Abstract
Alcohol intake is associated with numbers of different human cancers, such as hepatocellular carcinoma (HCC) and breast cancer. However, the molecular mechanism remains to be elucidated. RNA polymerase III-dependent genes (Pol III genes) deregulation elevates cellular production of tRNAs and 5S rRNA, resulting in an increase in translational capacity, which promote cell transformation and tumor formation. To explore a common mechanism of alcohol-associated human cancers, we have comparably analyzed that alcohol causes deregulation of Pol III genes in liver and breast cells. Our results reveal that alcohol enhances RNA Pol III gene transcription in both liver and breast cells. The induction of Pol III genes caused by alcohol in ER+ breast cancer lines or liver tumor lines are significantly higher than in their non-tumor cell lines. Alcohol increases cellular levels of Brf1 mRNA and protein, which is key transcription factor and specifically regulate Pol III gene activity. Alcohol activates JNK1 to upregulate transcription of Brf1 and Pol III genes, whereas inhibition of JNK1 by SP600125 or its siRNA significantly decreases the induction of these genes. Furthermore, alcohol increases the rates of transformation of liver and breast cells, repressed JNK1 and Brf1 expression decrease transcription of Pol III genes and reduce the rates of colony formation of AML-12 and MCF-10 cells. Together, these studies support the idea that alcohol induces deregulation of Brf1 and RNA Pol III genes in liver and breast cells, which share a common signaling pathway to promote cell transformation. Through the common mechanism, alcohol-induced deregulation of RNA Pol III genes brings about greater phenotypic changes.
Keywords: Alcohol, Brf1, Pol III genes, HCC, breast cancer, transformation
1. Introduction
Alcohol has been classified as carcinogen to humans (IARC, 2011; Cogliani et al., 2011; Shi et al., 2015). Targeted organs of alcohol-associated cancers include the breast, liver, stomach, pancreas, oral cavity, pharynx, esophagus, larynx, colon and ovary (Shi et al., 2015). Why alcohol is able to promoted cancer development in the different organs? Is there a common mechanism, which mediates this process? The questions remain to be studied. Cancer cells have a consistent cytological feature, nucleolar hypertrophy, where RNA polymerase III-dependent genes (Pol III genes) are transcribed. This feature provides possibility to explore the mechanism of alcohol-associated human cancers by determining the effect of alcohol on Brf1 (TFIIB-related factor 1) and Pol III genes (Shi et al., 2015). To investigate alcohol-induced deregulation of Brf1 and Pol III genes, we focus the studies on alcohol-associated hepatocellular carcinoma (HCC) and breast cancer in present study. Uncovering the underlying molecular mechanisms could lead to improving therapeutic approaches and preventative.
RNA Pol III transcribes a variety of untranslated RNAs, including tRNAs, 5S rRNAs, 7SL RNA, 7SK RNA and U6 RNA (Dieci et al., 2007; Ulu et al., 1984; Zhang et al., 2013), while tRNAs and 5S rRNAs control the translational and growth capacity of cells (Raha et al., 2010; White, 2001; Zhang et al., 2013). Oncogenic proteins (c-Myc, c-Jun, c-Fos) stimulate RNA Pol III gene transcription (Goodfellow et al., 2006; Johnson et al., 2008; Zhong et al., 2004, 2007); whereas tumor suppressors (BRCA1, PTEN, pRb, p53) inhibits transcription of this class of genes (Johnson et al., 2008A; White, 2001; Woiwode et al., 2008; Zhong et al., 2015). Studies have revealed that RNA Pol III transcription products are increased in both transformed and tumor cells, suggesting that they play an important role in tumorigenesis (Johnson et al., 2008; Winter et al., 2000; Zhong et al., 2015). Consistent with this idea, elevated Pol III transcription is required for cell transformation and tumor development (Johnson et al., 2008A; Zhang et al., 2013). The capacity of these oncogenes and tumor suppressor genes to alter Pol III transcription results from their ability to regulate the TFIIIB complex, which is composed of Brf1, Bdp1 and TBP (TATA box-binding protein). Brf1 and Bdp1 specifically regulate the transcription of Pol III genes (Zhang et al., 2013; Zhong et al., 2013A).
Alcohol is the dietary factor which is most consistently associated with risk of cancers (Hamajima et al., 2002; Macmahom B, 2006; Petri et al., 2004; Singlwtary KW and Gaspstur S., 2001). Alcohol induces liver injury, including liver steatosis and fibrosis, promotes liver cirrhosis, thereby increasing the risk of development of HCC (Lieber, 2000; Zhong et al., 2011). Alcohol in combination with viruses (hepatitis C or B), carcinogens (aflatoxin), obesity, or diabetes mellitus, promotes liver cancer development (Bagnardi et al., 2001; Seitz et al., 1998; Zhong et al., 2013A; Yuan et al., 2004). Emerging evidence indicates that alcohol intake is associated with breast cancer (Zhang et al., 2013). This association involves the estrogen receptor (ER), which is over-expressed (ER+) in approximately 80% of breast cancer cases (Deandrea et al., 2008). Alcohol is known to promote mammary tumorigenesis (Singletary et al., 1991, 1995; Watabiki et al., 2000). A cytological feature, nucleolar hypertrophy, has been used as a strong diagnostic indicator of cell transformation and neoplasia. This indicates that transformation in situ is tightly linked to the deregulation of RNA Pol I and III gene transcription, because the size of the nucleolus reflects the levels of rRNA synthesis (White R, 2001; Zhang et al., 2013). Although alcohol-associated human cancers were widely studied, the molecular mechanism remains to be elucidated (Zhang et al., 2013).
Our recent studies using both cell culture model and animal models have revealed that alcohol induces transcription of tRNALeu and 5S rRNA (Zhang et al., 2013; Zhong et al., 2011). This induction in mice fed with ethanol is associated with liver tumor development (Machida et al., 2009; Zhong et al., 2011). This implies that alcohol-induced deregulation of Pol III genes may play critical role in tumor development (Zhong et al., 2011). However, very little is known about the comparison study of alcohol-induced deregulation of Pol III genes and transformation in different human cancer cells. To comparably analyze the role of alcohol in this phenomenon, we treated liver and breast cells with ethanol. Our results indicate that ethanol increases tRNA and 5S rRNA transcription in both liver and breast cancer cells. The ER+ breast cancer cells is more sensitive to ethanol than liver cancer cells. Ethanol induces activation of JNK1, which mediates Pol III gene transcription. Further analysis reveals that inhibition of JNK1 by a chemical inhibitor (SP 600125) or JNK1 siRNA reduces Brf1 expression and Pol III gene transcription. Repressing Brf1 decreases alcohol-enhanced Pol III gene transcription, resulting in repression of alcohol-induced transformation of liver and breast cells. These studies support this idea that alcohol activates JNK1 to upregulate Brf1 expression and Pol III gene transcription, leading to transformation of liver and breast cells, which is a common mechanism of alcohol-promoted transformation of different cells. These novel findings will be of great interest both to the basic and clinical research communities and provide a potential approach of treatment for alcohol-associated cancer patients (Zhang et al., 2013).
2. Materials and methods
2.1. Cell lines, reagents and antibodies
Mouse hepatocyte line, AML-12 cell, which is a line with transgenic for human TGF-alpha; ER- human breast non-tumorigenic epithelial cell line (MCF-10A), ER+ human breast cancer cell lines (MCF-7 and T47D) were from ATCC. HepG2-ADH (alcohol dehydrogenase) was provided by Dr. DL Clemens (Nebraska University). Cell culture medium DMEM, DMEM/F12, Lipofectin reagent, Lipofectamine 2000, TRIzol reagent and OPTI-MEM were from Life Technologies. Antibodies against c-Jun, JNK1 (46kD) and β-actin were obtained from Santa Cruz Biotech. Brf1 (90kD) antibody was from Bethyl laboratories Inc. JNK and phosphor-JNK antibodies were from Cell Signaling. JNK inhibitor, SP600125 was from A.G. Scientific, Inc. The sequences of siRNAs of Brf1 and primers of Brf1, tRNALeu and 5S rRNA were listed in supplements. EGF and E2 (17β-estradiol) was from Sigma-Aldrich.
2.2. SDS-PAGE and immunoblot analysis
Human liver or breast cells were incubated with 50mM or 25mM ethanol for 60 min after starvation 3h. Cells were collected with lysis buffer and sonicated. The suspensions were centrifuged to save the supernatants. Protein concentrations were determined by the Bradford method using Fluostar Omega spectrometer (Cell Biology Core Laboratory of University of Southern California Research Center for Liver Diseases, P30DK DK048522). Lysates (50 μg of protein) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred from the SDS-PAGE gel to Hybond-P membrane and immunoblot analysis were performed with specific antibodies (Sun et al., 2016A; Song and Yin, 2016). Membranes were probed with these antibodies against Brf1, JNKs, phosphor-JNK and β-actin as described (Zhong et al., 2004; Zhong et al., 2013A). Bound primary antibody was visualized using horseradish peroxidase-conjugated secondary antibody (Vector Laboratories) and enhancing chemiluminescence reagents (Santa Cruz Biotech).
2.3. RNA isolation and RT-qPCR
Total RNA was isolated from liver and breast cells treated with ethanol using single step extraction method TRIzol reagent (Life Technologies). Total RNA samples were quantified and reverse-transcribed in a 20 μl reaction containing 1 x RT (reverse transcription) buffer. After first-strand cDNA synthesis, the cDNAs were diluted in DNase-free water and real time qPCR (RT-qPCR) were performed with specific primers (Table S2) and PCR reagent kits (Bio-Rad Biotech) in the ABI prism 7700 Sequence Detection System (Zhou et al., 2016). Precursor of tRNALeu and 5S rRNA transcripts and mRNAs of Brf1 were measured by RT-qPCR as described previously (Zhong et al., 2013B, 2016).
2.4. Transfection with siRNA and expression construct
For transient transfection assays, cells were transfected with plasmids of JNK1 expression or siRNAs of JNK1 and Brf1 as described previously (Fang et al., 2016; Sun et al, 2016B–C). Serum-free medium was added to each dish with Lipofectin-DNA or Lipofectamine 2000-siRNA complexes, and cells were further incubated for 4h. The medium was changed with 10% FBS DMEM or DMEM/F12 (phenol red-free) (Zhong et al., 2013A) and cells were incubated for 48h before harvesting. Cells were starved in DMEM for 3 h after rising twice with 1X PBS and treated with 50mM or 25mM ethanol for 60min to collect cell lysates or total RNA. Protein concentrations of the resultant lysates were measured by the Bradford method.
2.5. Cell Anchorage-independent growth
AML-12 cells and MCF-10A cells were transfected with mismatch RNA (siMM), JNK1 or Brf1 siRNAs (Table S1) as described (Zhang et al., 2011; Zhong et al., 2016). The transfected AML-12 cells or MCF-10A cells (1 × 104 cells/well in 6well plate) were suspended in 0.35% (w/v) agar in 10% FBS/DMEM/F12. AML-12 cells were treated with 20ng EGF with or without 50mM ethanol or both EGF and ethanol. MCF-10A cells were treated with or without 5nM E2, 25mM ethanol or both E2 and ethanol over a bottom layer of media with 0.5% (w/v) agar. Cells were fed fresh complete media with EGF or/and ethanol, or E2 or/and ethanol twice weekly. Colonies were counted 3 weeks or longer after plating as previously described (Zhang et al., 2011; Zhong et al., 2014, 2016).
3. Results
3.1. Alcohol induces expression of Brf1 and Pol III genes of liver and breast cells
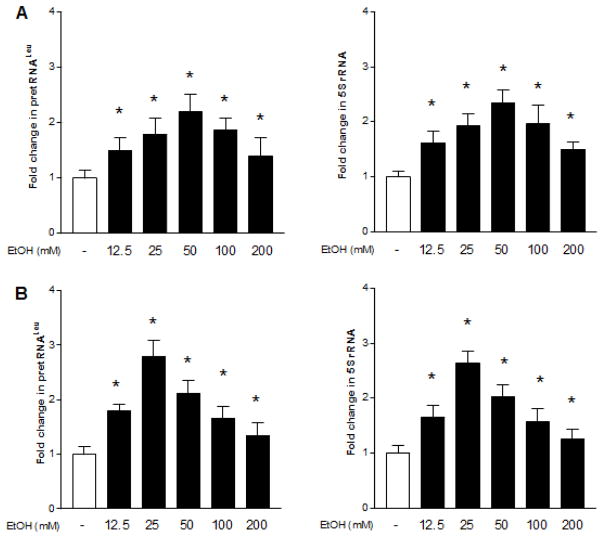
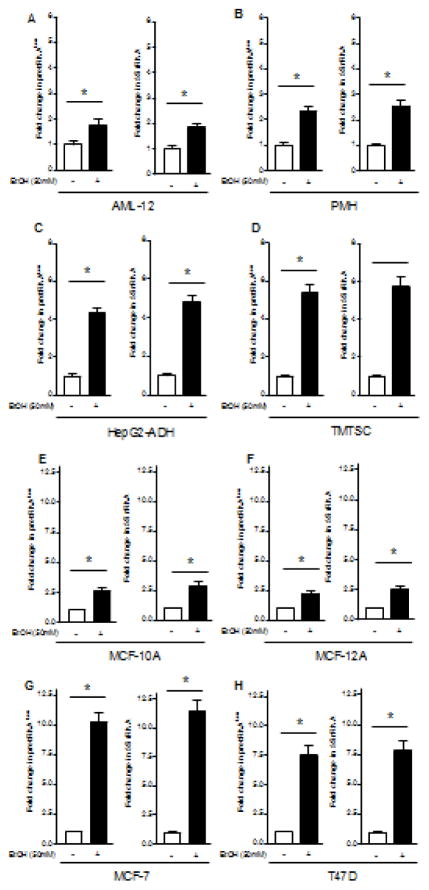
To determine the effect of alcohol on transcription of Pol III genes, non-tumor liver cell line AML-12 and breast cell line MCF-10A were exposed to ethanol. The cellular levels of precursor tRNALeu and 5S rRNA were tested by RT-qPCR. We performed the time curve and dose-dependent curve of ethanol-induced transcription of Pol III genes. We found that ethanol-caused Pol III gene transcription can reach high levels after 60min ethanol treatment (data not shown). Ethanol treatment of both AML12 and MCF-10A cells creates a dose-dependent increase in transcription of pre-tRNALeu and 5S rRNA genes (Zhang et al., 2013), where ethanol-caused maximum induction of Pol III genes was observed at 50mM ethanol for 60min of liver cells (Fig. 1A), whereas high peak of this induction in breast cells is 25mM (Fig. 1B). Therefore, we have used the conditions for the whole study. This results display that ethanol-caused induction of Pol III genes in breast cells is higher than in liver cells (Fig. 1). We further determined difference in cancer cell lines and non-tumor cells. Alcohol enhances transcription of pre-tRNALeu and 5S rRNA of liver cancer cells of HepG2-ADH and TSCML (tumor stem cells of mouse liver) (Fig. 2C,D) markedly higher than both non-tumor cells, AML-12 and primary mouse hepatocytes (PMH) (Fig 2A,B). Whereas ethanol-increased transcription of Pol III genes in ER+ breast cancer cell lines, MCF-7 and T47D (Fig 2G,H) is dramatically more than in ER- non-tumor breast cells, MCF-10A and MCF-12A (Fig. 2E,F). More interestingly, the inductions of pre-tRNALeu and 5S rRNA by ethanol in ER- breast cancer cells are similar to MCF-10A and MCF-12A (data not shown). These results indicate that ethanol-induced transcription of Pol III genes in cancer cells, such as liver HepG2-ADH and TSCML tumor cells, and ER+ breast MCF-7 and T47D cancer cells, is more than non-tumor liver and breast cells. This suggests that alcohol-caused transcription of Pol III genes is associated with oncogenic status (Zhong et al., 2015).
Fig. 1. Alcohol induces RNA Pol III-dependent transcription.
AML-12 cells and MCF-10A cells were starved in FBS/DMEM-F12 for 3h. Cells were treated with or without different amounts of ethanol as indicated. RNAs were isolated from these cells and RT-qPCR was performed to measure the amounts of pre-tRNALeu (A and B, left panel), 5S rRNA (A and B, right panel). The fold change was calculated by normalizing to the amount of GAPDH mRNA. High peak of the induction of Pol III genes are in 50mM ethanol of Liver cells and at 25mM ethanol of breast cells. *: P > 0.05. The columns represent Mean ± SE of at least three independent determinations.
Fig. 2. Pol III gene transcription is increased by alcohol.
(A–D): Ethanol increases Pol III gene transcription in liver cells. Non-tumor mouse liver line, AML-12 cells and PMH (primary mouse hepatocytes) (A–B), and liver tumor cells (C–D), HepG2 and TSCML (tumor stem cells of mouse liver) were grown to 85% confluency and starved in DMEM-F12 for 3 h and treated with 50mM ethanol for another hour. (E–H): Ethanol induces Pol III gene transcription in breast cells. Non-tumor breast cell lines (MCF-10A and MCF-12A) (E–F) and ER+ breast cancer cell lines (MCF-7 and T47D) (G–H) were treated with 25mM ethanol as described above. The total RNAs from these cells were extracted to measure pre-tRNALeu, 5S rRNA and GAPDH transcripts. The fold change was calculated by normalizing to the amount of GAPDH. *: P > 0.05. The columns represent Mean ± SE of at least three independent determinations.
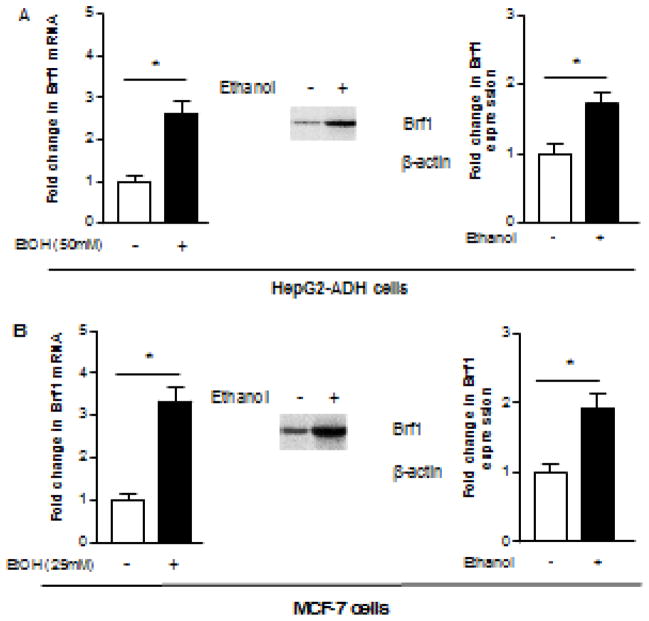
Brf1 is a subunit of TFIIIB, which specifically regulates tRNA and 5S rRNA transcription (Zhang et al., 2013). Our recent studies reveal that Brf1 is overexpression in human biopsies of HCC patients, and the patients of HCC with high expression of Brf1 show shorter period of overall survival (Zhong et al., 2016). Further studies indicate that Brf1 expression is also increases in human tumor foci of breast cancer (data not shown). Above studies show that alcohol is able to enhance Pol III gene transcription. Therefore, we further investigate whether alcohol affects Brf1 expression in liver and breast cell. The results indicate that ethanol treatments of either HepG2-ADH (Fig. 3A) or MCF-7 cells (Fig. 3B) increase the Brf1 protein and mRNA levels.
Fig. 3. Alcohol-mediated induction of Brf1 expression in liver and breast cells.
HepG2-ADH cells (A) and MCF-7 cells (B) were treated with ethanol respectively as described above. Resultant cell lysates and total RNAs were isolated from these cells. Immunoblot analysis was performed with a specific antibody against Brf1 (90kD) to determine changes in cellular levels of Brf1 (A and B, middle and right panel). A representative blot from three independent determinations and quantitative analysis are shown (middle and right) The total RNAs from these cells were extracted to measure mRNA of Brf1 by RT-PCR (A and B, left panel). The fold change was calculated by normalizing to the amount of GAPDH. *: P > 0.05. The values represent mean ± SE from three independent experiments.
3.2. Signal events of alcohol-induced cellular response which mediates Pol III gene transcription
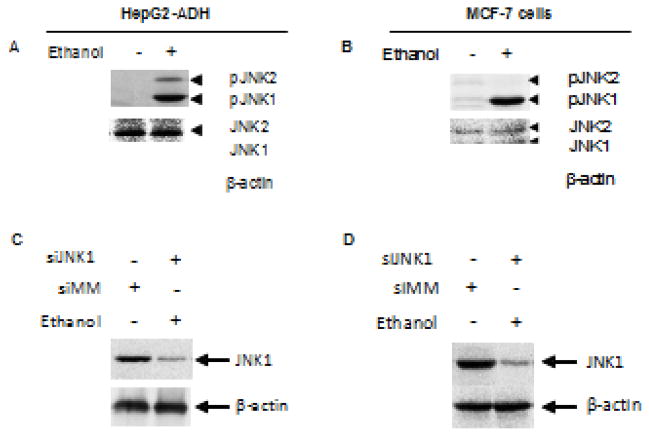
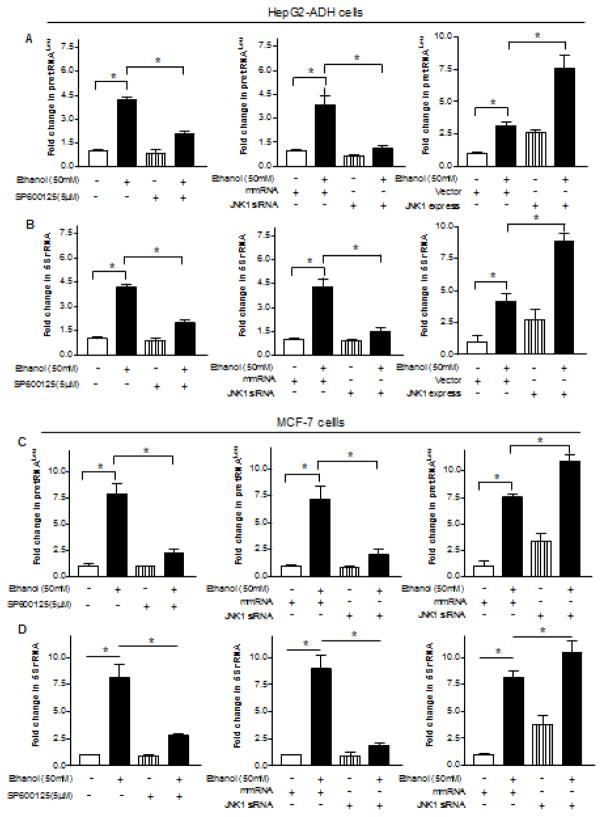
Since ethanol has been shown to induce JNK activation (Luedemann et al., 2005; Zhang et al., 2013) and the JNKs modulates Pol III gene transcription, we have determined whether JNKs mediates ethanol-induced Pol III gene transcription (Zhong et al., 2007; 2009). Ethanol strongly activates JNK1, but a weaker JNK2, in the HepG2-ADH cells (Fig. 4A). Therefore, we further assess whether ethanol induces activation of JNKs in breast cells. The results indicate that ethanol dramatically induced activation of JNK1 in MCF-7 cells (Fig. 4B). Our previous studies have identified that JNK1 and JNK2 have different function in Pol III gene transcription (Zhong et al., 2007; 2009). JNK1 positively, but JNK2 negatively, modulates transcription of Pol III genes (Zhong et al, 2007). This implies that alcohol-activated JNK1 may play a critical role in Pol III gene activity and cell transformation. Next, we investigate whether JNK1 affects Pol III gene transcription induced by alcohol. HepG2-ADH cells and MCF-7 cells were treated with 5μM SP600125, a JNK chemical inhibitor for one hour (Zhang et al., 2013). Hereafter, the cells were exposed to ethanol for another one hour. Fig. 5A–D (left panel) indicate that this JNK inhibitor significantly decreases transcription of pre-tRNALeu and 5S rRNA induced by alcohol. Then, we transfected HepG2-ADH and MCF-7 cells with JNK1 siRNA to determine alteration of transcription of Pol III genes. The results reveal that JNK1 siRNA specifically represses JNK1 expression (Fig. 4C–D), whereas JNK1 siRNA dramatically reduces alcohol-induced transcription of Pol III genes (Fig. 5A–D, Middle panel). In contrast, we found that increasing JNK1 expression by JNK1 expressing construct elevates alcohol-induced Pol III gene transcription in both HepG2-ADH cells and MCF-7 cells (Fig. 5A–D, Right panel). These studies demonstrate that alcohol-caused JNK1 activation indeed mediates transcription of Pol III genes.
Fig. 4. Alcohol induces activation of JNK1.
(A and B): Ethanol induces JNK1 activation. HepG2-ADH cells and MCF-7 cells were treated with or without ethanol as described above. Immunoblot analysis was performed using protein lysates derived from these cells and antibodies against phosphorylated JNK1/2 (46kD/54kD), JNK1/2 and β-actin as designated. (C and D): JNK1 siRNA decreases cellular levels of JNK1. HepG2-ADH cells and MCF-7 cells were transfected with mismatch RNA (siMM) and JNK1 siRNA (siJNK1) for 48 hours. The cell lysates were extracted from these cells to determine cellular levels of JNK1 and βactin (up panel) and quantitation analysis (bottom panel) as indicated. A representative blot from three independent determinations is shown.
Fig. 5. Alcohol-activated JNK1 mediates transcription of Pol III genes.
(A–D, left panel) JNK inhibitor SP600125 inhibits alcohol-induced Pol III gene transcription. HepG2-ADH cells and MCF-7 cells were pretreated with 5μM SP600125 and then treated with or without ethanol. (A–D, middle panel): Repression of JNK1 decreases transcription. HepG2-ADH cells and MCF-7 cells were transfected with either mismatch RNA (siMM) or JNK1-specific siRNA (siJNK1) for 48 hours and then treated with ethanol; (A–D, right panel): Overexpression of JNK1 enhances transcription of Pol III genes. HepG2-ADH cells and MCF-7 cells were transfected with either JNK1 expression construct or vector for 48 hours and treated with ethanol. RNAs was derived from these cells and RT-qPCR was performed to measure the amounts of pre-tRNALeu, (A and C), 5S rRNA (B and D), and GAPDH transcripts. The fold change was calculated by normalizing to the amount of GAPDH. *: P > 0.05. The values represent mean ± SE from three independent experiments.
3.3. Reduction of Brf1 expression represses cell transformation
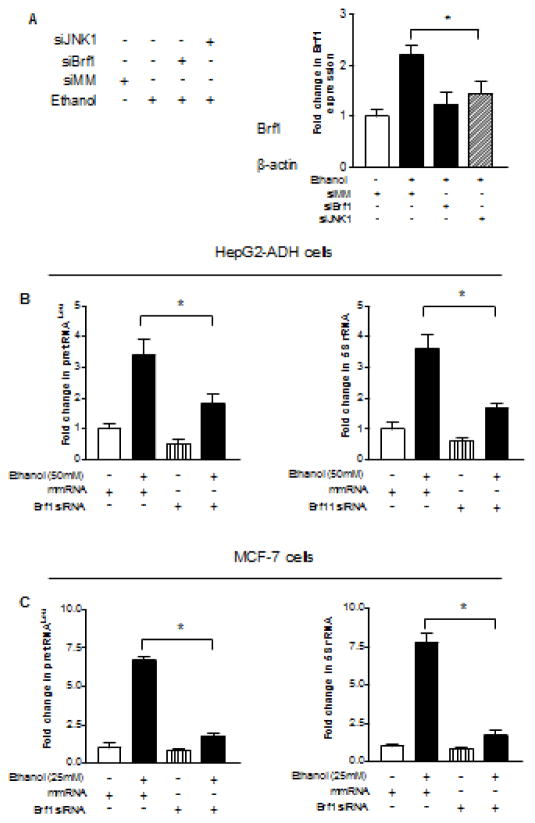
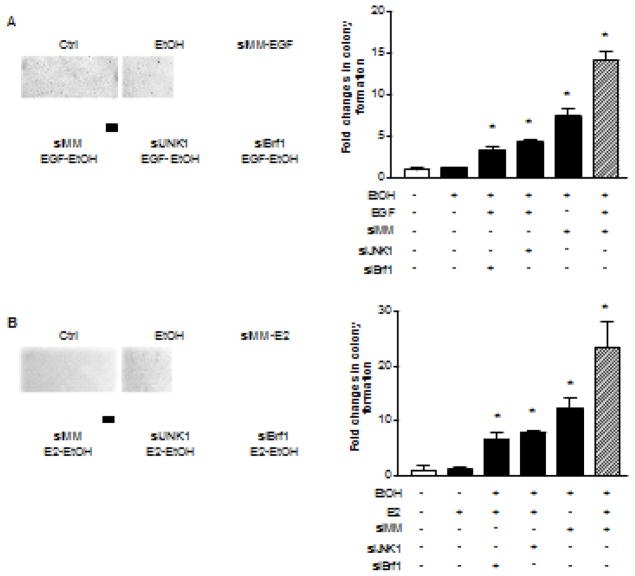
As mentioned above that Brf1 overexpression was in human HCC cases (Zhong et al., 2016), we determine whether alcohol-induced activation of JNK1 mediates Brf1 expression. HepG2-ADH cells were transfected with JNK1 and Brf1 siRNA, respectively and Brf1 alteration was determined. The results indicate that repressing JNK1 or Brf1 expression by their siRNA results in reduction of the cellular levels of Brf1 protein (Fig 6A). Then, we transfected HepG2-ADH and MCF-7 cells with Brf1 siRNA to further determine the alteration of Pol III genes. Fig. 6B–C show that repression of Brf1 by its siRNA decrease pre-tRNALeu (Left panel) 5S rRNA transcription (Right panel). Previous studies demonstrated that repression of Brf1 was sufficient for inhibition of cell transformation (Johnson S and Johnson D, 2008). Induction of Brf1 expression allowed anchorage-independent colonies to form and promoted tumor formation (Zhang et al., 2011, 2013; Zhong et al., 2013B, 2016). To further assess potential alterations of the rate of cell transformation by alcohol-increased Brf1 expression, we carried out soft agar assay to determine cell anchorage-independent growth. We seeded AML-12 cells in soft agar and treated the cells with EGF or EGF plus ethanol, or seeded MCF-10A cells in soft agar and treated the cells with E2 or E2 plus ethanol. The results indicate that ethanol at the concentrations alone hardly induce transformation of AML-12 cells (Fig. 7A) and MCF-10A cells (Fig. 7B), whereas EGF with ethanol or E2 with ethanol significantly enhance the rates of transformation of AML-12 cell of MCF-10A cells (Fig. 7). Given that Brf1 siRNA or JNK1 siRNA decreased cellular levels of Brf1 mRNA (Fig. 3 and 6) (Zhang et al., 2013), as well as the levels of Pol III genes (Fig. 5 and 6), repression of Brf1 and JNK1 by their siRNA markedly decreased ethanol-induced colony formation of these cells, compared to control RNA (Fig. 7A–B). These results demonstrate that alcohol activates JNK1 to increase cellular level of Brf1 and enhance transcription of Pre-tRNALeu and 5S rRNA, thereby promoting alcohol-induced transformation of liver and breast cells.
Fig. 6. Repression of Brf1 decreases the induction of Pol III genes.
(A) JNK1 and Brf1 siRNA reduce the levels of Brf1 in breast cells. MCF-7 cells were transfected with mismatch RNA (siMM), JNK1 siRNA (siJNK1) or Brf1 siRNA (siBrf1) 48 hours and then treated with ethanol for another 1 hour. The cell lysates were extracted from these cells to determine. Immuno-blots were performed for these sample to determine the cellular levels of Brf1. A representative blot from three independent determinations is shown (left panel) and quantitative analysis (right panel). (B–C) Pre-tRNALeu and 5S rRNA. HepG2-ADH cells and MCF-7 cells were transfected with siMM or siBrf1 for 48 hours and then treated with ethanol as described above. RNAs was derived from these cells and RT-qPCR was performed to measure the amounts of pre-tRNALeu (B and C, left panel), 5S rRNA (B–C, right panel), and GAPDH transcripts. The fold change was calculated by normalizing to the amount of GAPDH. *: P > 0.05. The values represent mean ± SE from three independent experiments.
Fig. 7. Down-regulating JNK1 and Brf1 expression decreases ethanol-induced anchorage-independent growth.
(A) Alcohol increases the rate of liver cell transformation. Parent AML-12 cells and AML-12 cells expressing JNK1 siRNA or Brf1 siRNAs were poured in triplicate into 6-well plate with 0.35% agar containing 50mM ethanol, 20ng EGF or ethanol plus EGF; (B) Alcohol promotes transformation of breast cells. Parent MCF-10A cells and MCF-10A cells expressing JNK1 siRNA or Brf1 siRNAs were poured in triplicate into 6-well plate with 0.35% agar containing 25mM ethanol, 5nM E2 or ethanol plus E2. AML-12 cells or MCF-10A cells were incubated at 37°C in 5% CO2 for 3 weeks or longer and were fed with fresh complete media with or without ethanol, ethanol + EGF or ethanol + E2 twice weekly. Colonies were counted at 3 weeks after plating. The fold changes were calculated by normalizing to the colonies amounts control. Bar = 200 μM. *: P > 0.05. Values are the means ± SE (n ≥ 3).
4. Discussion
Our studies have shown a comparable analysis, which characterizes how alcohol mediates the transcription of endogenous Pol III genes in both liver and breast cells. The ER+ breast cells is more sensitive to alcohol than liver cells. The results have indicated that ethanol induces activation of JNK1 of HepG2 and MCF-7 cells. Inhibiting JNK1 by an inhibitor, SP600125 or its siRNA decreases the expression of Brf1 and Pol III genes caused by ethanol, whereas enhancement of JNK1 by its expressing construct augments alcohol-induced transcription of Pol III genes of both liver and breast cells. These studies demonstrate that ethanol induces expression of Brf1 and RNA Pol III genes through the JNK1 pathway. Repression of JNK1 or Brf1 is sufficient to inhibit ethanol-induced anchorage-independent growth of AML-12 cells and MCF-10A cells. Therefore, the comparable analysis reveals a common mechanism, by which ethanol activates JNK1 to increase transcription of Brf1 and Pol III genes and to promote cell transformation, resulting in alteration of cellular phenotype (Fig. 8).
Fig. 8. Schematic illustration of a proposed mechanism of alcohol-induced deregulation of Pol III genes.
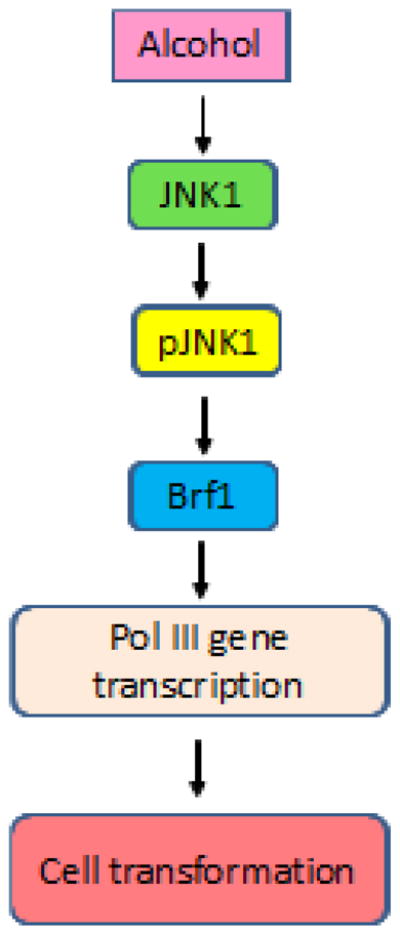
Ethanol induces activation of JNK1 to upregulate Brf1 expression, which in turn enhances Pol III gene transcription to promote alcohol-induced cell transformation.
Alcohol-caused liver injury, including liver steatosis and fibrosis, promotes liver cirrhosis, thereby increasing the risk of hepatocellular carcinoma (HCC) development (Hamajima et al., 2002; Shi et al., 2015; Zhong et al., 2013A). Alcohol combining with viruses (hepatitis C or B), carcinogens (aflatoxin), obesity, or diabetes mellitus, promotes liver cancer development (Suzuki et al., 2008; Zhong et al., 2013A). Alcohol intake has consistently been associated with an enhanced risk of breast cancer in both premenopausal and postmenopausal woman (Macmahon B, 2006; Petri et al., 2004; Zhang et al., 2013). Studies have indicated that alcohol enhanced MCP-1 and CRR2 expression, which promoted mammary tumor growth in alcohol-fed mice (Wang et al., 2011). Epidemiologic studies showed that alcohol intake was associated with ER+ breast cancer cases more than to ER- cases (Dumitrescu R & Shoelds P, 2005; Wang et al., 2011; Zhang et al., 2013). Ethanol increases ERα expression to promote breast tumor formation in mice (Wong et al., 2012). Literatures reveal that alcohol intake is associated with human cancers in more than ten organs (Bagnardi et al., 2001; Pudohit et al., 2005; Zhang et al., 2013). Why alcohol intake is related to different human cancers (Zhang et al., 2013), this question remains to be elucidated. Nucleolar hypertrophy is a consistent cytological feature of cancer cells, where products of Pol III genes are synthesized (Shi et al., 2015). The nucleolus size reflects the transcription level of Pol III genes. Thus, this cytological feature provides a possibility to identify a common mechanism of alcohol-associated cancers. Our recent studies have demonstrated that alcohol-feeding mice enhances Pol III gene transcription to promote liver tumor formation (Zhong et al., 2011). In present studies, these studies support the idea that alcohol indeed causes deregulation of Pol III genes to promote transformation of liver and breast cells (Zhong et al., 2016; Zhang et al., 2013).
Oncogenic proteins, c-Myc, cJun, c-Fos and Ras, stimulate Pol III gene transcription (Goodfellow et al., 2006: Johnson D & Johnson S, 2008; White R, 2001; Woiwode et al., 2008; Zhang et al., 2013, Zhong et al., 2004, 2007, 2015), whereas tumor suppressors, BRCA1, PTEN, p53 and pRb repress transcription of Pol III genes (Goodfellow et al., 2006: Johnson D & Johnson S, 2008; White R, 2001; Woiwode et al., 2008; Zhang et al., 2013, Zhong et al., 2004, 2007, 2015). The capacity of these oncogenes and tumor suppressor genes to regulate Pol III gene transcription result from their ability to modulate the TFIIIB complex (Zhong et al., 2013A, 2013B). The TFIIIB complex includes TATA box-binding protein (TBP) and its associated factors, Brf1 and Bdp1 (Zhang et al., 2013; Zhong et al., 2013B). In the present studies, the results indicate that ethanol increases transcription of Brf1 and Pol III genes (Fig. 2,3). We have demonstrated that the cases of human HCC with alcohol consumption reveal higher expression of Brf1 and Pol III genes (Zhang et al., 2013; Zhong et al., 2011; 2016). The animal studies further support that alcohol feeding mice promotes liver tumor formation (Machida et al., 2009; Zhong et al., 2011). Our recent results indicate that overexpression of Brf1 was in human cases of ER+ breast cancer (data not shown). This shows that alcohol-increased Brf1 expression is critically important during tumor development. Furthermore, these studies reveal that ethanol enhances Brf1 expression and Pol III gene transcription in breast and liver cell lines and the induction of these genes in cancer cell lines are higher than in non-tumor cell lines (Fig 2). Whereas, reduction of Brf1 expression inhibits Pol III gene transcription (Fig. 6) and the rates of transformation (Fig. 7). As Brf1 expression is elevated in human HCC cases (Zhong et al., 2016), high levels of Brf1 expression of HCC patients reveals shorter survival times (Zhong et al., 2016). This suggests that the high level of Brf1 reflects the oncogenesis status of cells. Brf1 may be as a novel biomarker of cancers. Whereas alcohol-caused deregulation of Brf1 and Pol III genes are a common mechanism of alcohol-associated cancers.
Our studies have shown that JNK1 and JNK2 have different roles in Pol III gene transcription (Zhong et al., 2007, 2009). JNK1 positively mediated transcription of these genes (Zhong et al., 2007). In the present studies, the results show that alcohol induces JNK1 activation in both breast and liver cells (Fig. 4). Inhibiting JNK1 reduces the induction of Brf1 and Pol III genes caused by ethanol (Fig. 5). In contrast, enhancement of JNK1 expression increases alcohol-caused Brf1 and Pol III gene induction. Alcohol may go through other MAP kinase subfamily member, such as p38 to promote breast cancer progress (Sakurai et al., 2006; Xu et al., 2016). However, JNK1 knock out mice is able to repress liver tumor development (Kalra et al., 2008; Naugker et al., 2007). Therefore, alcohol induces JNK1 activation to promote cell transformation, which may be a key step of alcohol-associated tumor development (Fig. 8) (Shi and Zhong, 2016).
In summary, the studies provide evidences that ethanol induces JNK1 activation to enhance Brf1 expression and Pol III gene transcription to promote AML-12 and MCF-10A cell transformation (Zhong et al., 2013B, 2016). The novel findings suggest a common mechanism, which mediates alcohol consumption-associated human cancers. It implies that the possibility that repression of Brf1 may be as a potential approach to inhibit alcohol-induced cell transformation and tumor development (Zhang et al., 2013; Shi & Zhong, 2016).
Supplementary Material
Highlights.
Alcohol induces deregulation of RNA Pol III genes in liver and breast cells;
The induction of Pol III genes by alcohol in breast cancer cells is more sensitive than liver cancer cells;
Alcohol-increased Pol III gene transcription is through MAP kinase, JNK1 pathway;
Alcohol upregulates Brf1 expression and Pol III gene transcription to promote cell transformation.
Acknowledgments
We want to thank Drs. M. R. Stallcup, Danial Levy and Neil Kaplowitz (University of Southern California) for scientific discussions. This work was supported by NIAAA/NIH grants AA017288, AA021114 and AA02324 to S.Z. and CNJ14C007 in China to Y.Y.
Abbreviation
- HCC
Hepatocellular carcinoma
- Pol III genes
RNA polymerase III-dependent genes
- Brf1
TFIIB-related factor 1
- ER
estrogen receptor
Footnotes
Declaration of interest
The authors declare that they have no conflict of interest.
Author Contributions
GS, ZW, NX, and SZ involved in conception and design and in analysis and interpretation of data. GS, SZ and NX involved in data acquisition and in writing and reviewing of the manuscript. YY, CH, YZ and JL involved in development of methodology. SC, ST, YZ, YY provided administrative, technical, or material support. SZ and GS supervised the study. All authors read and approved the nal manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagnardi V, Blangiardo M, La Vecchia C, Corrao G. A meta-analysis of alcohol drinking and cancer risk. Br J Cancer. 2001;85:1700–1705. doi: 10.1054/bjoc.2001.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogliani VJ, Baan R, Straif K, Crosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L. Wild CP. Preventable 33. exposures associated with human cancers. J Natl Cancer Inst. 2011;103:1827–1839. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deandrea S, Talamini R, Foschi R, Montella M, Falcini F, La Vecchia C, Franceschi S, Negri E. Alcohol and breast cancer risk defined by estrogen and progesterone receptor status: a case-control study. Cancer Epidemiol Biomarkers Prev. 2008;17:2025–2028. doi: 10.1158/1055-9965.EPI-08-0157. [DOI] [PubMed] [Google Scholar]
- Dieci G, Fiorino G, Castelnuoyo M, Telchmann M, Pageno A. The expanding RNA polymerase III transcriptome. Trends Genet. 2007;23:614–622. doi: 10.1016/j.tig.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Dumitrescu RG, Shoelds PG. The etiology of alcohol-induced breast cancer. Alcohol. 2005;35:213–225. doi: 10.1016/j.alcohol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Fang J, Sun CC, Gong C. Long non-coding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochem Biophys Res Commun. 2016;478:811–817. doi: 10.1016/j.bbrc.2016.08.030. [DOI] [PubMed] [Google Scholar]
- Goodfellow SJ, Innes F, Derbpay LE, Maclellan WR, Scott PH, White RJ. Regulation of RNA polymerase III transcription during hepertrophic growth. EMBO J. 2006;25:1522–1533. doi: 10.1038/sj.emboj.7601040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamajima N, Hirose K, Tajima K, Rohan T, Calle EE, Heath CW, Coates RJ, Liff JM, Talamini R, Chantarakul N, et al. Alcohol, tobacco and breast cancer--collaborative reanalysis of individual data from 53 epidemiological studies, including 58,515 women with breast cancer and 95,067 women without the disease. Br J Cancer, 2002. 2002;87:1234–1245. doi: 10.1038/sj.bjc.6600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC. A Review of Human Carcinogens. Vol. 100. Lyon, France: International Agency for Research on Cancer; 2011. [Accessed November 2, 2011]. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. http://monographs.iarc.fr/ENG/Monographs/PDFs/index.php. [Google Scholar]
- Johnson DL, Johnson SA. Cell biology, RNA metabolism and oncogenesis. Science. 2008;320:461–462. doi: 10.1126/science.1158680. [DOI] [PubMed] [Google Scholar]
- Johnson SA, Dubeau L, Johnson DL. Enhanced RNA polymerase III-dependent transcription is required for oncogenic transformation. J Biol Chem. 2008;283:19184–19191. doi: 10.1074/jbc.M802872200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalra M, Mayee J, Assefa S, Kaul AK, Kaul R. Role of sex steroid receptors in pathobiology of hepatocellular carcinoma. World J gastroenterology. 2008;14:5945–5961. doi: 10.3748/wjg.14.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber C. Hepatic, metabolic, and nutritional disorders of alcoholism: from pathogenesis to therapy. Crit Rev Clin Lab Sci. 2000;37:551–584. doi: 10.1080/10408360091174312. [DOI] [PubMed] [Google Scholar]
- Luedemann CE, Bord E, Qin G, Zhu Y, Goukassian D, Losordo DW, Kishore R. Ethanol modulation of TNF-alpha biosynthesis and signaling in endothelial cells: synergistic augmentation of TNF-alpha mediated endothelial cell dysfunctions by chronic ethanol. Alcohol Clin Exp Res. 2005;29:930–938. doi: 10.1097/01.alc.0000171037.90100.6b. [DOI] [PubMed] [Google Scholar]
- Machida KH, Tsukamoto H, Mkrtchyan L, Duan A, Dynnyk HM, Liu K, Asahina S, Govindarajan R, Ray JH, Ou E, Seki E, Deshaies R, Miyake K, Lai MM. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci USA. 2009;106:1548–53. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macmahon B. Epidemiology and the causes of breast cancer. Int J Cancer. 2006;118:2373–2378. doi: 10.1002/ijc.21404. [DOI] [PubMed] [Google Scholar]
- Naugler WE, Sakural T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differentces in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- Petri A, Tionneland A, Gambrog M, Johansen D, Hoidrup S, Sorensen T, Hoidrup S, Sorensne TI, Gronhback M. Alcohol intake, type of beverage, and risk of breast cancer in pre- and postmenopausal women. Alcohol Clin Exp Res. 2004;28:1084–1090. doi: 10.1097/01.alc.0000130812.85638.e1. [DOI] [PubMed] [Google Scholar]
- Purohit V, Khalsa J, Serrano J. Mechanisms of alcohol-associated cancers: introduction and summary of the symposium. Alcohol. 2005;35:155–160. doi: 10.1016/j.alcohol.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Raha D, Wang Z, Moqtaderi Z, Wu L, Zjang G, Gerstein, Struhl K, Snyder M. Close association of RNA polymerase II and many transcription factors with Pol III genes. Proc Natl Acad Sci USA. 2010;107:3639–3644. doi: 10.1073/pnas.0911315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullu E, Tschudi C. Alu sequences are processed 7SL RNA genes. Nature. 1984;312:171–172. doi: 10.1038/312171a0. [DOI] [PubMed] [Google Scholar]
- Sakural T, Maeda S, Chang L, Karin M. Loss of hepatic NF-kappa B activity enhances chemical hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1 activation. Proc Natl Acad Sci USA. 2006;103:10544–51. doi: 10.1073/pnas.0603499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz HK, Poschi G, Simanowski UA. Alcohol and cancer. Recent Dev Alcohol. 1998;14:67–95. doi: 10.1007/0-306-47148-5_4. [DOI] [PubMed] [Google Scholar]
- Shi G, Zhang Q, Levy D, Zhong S. Exploring common mechanism of alcohol-associated breast and liver cancers. Alcohol Clin Exp Res. 2015 Supplement P301A. [Google Scholar]
- Shi G, Zhong S. Alcohol-associated cancer and deregulation of Pol III genes. Gene. 2016 Sep 30; doi: 10.1016/j.gene.2016.09.046. pii: S0378-1119(16)30793-4. doi:10.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singletary KW, McNary MQ, Odoms AM, Nelshoppen J, Walling MA. Ethanol consumption and DMBA-induced mammary carcinogenesis in rats. Nutr Cancer. 1991;16:13–23. doi: 10.1080/01635589109514136. [DOI] [PubMed] [Google Scholar]
- Singletary KW, Nelshoppen J, Walling MA. Enhancement by chronic ethanol intake of N-methyl-N- nitrosourea-induced rat mammary tumorigenesis. Carcinogenesis. 1995;16:959–964. doi: 10.1093/carcin/16.4.959. [DOI] [PubMed] [Google Scholar]
- Singletary KW, Gaspstur SM. Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA. 2001;286:2143–2151. doi: 10.1001/jama.286.17.2143. [DOI] [PubMed] [Google Scholar]
- Song P, Yin SC. Long non-coding RNA EWSAT1 promotes human nasopharyngeal carcinoma cell growth in vitro by targeting miR-326/-330-5p. Aging (Albany NY) 2016;8:2948–2960. doi: 10.18632/aging.101103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CC, Li SJ, Li G, Hua RX, Zhou XH, Li DJ. Long intergenic noncoding RNA 00511 acts as an oncogene in non-small-cell lung cancer by binding to EZH2 and suppressing p57. Mol Ther Nucleic Acids. 2016A;5:e385. doi: 10.1038/mtna.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Li S, Zhang F, Xi Y, Wang L, Li D. Long non-coding RNA NEAT1 promotes non-small-cell lung cancer progression through regulationof miR-377-3p-E2F3 pathway. Oncotarget. 2016B;7:51784–51814. doi: 10.18632/oncotarget.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CC, Li SJ, Zhang F, Zhang YD, Zou ZY, Xi YY, Wang L, Li D. The novel miR-9600 suppresses tumor progression and promotes paclitaxel sensitivety in non-small-cell lung cancer progression through altering STAT3 expression. Mol Ther Nucleic Acids. 2016C;5:e387. doi: 10.1038/mtna.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki R, Orsini R, Mignone L, Saji S, Wolk A. Alcohol intake and risk of breast cancer defined by estrogen and progesterone receptor status--a meta-analysis of epidemiological studies. Int J Cancer. 2008;122:1832–1841. doi: 10.1002/ijc.23184. [DOI] [PubMed] [Google Scholar]
- Wang S, Xu M, Li F, Wang X, Bower KA, Frank JA, Lu Y, Chen G, Zhang Z, Ke Z, Shi X, Luo J. Ethanol promotes mammary tumor growth and angiogenesis: the involvement of chemoattractant factor MCP-1. Breast Cancer Res Treat. 2011;133:1037–1048. doi: 10.1007/s10549-011-1902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabiki T, Okii Y, Tokiyasu T, Yoshimura S, Yoshida M, Akane N, Shikata N, Tsubura A. Long-term ethanol consumption in ICR mice causes mammary tumor in females and liver fibrosis in males. Alcohol Clin Exp Res. 2000;24:117S–122S. [PubMed] [Google Scholar]
- White RJ. RNA polymerase III transcription and cancer. Oncogene. 2001;23:3208–3216. doi: 10.1038/sj.onc.1207547. [DOI] [PubMed] [Google Scholar]
- Winter A, Souvinos S, Allison SJ, Tosh K, Scott OH, Spandidos DA, White RJ. RNA polymerase III transcription factor TFIIIC2 is overexpressed in ovarian tumors. Proc Natl Acad Sci USA. 2000;97:12619–21264. doi: 10.1073/pnas.230224097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woiwode A, Johnson SA, Zhong S, Roeder RG, Taichmann M, Johnson DL. PTEN represses RNA polymerase III-dependent transcription by targeting the TFIIIB complex. Mol Cell Biol. 2008;28:4204–4214. doi: 10.1128/MCB.01912-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AW, Dunlap SM, Holcomb VB, Nunez NP. Alcohol Promotes Mammary Tumor Development via the Estrogen Pathway in Estrogen Receptor Alpha-Negative HER2/neu Mice. Alcohol Clin Exp Res. 2012;36:577–587. doi: 10.1111/j.1530-0277.2011.01654.x. [DOI] [PubMed] [Google Scholar]
- Xu M, Wang S, Ren Z, Franl JA, Yang XH, Zhang Z, Shi X, Luo J. Chronic ehtnal exposure chances the aggressiveness of breast cancer: the role of p38γ. Oncotarget. 2016;7:3489–505. doi: 10.18632/oncotarget.6508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan JM, Govindaraian S, Arakawa K, Yu MC. Synergism of alcohol, diabetes, and viral hepatitis on the risk of hepatocellular carcinoma in blacks and whites in the U.S. Cancer. 2004;101:1009–17. doi: 10.1002/cncr.20427. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Jin J, Zhong Q, Yu XL, Levy D, Zhong S. ERα mediates alcohol-induced deregulation of Pol III genes in breast cancer cells. Carcinogenesis. 2013;34:28–3. doi: 10.1093/carcin/bgs316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Zhong Q, Evans AG, Levy D, Zhong S. Phosphorylation of histone H3 serine 28 modulates RNA polymerase III-dependent transcription. Oncogene. 2011;30:3943–3952. doi: 10.1038/onc.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Shi G, Zhang Y, Lu L, Levy D, Zhong S. Alteration of BRCA1 expression affects alcohol-induced transcription of RNA Pol III-dependent genes. Gene. 2015;556:74–79. doi: 10.1016/j.gene.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Shi G, Zhang Y, Levy D, Zhong S. Elk-1 and AP-1 sites in the TBP promoter mediate alcohol-induced deregulation of Pol III-dependent genes. Gene. 2013A;526:54–60. doi: 10.1016/j.gene.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Shi G, Zhang QS, Zhang YM, Levy D, Zhong S. Role of phosphorylated histone H3 serine 10 in DEN-induced deregulation of Pol III genes and cell proliferation and transformation. Carcinogenesis. 2013B;34:2460–2469. doi: 10.1093/carcin/bgt219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Shi G, Zhang Q, Lu L, Levy D, Zhong S. Tamoxifen represses alcohol-induced transcription of RNA polymerase III-dependent genes. Oncotarget. 2014;5:12410–12417. doi: 10.18632/oncotarget.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Q, Xi S, Liang J, Shi GG, Huang Y, Zhang YM, Levy D, Zhong S. The significance of Brf1 overexpression in human hepatocellular carcinoma. Oncotarget. 2016;7:6243–6254. doi: 10.18632/oncotarget.6668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Fromm J, Johnson DL. TBP is differentially regulated by JNK1 and JNK2 through Elk-1, controlling c-Jun expression and cell proliferation. Mol Cell Biol. 2007;27:54–64. doi: 10.1128/MCB.01365-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Mechida K, Tsukamoto H, Johnson DL. Alcohol induces RNA polymerase III-dependent transcription through c-Jun by coregulating TBP and Brf1 expression. J Biol Chem. 2011;286:2393–2401. doi: 10.1074/jbc.M110.192955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Johnson DL. The JNKs differentially regulate RNA polymerase III transcription by coordinately modulating the expression of all TFIIIB subunits. Proc Natl Acad Sci USA. 2009;106:12682–12687. doi: 10.1073/pnas.0904843106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong S, Zhang C, Johnson DL. Epidermal Growth Factor enhances cellular TBP levels and induces RNA polymerase I- and III-dependent gene activity. Mol Cell Biol. 2004;24:5119–5129. doi: 10.1128/MCB.24.12.5119-5129.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Wang F, Chen H, Tan Q, Qiu S, Chen S, Jing W, Yu M, Liang C, Ye S, Tu J. Increased expression of long-noncoding RNA ZFAS1 is associated with epithelial-mesenchymal transition of gastric cancer. Aging (Albany NY) 2016;8:2023–2038. doi: 10.18632/aging.101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.