suppressive properties of Gal-9 and the potential therapeutic approach for T cell–mediated diseases.(25)
LIVER ISCHEMIC REPERFUSION INJURY
Liver ischemia/reperfusion injury (IRI) remains an important problem in clinical transplantation and after hepatic resection. Both innate and adaptive immunity have been implicated in mediating IRI.(26) For example, KCs trigger recruitment of CD4+ T cells in the postischemic liver by releasing mediators, and CD4+ T cells in turn influence the activation of KCs.(27) TIM-3/Gal-9 has been implicated in this crosstalk, as described in several elegant studies(27–30) (Fig. 1). TIM-3 blockade exacerbates local inflammation and liver damage, increasing expression of TLR4 and chemokine (C-X-C motif) ligands 1 and 2, in a murine model of partial liver warm ischemia (90 minutes) followed by reperfusion, whereas TIM-3 engagement ameliorates neutrophil, T-cell, and macrophage sequestration in IRI livers.(28) The concentrations of Gal-9 in the circulation and hepatic tissues of mice that have undergone warm ischemia/reperfusion are increased,(29) with hepatic Gal-9 mRNA increasing progressively during reperfusion, peaking at 6 hours and decreasing thereafter. Moreover, the IRI-related damage is profoundly exacerbated in Gal-9-deficient mice compared with wild-type mice, whereas pretreatment with recombinant Gal-9 attenuates liver IRI,(29) profoundly decreasing the expression of TLR4, proinflammatory cytokines (TNF-α, IL-6, IL-1β, and IFN-γ), and apoptosis of hepatocytes. Using mice that selectively overexpress TIM-3 on their T cells (TIM-3Tg) in a clinically relevant liver transplant model, investigators found that higher TIM-3 expression by recipient CD4+ T cells protected liver grafts from IRI.(30) Furthermore, hepatocytes are major Gal-9 producers in vivo and within IR-stressed hepatocyte cultures.(30) Taken together, these data point to Gal-9 as an alarmin in liver IRI that provides cytoprotection against tissue damage.(29)
NONALCOHOLIC FATTY LIVER DISEASE
Natural killer T (NKT) cells exhibit features of both innate and adaptive immunity and, although enriched within the normal liver (relative to peripheral compartment), are depleted in nonalcoholic fatty liver disease (NAFLD).(31) Accordingly, up-regulation of hepatic NKT cells has been shown to improve high fat (HF) diet–induced NAFLD and insulin resistance.(32) Gal-9 induces apoptosis of hepatic NKT cells in a dose-dependent fashion and is blocked by α-lactose (competitive inhibitor of Gal-9) or anti-TIM-3 antibody(33); in mice fed an HF diet, TIM-3pos NKT cells are more prone to apoptosis. Surprisingly, Gal-9 increases hepatic NKT cells in mice fed an HF diet, selectively enhancing the CD4+ NKT cell population. In the livers of mice fed an HF diet, Gal-9 treatment significantly increased both mRNA and protein expression of IL-15, known to induce proliferation and maintenance of NKT cells. Furthermore, blocking IL-15 or depleting KCs abolished Gal-9-induced hepatic NKT proliferation in these mice.(33) The fact that Gal-9 treatment improved steatosis in mice fed the HF diet underscores the complex roles of the Tim-3/Gal-9 pathway during various phases of the immune response to maintain homeostasis: activation of NKT cells leads to secretion of IFN-γ, which leads to both up-regulation of TIM-3 and production of Gal-9 by KCs that in turn leads to TIM-3+-dependent and TIM-3+-independent apoptosis of NKT cells, limiting destructive immunity.(33) Concurrently, Gal-9 also interacts with Tim-3 expressed on KCs to produce IL-15 that induces the proliferation of NKT cells (Fig. 2).
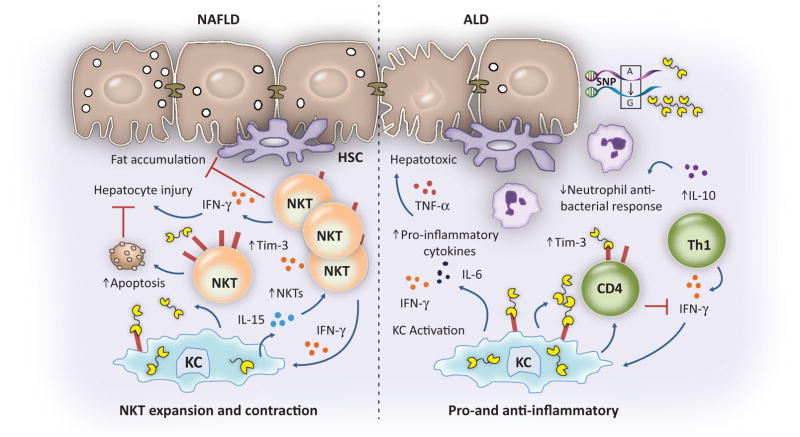
FIG. 2.
Putative roles for Gal-9 in NAFLD and alcohol-associated liver disease. NKT cells are protective in NAFLD. Direct engagement of Gal-9 by Tim-3 expressed on NKT cells induces apoptosis, while Gal-9 increases expression of IL-15 from KCs, which expands the hepatic NKT cell population. Activation of KCs by Gal-9 in the setting of alcoholic liver disease induces several proinflammatory cytokines that have hepatotoxic effects. T cells in patients with alcoholic hepatitis expressed high levels of TIM-3 and Gal-9, correlating with an exaggerated IL-10-mediated antimicrobial T-cell response that leads to immune exhaustion and a subsequent failure to support innate immunity, through impaired bacteria-specific IFN-γ responses. Functional single-nucleotide polymorphisms are associated with development of alcoholic liver disease. Thus, the effects of Gal-9 are disease context–specific.(33,35,36) Abbreviations: ALD, alcoholic liver disease; HSC, hepatic stellate cell; NAFLD, non-alcoholic fatty liver disease; SNP, single-nucleotide polymorphism.
ALCOHOLIC LIVER DISEASE
Recombinant Gal-9 induces proinflammatory mediators such as TNF-α, IL-1β, and IFN-γ from peripheral and liver-derived mononuclear cells of study subjects(15); and these cytokines are central effectors in alcohol-related liver injury.(34) A recent study found that patients with acute alcoholic hepatitis have higher plasma Gal-9 levels compared to healthy controls or patients with alcoholic cirrhosis.(35) T cells in patients with alcoholic hepatitis expressed higher levels of TIM-3 and Gal-9, correlating with an exaggerated IL-10-mediated antimicrobial T-cell response, which leads to immune exhaustion, and a subsequent failure to support innate immunity, through impaired bacteria-specific IFN-γ responses.(35) These results suggest that a marked immunosuppressive state (compensatory anti-inflammatory response syndrome), rather than the initial proinflammatory cytokine storm, might account for sepsis-related morbidity and mortality in these patients.(35) In a recent study of 575 individuals with at-risk alcohol consumption, we found that LGALS9 polymorphisms are associated with development of alcoholic liver disease.(36) The presence of single-nucleotide polymorphisms rs4239242 and rs4794976 is associated with higher transcription and protein expression of Gal-9 following stimulation of monocytes with IFN-γ and ethanol. The seemingly opposite effects of Gal-9 in NAFLD and alcohol-associated liver disease are consistent with the concept that although these two diseases share some aspects of dysregulated innate immunity (including KC activation), distinct immunopathogenic mechanisms are likely operant in NAFLD versus alcoholic liver disease (Fig. 2).
HEPATOCELLULAR CARCINOMA
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide, and the majority of cases are linked to chronic infection with viral hepatitis.(37) In HBV-related HCC, Gal-9pos KC and TIM-3pos T cells colocalize, and higher immunohistochemical expression of TIM-3 on CD4+ T cells is associated with shorter survival when compared to the low Tim-3 expressing group, consistent with the concept that Gal-9 impairs liver tumor immunity.(38) Another potential target for Gal-9 in immune suppression is the natural killer (NK) cell population, which comprises important antitumor effectors in the liver. We found that Gal-9 ligation on NK cells down-regulated multiple immune-activating genes, including eight that are involved in the NK cell–mediated cytotoxicity pathway, impaired lymphokine-activated killing, and decreased IFN-γ production independently of Tim-3.(39) Gal-9 is up-regulated at the gene and protein levels in human liver cancer cell lines (HepG2 and SMMC7721) compared to normal hepatocytes (Lo2) and induces apoptosis of peripheral blood mononuclear cells.(40) Furthermore, microRNA 22, which directly inhibits Gal-9 expression, is down-regulated in cancer tissues and in HCC lines.(40) Moreover, up-regulated Gal-9 expression on endothelial cells may also contribute to immune dysfunction.(41) In contrast to the immunosuppressive activities of Gal-9 in promoting tumor escape,(17) Gal-9 also has been associated with antimetastatic potential in HCC, (for example, relative down-regulation of Gal-9 in human HCC was associated with the histopathologic grade of the tumor, lymph node metastasis, vascular invasion, intrahepatic metastasis, and decreased survival),(42) with the likely mechanism that cytoplasmic Gal-9 induces cancer cell aggregation (stabilizing cell–cell adhesion junctions), and inhibits cell invasion, detachment from tumor, and attachment to vascular endothelium (Fig. 3), as shown in breast cancer.(43) Additional antitumor roles for Gal-9 are supported by a recent study in which Gal-9 induced apoptosis of HCC lines in vitro in a dose-dependent and time-dependent manner and inhibited the growth of human HCC cells in a xeno-graft athymic murine model.(44) TIM-3-independent apoptosis (without arrest of the cell cycle) occurred through endoplasmic reticulum stress and an intrinsic mitochondrial pathway (caspase-9) involving up-regulation of the oncogene microRNA 1246.(44) Of note, while the apoptotic effect was observed for two HCC lines (HLE and Li-7), Huh-7 cells were resistant, suggesting that not all HCCs will behave the same. These data suggest that Gal-9 holds promise as a potential adjuvant to conventional chemotherapy for some HCCs,(44) particularly because of different mechanisms of action (i.e., sorafenib induces cell cycle arrest). Of interest, Gal-9 also induces apoptosis of cholangiocarcinoma cells in vitro and in vivo and induces the tumor suppressor microRNA 198 in these cells.(45) The potential duality of Gal-9 in HCC is shown in Fig. 3. It has been proposed that after its initial up-regulation establishes a tolerogenic environment, Gal-9 expression might be lost during tumor progression. Thus, targeting of Gal-9 may need to be cell-specific to harness the therapeutic potential in the context of HCC.(6)
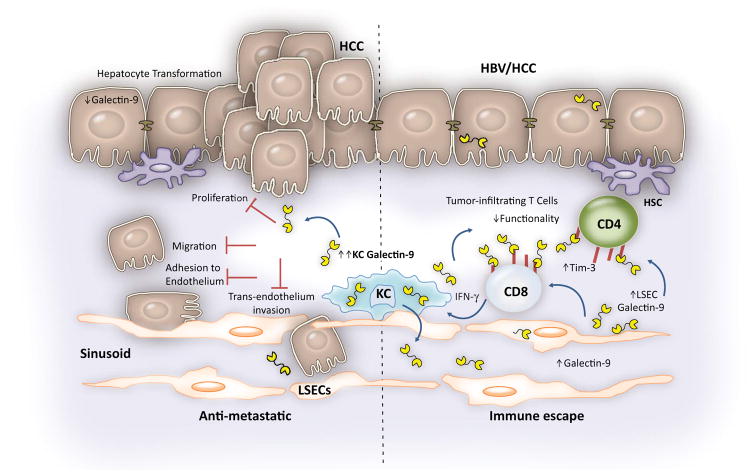
FIG. 3.
The dichotomous roles of Gal-9 in HCC. Gal-9 can display both beneficial and detrimental effects in the context of HCC. KC Gal-9 is highly up-regulated in HBV-associated HCC. In normal liver, Gal-9 is expressed in hepatocytes and down-regulated on transformation. Gal-9 can restore antimetastatic functions by blocking proliferation, migration, adhesion to endothelium, and trans-endothelium invasion of carcinoma cells. On the other hand, Gal-9 promotes immune escape by induction of apoptosis and inhibition of effector functions of tumor-infiltrating T cells which express high levels of Tim-3. Increased Gal-9 expression on liver endothelial cells may contribute to immune suppression.(38,39,41,42,44) Abbreviations: HSC, hepatic stellate cell; LSEC, liver sinusoidal endothelial cell.
ACUTE LIVER FAILURE
Acute liver failure (ALF) is comprised of severe liver injury in combination with progressive encephalopathy in a previously healthy patient and is associated with a high risk of progression to multiorgan failure.(46) The mechanisms by which drugs induce liver injury have been incompletely characterized,(47) but the innate immune response has been implicated. KCs play key roles in the development of acetaminophen and non-acetaminophen hepatotoxicity.(48,49) In a recent study, we found that patients (n = 149) with ALF related to either acetaminophen or idiosyncratic non-acetaminophen had higher circulating plasma levels of Gal-9, which was associated with the development of systemic inflammatory response syndrome, and predicted early mortality independently of the Model for End-Stage Liver Disease.(50) We developed competing risk multivariate models that defined the hazard ratios, finding that in ALF patients with identical Model for End-Stage Liver Disease scores, patients with Gal-9 levels ≥690 pg/mL had an almost 3-fold greater risk of dying within 21 days than patients with Gal-9 levels <690. Our results suggest that Gal-9-mediated impairment of immune function might predispose patients to infectious complications because Gal-9 is known to inhibit T-cell(8) and NK-cell(39) function. Development of neutralizing strategies to block Gal-9 effects might provide important insights into pathogenic mechanisms and novel therapeutic targets in the ALF setting.(21)
Summary
Gal-9 has a wide variety of biological functions in innate and adaptive immunity that are instrumental in the maintenance of hepatic homeostasis. Gal-9 may exhibit a “double-edged sword” effect with opposing biological outcomes depending on the localization, cell of origin (e.g., KC, hepatocyte), multiple target cells, and disease process (Table 1). For example, KC-derived Gal-9 favors persistence of viral hepatitis through expansion of Tregs and modulation of Th1 and Th17 immunity through induction of apoptosis. In ischemic liver injury, hepatocyte-derived Gal-9 is both diagnostic and cytoprotective. For Gal-9 to exert therapeutic benefit in NAFLD, it requires driving IL-15 production from KCs, which in turn leads to proliferation of NKT cells. Moreover, emerging work in NAFLD and IRI indicates the possibility that Gal-9 may synergize with TLRs to affect a range of hepatic inflammatory reactions. Ongoing studies are warranted to better elucidate the pathophysiology of hepatic immune-mediated diseases and to develop new therapeutic interventions using glycan-binding proteins.
TABLE 1.
Cellular Targets of Gal-9 and Potential Functions in Liver Disease
| Cell Population | Function and/or Effect | Role in Liver Disease |
|---|---|---|
| T cells | Inhibits proliferation of and cytokine production by CD4+ helper T cells Induces apoptosis of antigen-specific CD8+ T cells Attenuates IFN-γ production by CD8+ T cells |
|
| Tregs | Expands Tregs Effector molecule |
|
| NKT cells | Induces apoptosis of NKT cells Expands NKT cells indirectly through up-regulation of IL-15 in macrophages |
|
| KCs | Activation of this population resulting in production of TNF-α, IL-1β, IL-10, and IL-15 |
|
| Hepatocytes | Alarmin produced upon cell injury |
|
Abbreviations: ALD, alcoholic liver disease; ECM, extracellular matrix; NASH, nonalcoholic steatohepatitis.
Acknowledgments
Supported by National Institutes of Health grants RO1 DK106491 (to L.G.-M.) and R01AI120622, R01HD075549, and R21-AI103361, as well as a merit review grant (to H.R.R.).
Abbreviations
- AIH
autoimmune hepatitis
- AILD
autoimmune liver disease
- ALF
acute liver failure
- CD
cluster of differentiation
- CRD
carbohydrate recognition domain
- Gal-9
galectin-9
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HF
high-fat
- IFN-γ
interferon gamma
- IL
interleukin
- IRI
ischemia/reperfusion injury
- KC
Kupffer cell
- NAFLD
nonalcoholic fatty liver disease
- NK
natural killer
- NKT
natural killer T
- Th17
T helper 17
- TIM-3
T-cell immunoglobulin and mucin domain-containing molecule 3
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor alpha
- Treg
regulatory T cell
Footnotes
Potential conflict of interest: Nothing to report.
References
Author names in bold designate shared co-first authorship.
- 1.Schnaar RL. Glycobiology simplified: diverse roles of glycan recognition in inflammation. J Leukoc Biol. 2016;99:825–838. doi: 10.1189/jlb.3RI0116-021R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.John S, Mishra R. Galectin-9: from cell biology to complex disease dynamics. J Biosci. 2016;41:507–534. doi: 10.1007/s12038-016-9616-y. [DOI] [PubMed] [Google Scholar]
- 3.Nagae M, Nishi N, Nakamura-Tsuruta S, Hirabayashi J, Wakatsuki S, Kato R. Structural analysis of the human galectin-9 N-terminal carbohydrate recognition domain reveals unexpected properties that differ from the mouse orthologue. J Mol Biol. 2008;375:119–135. doi: 10.1016/j.jmb.2007.09.060. [DOI] [PubMed] [Google Scholar]
- 4.Liu FT, Yang RY, Hsu DK. Galectins in acute and chronic inflammation. Ann N Y Acad Sci. 2012;1253:80–91. doi: 10.1111/j.1749-6632.2011.06386.x. [DOI] [PubMed] [Google Scholar]
- 5.Rabinovich GA, Toscano MA. Turning “sweet” on immunity: galectin–glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 6.Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol. 2013;19:8831–8849. doi: 10.3748/wjg.v19.i47.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsumoto R, Matsumoto H, Seki M, Hata M, Asano Y, Kanegasaki S, et al. Human ecalectin, a variant of human galectin-9, is a novel eosinophil chemoattractant produced by T lymphocytes. J Biol Chem. 1998;273:16976–16984. doi: 10.1074/jbc.273.27.16976. [DOI] [PubMed] [Google Scholar]
- 8.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 9.Wiersma VR, de Bruyn M, Helfrich W, Bremer E. Therapeutic potential of Galectin-9 in human disease. Med Res Rev. 2013;33(Suppl 1):E102–E126. doi: 10.1002/med.20249. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Feng J, Geng S, Geng S, Wei H, Chen G, et al. The N-and C-terminal carbohydrate recognition domains of galectin-9 contribute differently to its multiple functions in innate immunity and adaptive immunity. Mol Immunol. 2011;48:670–677. doi: 10.1016/j.molimm.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Blidner AG, Mendez-Huergo SP, Cagnoni AJ, Rabinovich GA. Re-wiring regulatory cell networks in immunity by galectin–glycan interactions. FEBS Lett. 2015;589:3407–3418. doi: 10.1016/j.febslet.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Rosen HR. Emerging concepts in immunity to hepatitis C virus infection. J Clin Invest. 2013;123:4121–4130. doi: 10.1172/JCI67714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, et al. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, et al. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. J Clin Invest. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mengshol JA, Golden-Mason L, Arikawa T, Smith M, Niki T, McWilliams R, et al. A crucial role for Kupffer cell–derived galectin-9 in regulation of T cell immunity in hepatitis C infection. PLoS One. 2010;5:e9504. doi: 10.1371/journal.pone.0009504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwood NM, Golden-Mason L, Cheng L, Rosen HR, Mengshol JA. HCV-infected cells and differentiation increase monocyte immunoregulatory galectin-9 production. J Leukoc Biol. 2016;99:495–503. doi: 10.1189/jlb.5A1214-582R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lv K, Zhang Y, Zhang M, Zhong M, Suo Q. Galectin-9 promotes TGF-beta1-dependent induction of regulatory T cells via the TGF-beta/Smad signaling pathway. Mol Med Rep. 2013;7:205–210. doi: 10.3892/mmr.2012.1125. [DOI] [PubMed] [Google Scholar]
- 18.Kared H, Fabre T, Bedard N, Bruneau J, Shoukry NH. Galectin-9 and IL-21 mediate cross-regulation between Th17 and Treg cells during acute hepatitis C. PLoS Pathog. 2013;9:e1003422. doi: 10.1371/journal.ppat.1003422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seki M, Oomizu S, Sakata KM, Sakata A, Arikawa T, Watanabe K, et al. Galectin-9 suppresses the generation of Th17, promotes the induction of regulatory T cells, and regulates experimental autoimmune arthritis. Clin Immunol. 2008;127:78–88. doi: 10.1016/j.clim.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 20.Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PLoS One. 2012;7:e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barjon C, Niki T, Verillaud B, Opolon P, Bedossa P, Hirashima M, et al. A novel monoclonal antibody for detection of galectin-9 in tissue sections: application to human tissues infected by oncogenic viruses. Infect Agent Cancer. 2012;7:16. doi: 10.1186/1750-9378-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liberal R, Grant CR, Holder BS, Ma Y, Mieli-Vergani G, Vergani D, et al. The impaired immune regulation of autoimmune hepatitis is linked to a defective galectin-9/tim-3 pathway. Hepatology. 2012;56:677–686. doi: 10.1002/hep.25682. [DOI] [PubMed] [Google Scholar]
- 23.Longhi MS, Hussain MJ, Mitry RR, Arora SK, Mieli-Vergani G, Vergani D, et al. Functional study of CD4+CD25+ regulatory T cells in health and autoimmune hepatitis. J Immunol. 2006;176:4484–4491. doi: 10.4049/jimmunol.176.7.4484. [DOI] [PubMed] [Google Scholar]
- 24.Ferri S, Longhi MS, De Molo C, Lalanne C, Muratori P, Granito A, et al. A multifaceted imbalance of T cells with regulatory function characterizes type 1 autoimmune hepatitis. Hepatology. 2010;52:999–1007. doi: 10.1002/hep.23792. [DOI] [PubMed] [Google Scholar]
- 25.Lv K, Zhang Y, Zhang M, Zhong M, Suo Q. Galectin-9 ameliorates Con A-induced hepatitis by inducing CD4+CD25low/int effector T-cell apoptosis and increasing regulatory T cell number. PLoS One. 2012;7:e48379. doi: 10.1371/journal.pone.0048379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, et al. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 27.Hanschen M, Zahler S, Krombach F, Khandoga A. Reciprocal activation between CD4+ T cells and Kupffer cells during hepatic ischemia–reperfusion. Transplantation. 2008;86:710–718. doi: 10.1097/TP.0b013e3181821aa7. [DOI] [PubMed] [Google Scholar]
- 28.Uchida Y, Ke B, Freitas MC, Yagita H, Akiba H, Busuttil RW, et al. T-cell immunoglobulin mucin-3 determines severity of liver ischemia/reperfusion injury in mice in a TLR4-dependent manner. Gastroenterology. 2010;139:2195–2206. doi: 10.1053/j.gastro.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirao H, Uchida Y, Kadono K, Tanaka H, Niki T, Yamauchi A, et al. The protective function of galectin-9 in liver ischemia and reperfusion injury in mice. Liver Transpl. 2015;21:969–981. doi: 10.1002/lt.24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y, Ji H, Zhang Y, Shen X, Gao F, He X, et al. Recipient T cell TIM-3 and hepatocyte galectin-9 signalling protects mouse liver transplants against ischemia–reperfusion injury. J Hepatol. 2015;62:563–572. doi: 10.1016/j.jhep.2014.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880–885. doi: 10.1002/hep.20826. [DOI] [PubMed] [Google Scholar]
- 32.Ma X, Hua J, Li Z. Probiotics improve high fat diet–induced hepatic steatosis and insulin resistance by increasing hepatic NKT cells. J Hepatol. 2008;49:821–830. doi: 10.1016/j.jhep.2008.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang ZH, Liang S, Potter J, Jiang X, Mao HQ, Li Z. Tim-3/galectin-9 regulate the homeostasis of hepatic NKT cells in a murine model of nonalcoholic fatty liver disease. J Immunol. 2013;190:1788–1796. doi: 10.4049/jimmunol.1202814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mandrekar P, Bataller R, Tsukamoto H, Gao B. Alcoholic hepatitis: translational approaches to develop targeted therapies. Hepatology. 2016;64:1343–1355. doi: 10.1002/hep.28530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Markwick LJ, Riva A, Ryan JM, Cooksley H, Palma E, Tranah TH, et al. Blockade of PD1 and TIM3 restores innate and adaptive immunity in patients with acute alcoholic hepatitis. Gastroenterology. 2015;148:590–602. doi: 10.1053/j.gastro.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 36.Rosen HR, Golden-Mason L, Daly AK, Yang I, Day CP. Variants in the LGALS9 gene are associated with development of liver disease in heavy consumers of alcohol. Clin Gastroenterol Hepatol. 2016;14:762–768. doi: 10.1016/j.cgh.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 37.McMahon B, Block J, Block T, Cohen C, Evans AA, Hosangadi A, et al. Hepatitis-associated liver cancer: gaps and opportunities to improve care. J Natl Cancer Inst. 2016;108:djv359. doi: 10.1093/jnci/djv359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li H, Wu K, Tao K, Chen L, Zheng Q, Lu X, et al. Tim-3/galectin-9 signaling pathway mediates T-cell dysfunction and predicts poor prognosis in patients with hepatitis B virus–associated hepatocellular carcinoma. Hepatology. 2012;56:1342–1351. doi: 10.1002/hep.25777. [DOI] [PubMed] [Google Scholar]
- 39.Golden-Mason L, McMahan RH, Strong M, Reisdorph R, Mahaffey S, Palmer BE, et al. Galectin-9 functionally impairs natural killer cells in humans and mice. J Virol. 2013;87:4835–4845. doi: 10.1128/JVI.01085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Q, Jiang W, Zhuang C, Geng Z, Hou C, Huang D, et al. microRNA-22 downregulation of galectin-9 influences lymphocyte apoptosis and tumor cell proliferation in liver cancer. Oncol Rep. 2015;34:1771–1778. doi: 10.3892/or.2015.4167. [DOI] [PubMed] [Google Scholar]
- 41.Heusschen R, Schulkens IA, van Beijnum J, Griffioen AW, Thijssen VL. Endothelial LGALS9 splice variant expression in endothelial cell biology and angiogenesis. Biochim Biophys Acta. 2014;1842:284–292. doi: 10.1016/j.bbadis.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Zhang ZY, Dong JH, Chen YW, Wang XQ, Li CH, Wang J, et al. Galectin-9 acts as a prognostic factor with antimetastatic potential in hepatocellular carcinoma. Asian Pac J Cancer Prev. 2012;13:2503–2509. doi: 10.7314/apjcp.2012.13.6.2503. [DOI] [PubMed] [Google Scholar]
- 43.Irie A, Yamauchi A, Kontani K, Kihara M, Liu D, Shirato Y, et al. Galectin-9 as a prognostic factor with antimetastatic potential in breast cancer. Clin Cancer Res. 2005;11:2962–2968. doi: 10.1158/1078-0432.CCR-04-0861. [DOI] [PubMed] [Google Scholar]
- 44.Fujita K, Iwama H, Sakamoto T, Okura R, Kobayashi K, Takano J, et al. Galectin-9 suppresses the growth of hepatocellular carcinoma via apoptosis in vitro and in vivo. Int J Oncol. 2015;46:2419–2430. doi: 10.3892/ijo.2015.2941. [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi K, Morishita A, Iwama H, Fujita K, Okura R, Fujihara S, et al. Galectin-9 suppresses cholangiocarcinoma cell proliferation by inducing apoptosis but not cell cycle arrest. Oncol Rep. 2015;34:1761–1770. doi: 10.3892/or.2015.4197. [DOI] [PubMed] [Google Scholar]
- 46.Bernal W, Hyyrylainen A, Gera A, Audimoolam VK, McPhail MJ, Auzinger G, et al. Lessons from look-back in acute liver failure? A single centre experience of 3300 patients. J Hepatol. 2013;59:74–80. doi: 10.1016/j.jhep.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 47.Chung RT, Stravitz RT, Fontana RJ, Schiodt FV, Mehal WZ, Reddy KR, et al. Pathogenesis of liver injury in acute liver failure. Gastroenterology. 2012;143:e1–e7. doi: 10.1053/j.gastro.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu ZX, Kaplowitz N. Role of innate immunity in acetaminophen-induced hepatotoxicity. Expert Opin Drug Metab Toxicol. 2006;2:493–503. doi: 10.1517/17425255.2.4.493. [DOI] [PubMed] [Google Scholar]
- 49.Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci. 2007;96:2–15. doi: 10.1093/toxsci/kfl173. [DOI] [PubMed] [Google Scholar]
- 50.Rosen HR, Biggins SW, Niki T, Gralla J, Hillman H, Hirashima M, et al. Association between plasma level of galectin-9 and survival of patients with drug-induced acute liver failure. Clin Gastroenterol Hepatol. 2016;14:606–612. doi: 10.1016/j.cgh.2015.09.040. [DOI] [PubMed] [Google Scholar]